Abstract
Objective
A startling loud acoustic stimulus can involuntarily elicit planned movements, a phenomenon referred to as startReact. Following stroke, startReact elbow flexion in stroke survivors are improved from voluntary movements. Specifically, startReact elbow flexion in unimpaired individuals is not statistically different from stroke survivors in terms of onset latency and muscle activation patterns. As hand movements are particularly impacted by stroke, our objective was to determine if startReact was intact in the hand following stroke.
Methods
Data were collected in 8 stroke survivors and 10 age-matched subjects performing hand extension following two non-startling acoustic stimuli representing “get ready” and “go” respectively. Randomly, the “go” was replaced with a startling acoustic stimulus. We hypothesized that 1) startReact would be intact during hand extension in stroke survivors and 2) that the latency of movement would be the same as in age-matched subjects.
Results
We found that startReact was intact in stroke subjects and further that the onset latency of these movements was not different from age-matched subjects.
Conclusions
We conclude that startReact is intact in the hand following stroke.
Significance
An intact startReact response indicates that this reflex may be an attractive therapeutic target for initiating hand extension in stroke survivors.
Keywords: Startle, startReact, stroke, hand
INTRODUCTION
Movement deficits following stroke are particularly prevalent in the hand leading to significant reduction in independence and the ability to participate in daily functions (Latham, 1989). Therefore, new therapies that target the hand are extremely valuable. Startle-elicited movements have recently been implicated as a possible therapy target. The classic startle reflex that occurs during exposure to a startling stimulus, e.g. loud sound, results in the adoption of a protective stance (flexion of the upper joints). However, if the subject is planning a movement when the startling stimulus occurs, the subject involuntarily releases the planned movement a phenomenon referred to as startReact (Valls-Sole et al. , 1999). It was demonstrated that startReact elbow flexion movements are not statistically different between unimpaired individuals and stroke survivors in onset latency and muscle activation patterns (Honeycutt and Perreault, 2012) indicating that startReact may be an attractive therapeutic target in stroke survivors. As hand movements are particularly impacted by stroke, our objective was to determine if startReact was intact in the hand following stroke and to compare the responses to age-matched controls.
There is evidence that the startReact response may be more difficult to achieve in the hand. First, the startReact response is likely mediated in part via the reticulospinal tract (Davis et al. , 1982, Davis and Gendelman, 1977a, Groves et al. , 1974, Hammond, 1973, Rothwell, 2006). As movement of the hand relies heavily on corticospinal control, it was unclear if startReact could be elicited in the hand. Still, there is evidence from primates (Riddle and Baker, 2010) that reticulospinal projections extend to the muscles of the hand, an observation that likely extends to humans (Honeycutt et al. , 2013). Furthermore, it has been suggested that following a stroke individuals rely more heavily on reticulospinal pathways for movement execution (Dewald et al. , 1995). We hypothesized that 1) startReact would be intact during hand extension in stroke survivors and that 2) the latency of movement would be the same as in age-matched, unimpaired subjects. If true, it would suggest that startReact is an appropriate alternative pathway to activate muscles of the hand.
METHODS
Subjects
Data were collected from 8 chronic stroke subjects (age: 59 ± 11;Table 1) and 10 unimpaired, age-matched subjects (age: 66 ± 10). A two-sample, unpaired t-test confirmed that no difference existed between the ages of each population (p = 0.18). Subjects had no self-reported hearing damage and were capable of understanding the task and providing oral, informed consent. All experiments were approved by Northwestern University Institutional Review Board IRB (STU9204).
Table 1.
Subject Characteristics.
| Subject # | Sex | Age | Paretic Limb | Dominant Arm | Years since Stroke | Chedoke Hand | Multiple Strokes |
|---|---|---|---|---|---|---|---|
| 1 | M | 63 | R | R | 5 | 4 | N |
| 2 | F | 58 | L & R | R | 9 | 6 | Y |
| 3 | M | 70 | R | R | 3 | 4 | N |
| 4 | M | 36 | R | R | 1 | 6 | N |
| 5 | M | 55 | R | L | 5 | 4 | N |
| 6 | F | 61 | R | R | 27 | 3 | N |
| 7 | M | 59 | R | R | 10 | 7 | N |
| 8 | F | 70 | R | R | 6 | 4 | N |
Bipolar electromyography (EMG) electrodes recorded activity from the right extensor digitorum communis (EDC) and the left and right sternocleidomastoid muscles (SCM). EMG signals were pre-amplified and filtered with a band-pass filter of 10-1000 Hz. The resulting signals were anti-alias filtered by 5th order Bessel filters with a 500 Hz cut-off frequency and sampled at 2500 Hz.
Protocol
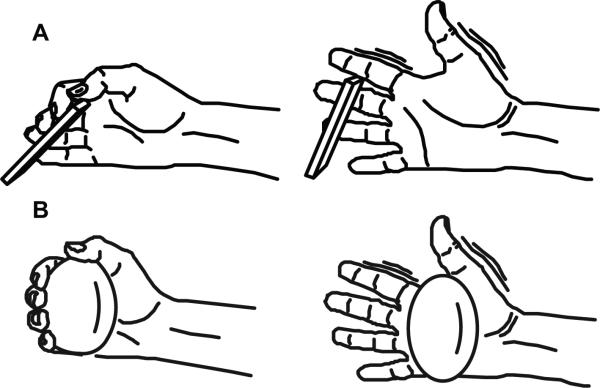
Subjects were seated in a chair with the right elbow joint flexed at 90 degrees. Subjects were asked to extend their right hand from a neutral, resting position with the palm oriented in the sagittal plane and the thumb on the top. In unimpaired subjects, a switch device was placed such that the switch was pressed when participants were in a resting position and was released when they extended their hand (Figure 1A). The switch device was used during data processing to ensure that the task was executed correctly. Due to decreased hand function, stroke subjects were asked to relax their hand around a ball and then extend their grasp (Figure 1B). An electrogoniometer was fixed on the right index finger in 6 of 8 stroke subjects to quantify hand extension.
Figure 1. Task depiction.
A) In unimpaired, age-matched subjects a switch device was placed such that the switch was pressed when participants were in a resting position and was released when they extended their hand. B) Stroke subjects extended their hand from a neutral, resting position while holding a ball.
Subjects were instructed to wait in a relaxed position for two non-startling, low-intensity acoustic stimuli (80 dB). The first sound (WARNING) signaled the subject to get ready and prepare the extension task. The second sound (GO) was the cue to initiate the extension movement as fast as possible. The time between the WARNING and the GO signal was variable (± 2s) to avoid anticipation. WARNING and GO cues were delivered by a small speaker located in front of the subject. Following training, participants performed six blocks of fifteen trials. During each block 3-5 trials were randomly selected to be startReact trials. During startReact trials, the GO signal was replaced by a startling, high-intensity acoustic stimulus (128 dB) emitted from a loud speaker located behind the subject's head. StartReact protocols are similar to those in previous reports (Honeycutt, Kharouta, 2013, Honeycutt and Perreault, 2012, Honeycutt and Perreault, 2013). Command signals for WARNING, GO, and the startling acoustic stimulus were generated in MatLab and their analog signals recorded to ensure they were delivered at the appropriate latency. Finally, we performed a linear regression at a confidence interval of 95% with impairment (Chedoke score) as the independent variable and the HI:SCM+ onset latency and probability of SCM+ as the dependent variables.
Data analysis
Onset latency of the EDC and SCM muscles was determined for all trials. EMG signals were rectified and filtered with a 10-point moving average filter. Onsets were identified with a Matlab (R2011b, the MathWorks) automatic detection file. The latency of muscle activity onset was calculated from the rectified EMG recorded in each trial. The average background activity and standard deviation prior to the stimulus were calculated. Next an automated program identified the time at which the processed EMG increased above 2.5 times the standard deviation of the background activity for a period of 15ms (for EDC) or rose above the maximum background activity for a period of 5ms (for SCM). Following the automatic detection of EMG onset, all trials were visually inspected for accuracy and to exclude trials when the subjects did not move or moved before the GO signal. Trial type was blinded to the reviewer.
Next we assessed the presence of a startle during all trials. Right or left SCM muscle onset latency within 120ms of the GO signal was used as an indicator of startle (Carlsen et al. , 2011). Trials where a startle was detected were designated SCM+, while those without were designated SCM-.
To determine if a movement was susceptible to startReact, it was first necessary to differentiate between the intensity-dependent and startle-dependent effects on reaction time. Faster onset latencies occur in the presence of a startle (startle-dependent effect)(Valls-Sole, Rothwell, 1999) and when the intensity of the GO stimulus is increased (intensity-dependent effect)(Kohfeld, 1969). To differentiate between these two factors, low-intensity SCM- trials are compared to high-intensity SCM- trials to quantify the intensity-dependent effect and high-intensity SCM+ and SCM- trials are compared to determine if the response is susceptible to startReact (startle-dependent effect). This results in 3 important trial types:1) Low-intensity SCM- trials (LI SCM-), 2) high-intensity SCM- trials (HI SCM-), 3) high-intensity SCM+ trials (HI SCM+).
We hypothesized that 1) startReact would be intact during hand extension in stroke survivors and that the latency of movement would be the same as in age-matched controls. If true, it would suggest that startReact is an appropriate alternative pathway to activate muscles of the hand. An intact startReact response would be indicated if muscle activity onset in HI SCM+ trials was significantly faster than HI SCM-. This hypothesis was tested using an ANOVA of a linear mixed-effect model with trial type (LI SCM-, HI SCM+, HI SCM-) and impairment (stroke subjects, unimpaired subjects) as the independent factors while onset latency was the dependent factor. Subjects were treated as a random effect. Equal variance was not assumed.
All individual trials were included in the analysis to decrease the probability of statistical errors by capturing all the variability of the data set (Hedeker, 2006). Tukey's Honestly Significant Difference (TukeyHSD) was applied for all post-hoc comparisons. The probability of eliciting a startReact (SCM+) was compared between unimpaired and stroke subjects using a two-sample, unpaired t-test. Electrogoniometer traces were assessed for similarity between HI:SCM+ and LI:SCM- trials in stroke subjects utilizing cross-correlation. ANOVA statistical analyzes were computed utilizing R (R Development Core Team, 2006) while t-tests and cross-correlations were performed utilizing MatLab R2007b. Differences with a probability lower than 0.05 were considered to be significant. All error bars in figures relate to standard deviations.
RESULTS
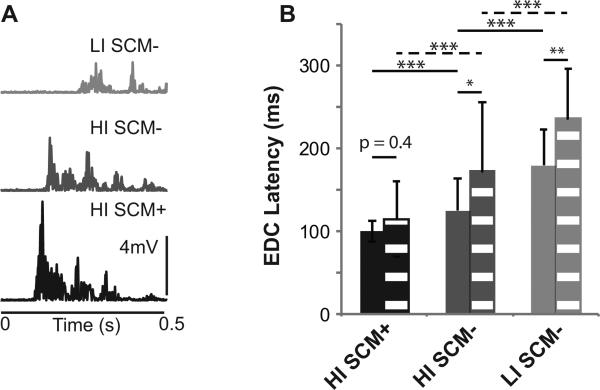
Hand extension was susceptible to startReact in both unimpaired and stroke subjects. In unimpaired subjects (Figure 2B – solid bars) and stroke subjects (Figure 2A, 2B – dashed bars), EDC muscle onset latencies were faster in HI SCM+ (unimpaired: 100 ± 12ms; stroke: 115 ± 45ms) trials compared to HI SCM- (unimpaired: 125 ± 39ms; stroke: 172 ± 83ms) and LI SCM-trials (unimpaired: 179 ±43ms; stroke: 236 ± 60ms). This result was confirmed by group results with latency significantly influenced by trial type (F2,1142 = 404.6; p < 0.0001) and impairment (F1,16 = 4.6; p = 0.04). Post-hoc tests confirmed that HI SCM+ trials were significantly faster than HI SCM- and LI SCM- trials (all: p 0) in both unimpaired and stroke subjects.
Figure 2. Sample data and EDC onset latency.
A) Representative EMG from the EDC muscle is shown for all trial types in stroke subjects. B) Group results comparing EDC onset latencies between trial types (HI SCM+, HI SCM-, and LI SCM- trial types) and stroke (dashed) and unimpaired (solid) subjects. ***P < 0.0001.
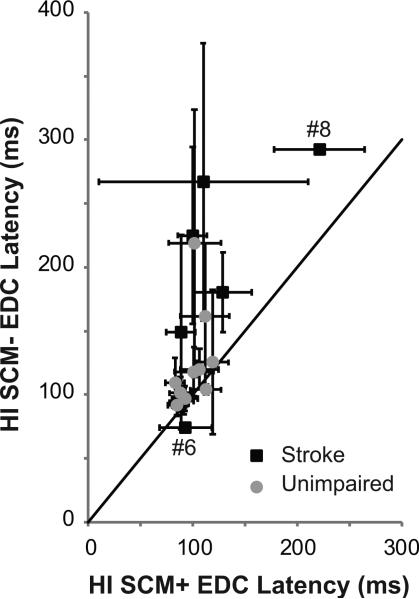
While differences were found during SCM- trials, SCM+ trials were similar in both unimpaired and stroke subjects. Both SCM- conditions (HI: p = 0.02 and LI: p = 0.005) were significantly affected by impairment; however, HI:SCM+ EDC onset latency was not affected by impairment (p = 0.44) (Figure 2B). With the exception of subject # 8, the HI:SCM+ EDC onset latencies of stroke subjects (Figure 3 – black) overlapped with those of unimpaired subjects (Figure 3 – gray). The probability of eliciting a SCM+ was also not different between unimpaired (0.55 ± 0.26) and stroke subjects (0.43 ± 0.26)(p = 0.33). Still, subject # 6, who had the most significant impairment (Chedoke 3), did not have an intact startReact (stroke subject lying below the unity line in Figure 3). Subject 6 did show an increase in detectable muscle activity. During LI SCM-trials, muscle activity was only detectable during 6% of trials compared to 31% of HI SCM+ trials. Despite this, no relationship was found between impairment level (Chedoke score) and HI:SCM+ latency (p = 0.4) or the probability of eliciting SCM+ (p = 0.5).
Figure 3. Relationship between SCM+ and SCM- latencies.
HI SCM+ EDC onset latency is graphed as a function of HI SCM- onset latency for stroke (black) and unimpaired (gray) subjects. A unity line is presented in black. Stroke subjects # 6 and 8 are identified on the figure.
While HI SCM+ trials showed faster onset latencies, the trajectory of these movements was unchanged from HI SCM- trials (Figure 4). Indeed, even spastic movements exhibited during HI SCM- trials were exhibited during HI SCM+ trials (Figure 4C). Cross-correlation results confirmed this with HI:SCM+ trials showing an average 0.90 ± 0.08 correlation to LI:SCM- trials across stroke subjects. Consistent with EDC muscle activity, movement was initiated faster.
Figure 4. Electrogoniometer.
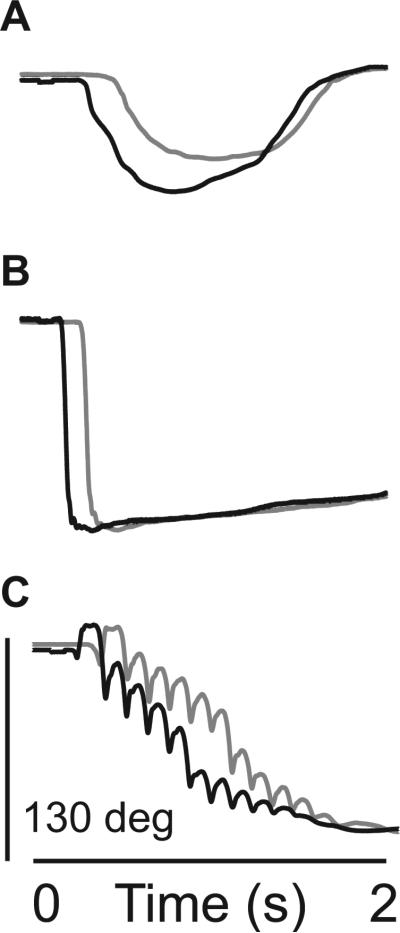
Representative goniometer data from HI SCM+ (black) and HI SCM- (gray) trials from stroke subjects with moderate (A: Chedoke = 4), mild/moderate (B: Chedoke = 6), and mild (C: Chedoke = 7) impairment.
DISCUSSION
Summary
Our result that HI SCM+ EDC onset latency was significantly faster than HI SCM- and LI SCM-trials indicates that startReact hand extension is intact in stroke survivors. Movements were significantly faster when a startle was detected compared to those where startle was absent in both stroke and unimpaired subjects. While voluntary (LI:SCM-) trials were slower in stroke subjects, startReact (HI:SCM+) trials were not different in latency between stroke and unimpaired subjects. Despite this faster muscle activity onset latency, the trajectory of movement was unchanged in stroke survivors between startReact and voluntary trials. Indeed, spastic movements exhibited during voluntary movement were similarly exhibited during startReact movements (Figure 4C). StartReact did not appear to be present in the most significantly impaired subject though this subject saw an increase in detectable muscle activity during startReact trials. These results indicate that startReact is intact in the hand of stroke subjects and can be utilized to decrease movement onset but that the movement trajectory is not improved.
Comparison between unimpaired and stroke
While voluntary trials were initiated slower in stroke subjects, startReact movements were initiated at latencies that were not significantly different (p = 0.4). This result is similar to a previous report at the elbow demonstrating the same trend (Honeycutt and Perreault, 2012). Still, the decrease in onset latency did not lead to changes in the movement trajectory. Movement traces were highly correlated (0.90) between voluntary and startReact trials indicating few differences between these movements.
Previous reports at the elbow showed inappropriate flexor activity that lead to deviations away from stroke subject's intended target (Honeycutt and Perreault, 2012, Honeycutt and Perreault, 2013); however we did not find evidence of this in the hand indicated by a strong similarity between voluntary and startReact movement traces. It was previously hypothesized that the inappropriate flexor activity results from an unsuppressed classic startle reflex - the adoption of a crouched stance that is dominated by flexor activity in the upper limb (Honeycutt and Perreault, 2012). As the classic startle reflex does not result in strong activation in the hand (Brown et al. , 1991), this could explain why we did not see inappropriate activity. Further, we evaluated only moderately to mildly impaired individuals. The inappropriate flexor activity at the elbow is more dominant in more severely impaired individuals (Honeycutt and Perreault, 2013).
It was noted that the probability of eliciting startReact was less in the hand (stroke: 0.42) than at the elbow (0.80); however lower probability of startReact was seen in both stroke and unimpaired groups indicating that the decrease is the result of the task and not the stroke population. Further, the lower probability does not likely relate to increased impairment as the population evaluated here was less impaired than the population where elbow startReact was evaluated.
A diminished probability of startReact in the hand is not unexpected given that the startReact response is likely mediated in part through the reticulospinal tract (Davis, Gendelman, 1982, Davis and Gendelman, 1977a, Groves, Wilson, 1974, Hammond, 1973). While this pathway has recently been shown to have connections to the hand muscles in the primate (Riddle and Baker, 2010), an observation that likely extends to humans (Honeycutt, Kharouta, 2013), these connections are fewer and weaker than their expression at the elbow.
Though the specific neural mechanisms driving the startReact response remain debated (Alibiglou and MacKinnon, 2012, Honeycutt, Kharouta, 2013, MacKinnon et al. , 2013, Marinovic et al. , 2014), numerous studies indicate the reticular formation is utilized during the response. Specifically, the startle reflex is completely blocked by lesions either of the caudal pontine reticular formation or medullary reticular formation (Davis, Gendelman, 1982, Groves, Wilson, 1974, Hammond, 1973) but remains intact following lesion (Davis, Gendelman, 1982) or removal (Davis and Gendelman, 1977b) of the cerebral cortices. Further in humans, individuated movements of the hand that are expressed predominately through the corticospinal tract (Kuypers, 1981, Lawrence and Kuypers, 1968, Lemon et al. , 2012, Schieber, 2004, 2011) are not susceptible to startReact (Carlsen et al. , 2009, Honeycutt, Kharouta, 2013). Finally, startReact is intact in hereditary spastic paraplegic patients with selective corticospinal degradation indicating that these responses are mediated via the reticulospinal tract (Nonnekes et al. , 2014).
It is important to note that the cortex and the corticospinal tract are known to modulate the startReact response though their specific role in the response remains uncertain (Alibiglou and MacKinnon, 2012, MacKinnon, Allen, 2013, Marinovic, Tresilian, 2014). Therefore, the decreased probability of startReact could also be due to an alternative mechanism such as a decreased proficiency at the task. StartReact is not intact when subjects cannot prepare movement in advance (Carlsen et al. , 2008). In the case of stroke subject #6, who was only able to achieve muscle activation during 6 percent of voluntary trials, startReact was not intact. Similarly, hand extension is more challenging for stroke survivors than elbow movement and therefore may be more difficult to prepare in advance.
Functional significance
Still, the result that startReact hand extension exists in stroke survivors indicates that the reticulospinal connections to the hand remain intact following stroke and further that they are strong enough to elicit faster movement. The corticospinal tract, the dominant neural pathway mediating hand movement (Lemon, 2008), is severely damaged following stroke (Zhu et al. , 2010). Therefore, an intact reticulospinal tract to the hand following stroke suggests that the reticulospinal tract may serve as an appropriate alternative pathway for voluntary movement of the hand and should be considered as a potential therapeutic target following stroke. Though reticular neurons are strongly mediated during fine finger movements (Soteropoulos et al. , 2012), startReact does not appear to be intact during individuated movements of the fingers (Honeycutt, Kharouta, 2013) Therefore, the usefulness of the startReact phenomenon may not extend to fine finger control.
Limitations
Our report only evaluated mild to moderately impaired subjects, more experiments are required to ascertain whether startReact movements can be activated in more severely impaired individuals and if startReact movements are improved compared to voluntary movements in stroke survivors. Indeed, our most significantly impaired subject did not appear to have an intact startReact (Figure 3C). While subjects were asked to self-report hearing loss, hearing deficits were not quantified. StartReact responses remained intact indicating that hearing loss was not a dominant factor; however the probability of eliciting startReact was diminished which could be driven in part by less hearing sensitivity. Finally, specific lesion data were not available for the subjects in this study. This information could have provided additional information to inform the mechanisms driving startReact responses in the hand.
HIGHLIGHTS.
We found hand extension is susceptible to the startReact reflex (involuntary release of planned movement by a startling stimulus) in stroke survivors.
While voluntary movements were delayed in stroke subjects compared to age-matched unimpaired subjects, startReact onset latencies were not different between the populations.
Though previous reports demonstrated abnormal elbow movement trajectories during startReact following stroke, these deficits were not seen in the hand.
ACKNOWLEDGMENTS
The authors would like to thank Tim Goetz-Haswell his technical and scientific expertise. This work was supported by the National Institutes of Health grants R01 NS053813 and K99 HD073240.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alibiglou L, MacKinnon CD. The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex. J Physiol-London. 2012;590:919–936. doi: 10.1113/jphysiol.2011.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain. 1991;114:1891–902. doi: 10.1093/brain/114.4.1891. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Dakin CJ, Sanderson DJ, Inglis JT, Franks IM. Startle reveals an absence of advance motor programming in a Go/No-go task. Neurosci lett. 2008;434:61–5. doi: 10.1016/j.neulet.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Differential effects of startle on reaction time for finger and arm movements. J neurophysiol. 2009;101:306–14. doi: 10.1152/jn.00878.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM. Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev. 2011;35:366–76. doi: 10.1016/j.neubiorev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Gendelman PM. Plasticity of the acoustic startle response in the acutely decerebrate rat. J Comp Physiol Psychol. 1977a;91:549–63. doi: 10.1037/h0077345. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman PM. Plasticity of the acoustic startle response in the acutely decerebrate rat. J Comp Physiol Psychol. 1977b;91:549–63. doi: 10.1037/h0077345. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Groves PM, Wilson CJ, Boyle RD. Brain stem pathways, cortical modulation, and habituation of the acoustic startle response. Behav Biol. 1974;10:391–418. doi: 10.1016/s0091-6773(74)91975-0. [DOI] [PubMed] [Google Scholar]
- Hammond GR. Lesions of pontine and medullary reticular formation and prestimulus inhibition of the acoustic startle reaction in rats. Physiol Behav. 1973;10:239–43. doi: 10.1016/0031-9384(73)90304-1. [DOI] [PubMed] [Google Scholar]
- Hedeker DGRD. Longitudinal data analysis. 2006:337. [Google Scholar]
- Honeycutt CF, Kharouta M, Perreault EJ. Evidence for reticulospinal contributions to coordinated finger movements in humans. J Neurophysiol. 2013;110:1476–83. doi: 10.1152/jn.00866.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ. Planning of Ballistic Movement following Stroke: Insights from the Startle Reflex. PLoS ONE. 2012;7:e43097. doi: 10.1371/journal.pone.0043097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ. Deficits in startle-evoked arm movements increase with impairment following stroke. Clin Neurophysiol. 2013 doi: 10.1016/j.clinph.2013.12.102. doi 10.1016/j.clinph.2013.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohfeld DL. Effects of the intensity of auditory and visual ready signals on simple reaction time. J Exp Psychol. 1969;82:88–95. doi: 10.1037/h0028033. [DOI] [PubMed] [Google Scholar]
- Kuypers H. Handbook of physiology Sect I The nervous system: Am Physiol Soc. Bethesda, MD: 1981. Anatomy of descending pathways. pp. 597–666. [Google Scholar]
- Latham CAT. Occupational therapy for physical dysfunction. 3rd ed. Williams & Wilkins; Baltimore: 1989. [Google Scholar]
- Lawrence DG, Kuypers HGJ. Functional Organization of Motor System in Monkey. Brain. 1968;91:1. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Landau W, Tutssel D, Lawrence DG. Lawrence and Kuypers (1968a, b) revisited: copies of the original filmed material from their classic papers in Brain. Brain. 2012;135:2290–5. doi: 10.1093/brain/aws037. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Allen DP, Shiratori T, Rogers MW. Early and unintentional release of planned motor actions during motor cortical preparation. PLoS One. 2013;8:e63417. doi: 10.1371/journal.pone.0063417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinovic W, Tresilian JR, de Rugy A, Sidhu S, Riek S. Corticospinal modulation induced by sounds depends on action preparedness. J Physiol. 2014;592:153–69. doi: 10.1113/jphysiol.2013.254581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnekes J, Oude Nijhuis LB, de Niet M, de Bot ST, Pasman JW, van de Warrenburg BP, et al. StartReact restores reaction time in HSP: evidence for subcortical release of a motor program. J Neurosci. 2014;34:275–81. doi: 10.1523/JNEUROSCI.2948-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol. 2010;103:2821–32. doi: 10.1152/jn.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC. The startle reflex, voluntary movement, and the reticulospinal tract. Suppl Clin Neurophysiol. 2006;58:223–31. doi: 10.1016/s1567-424x(09)70071-6. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Motor control: basic units of cortical output? Curr Biol. 2004;14:R353–4. doi: 10.1016/j.cub.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Dissociating motor cortex from the motor. J Physiol. 2011;589:5613–24. doi: 10.1113/jphysiol.2011.215814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soteropoulos DS, Williams ER, Baker SN. Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J Physiol. 2012;590:4011–27. doi: 10.1113/jphysiol.2011.225169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516:931–8. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–5. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]