Abstract
Histone proteins undergo various types of post-translational modifications (PTMs) to regulate dynamic processes in the cell, including replication, transcription and DNA damage repair. One type of histone PTM is the attachment of a small protein, ubiquitin (Ub). In eukaryotic organisms, a single Ub is attached to specific lysine residues of histones H2A and H2B in a modification that, unlike many other forms of ubiquitination in the cell, does not signal degradation. Instead, both attachment and removal of Ub to these histones has been shown to affect gene transcription, pre-mRNA splicing, and DNA damage repair, but the mechanisms by which histone ubiquitination governs these processes are not well understood. In an effort to identify “readers” of Ub-histones, we developed a straightforward crosslinking strategy to generate nonhydrolyzable Ub-histone mimics. These mimics were assembled into Ub-histone-containing dimers or nucleosomes. We demonstrate that they can be used in pulldown assays to identify proteins that differentiate unmodified and ubiquitinated histones.
Keywords: ubiquitin, histone modification, effector proteins
1. Introduction
A large and growing number of post-translational modifications (PTMs) have been mapped to histone proteins, which compact genomic DNA and control its access in eukaryotes [1, 2]. Dynamic addition and removal of these modifications have important roles in the regulation of many cellular processes including DNA damage repair, DNA replication, and transcription. Some histone modifications directly influence the structure of chromatin by modulating the stability of nucleosomes or higher-order chromatin structure. The vast majority of the modifications, however, function by recruiting or excluding effector proteins that interact with chromatin. These effector proteins, many of which are “readers” of the modifications, are identified by their abilities to distinguish modified versus unmodified forms of histones or nucleosomes. The use in pulldown assays of biotinylated synthetic histone peptides that have been methylated, acetylated, or phosphorylated at specific residues has proven to be a powerful tool for the discovery of effector proteins [3–5]. However, this approach may not be applicable to histones modified by ubiquitination.
In comparison to most other PTMs, ubiquitination stands out because of the much larger size of the modifier. Ubiquitin (Ub) is a 76-amino acid protein that is attached via an isopeptide bond formed between its C-terminal carboxylate and a lysine side chain of the substrate protein. Ub can also be attached to other Ub molecules to form polyUb chains. Although polyubiquitination and degradation of histones have been reported under specific conditions, such as DNA damage repair or spermatogenesis [6], the major form of histone ubiquitination is attachment of monoUb, which does not lead to degradation (Table 1). In all eukaryotes, histone H2B is modified by monoUb on a specific lysine residue in its C-terminal tail (K123 in yeast and K120 in humans). In metazoans, a significant fraction of histone H2A (5–15%) is also modified by monoUb in its C-terminal tail (K119 in humans) [7]. Whereas H2A ubiquitination is associated with transcriptional repression and silencing, H2B ubiquitination is associated with actively transcribed regions and has multiple roles in initiation, elongation and mRNA processing (reviewed in [8, 9]). Both H2A and H2B ubiquitination have also been implicated in DNA double strand break (DSB) repair [10]. In addition, H2B ubiquitination has been reported to enhance nucleosome assembly during DNA replication in yeast [11]. Despite accumulating evidence of the functional significance of histone ubiquitination, surprisingly little is known about how the modification elicits specific functions. This is in part due to difficulty in obtaining chemically-defined Ub-histone conjugates for in vitro studies.
Table 1.
Histone mono-ubiquitination at different sites are associated with different functions.
| Histone (human) | Mono-ubiquitination Sites | Associated functions |
|---|---|---|
| H2A | 13, 15 | DNA damage response [36, 37] |
| H2A | 119 | Transcription silencing and DNA damage response. Corresponding positions in H2A.X, H2A.Z, and MacroH2A1.2 have also been reported to undergo similar ubiquitination (reviewed in [8, 9]). |
| H2B | 34 | Transcription activation [15] |
| H2B | 120 | Transcription initiation, elongation, pre-mRNA splicing and mRNA export (reviewed in [8, 9]). |
| H3 | 23 | Maintenance of DNA methylation during DNA replication [38] |
| H4 | 31 | Transcription activation [39] |
| H4 | 91 | DNA damage response [40] |
In cells, attachment of Ub to a substrate protein is the product of the action of three enzymes, E1, E2, and E3; E3 enzymes are the Ub–protein ligases that select the protein targets for ubiquitination. Deubiquitinating enzymes (DUBs) reverse these modifications, and typically are highly regulated or act on specific Ub-protein conjugates. For histone ubiquitination, in most cases the responsible E2 and E3 enzymes have been identified through genetic studies (reviewed in [8, 9]). However, reconstitution of an in vitro system that can produce quantities of ubiquitinated histones sufficient for biochemical studies has been challenging. In the case of human H2B, using recombinant E1, E2 (RAD6A or RAD6B), E3 (RNF20/RNF40) and nucleosome substrates, the yield of ubiquitinated products is typically less than 5%. Additionally, depending on the condition, these reactions often result in non-specific ubiquitination at sites other than H2BK120 [12]. As an alternative approach, ubiquitinated histones have been purified from cells [13, 14]. This is often assisted by expression of epitope-tagged Ub in order to facilitate enrichment of ubiquitinated species. However, native histones are inherently heterogenous due to the large variety of other naturally-occurring PTMs. In addition, the presence of cellular DUBs can significantly lower the yield by nonspecific deubiquitination. Such an approach is also not practical for the isolation of Ub-histone species that are inherently of low abundance, such as H2B ubiquitinated at K34 [15]. Recent advances in chemical biology have led to the development of several semi-synthetic strategies to obtain histones ubiquitinated at a specific site. These methods use a combination of expressed protein ligation (EPL) and solid phase peptide synthesis (SPPS) techniques that can produce homogeneously-modified histones [16–19]. The drawback, however, is that these methods are often cumbersome and technically challenging. Moreover, procedures developed for a Ub-histone conjugate in which the Ub is attached to one particular lysine residue often are not easily modified to synthesize conjugates with Ub attached at different sites.
For many applications, a native isopeptide bond that links Ub to histone is not required. For example, Chatterjee et al. developed a Ub-H2B mimic in which Ub is linked to H2B at residue 120 via a disulfide bond (uH2Bss). Despite that a disulfide bond is ~2.4 Å longer than a native isopeptide bond, this mimic was successfully used to probe the activation mechanism of histone methyltransferase hDot1L [20] and to study how H2B ubiquitination affects chromatin compaction [21]. Unfortunately, the labile nature of the disulfide bond prevents broad application of this mimic. To overcome these obstacles, we have developed a strategy to synthesize a nonhydrolyzable Ub-histone mimic that we can purify to homogeneity in large amounts and is impervious to disassembly by cellular DUBs. Such mimics can be readily assembled into H2A/H2B dimers, histone octamers, or nucleosomes in vitro. Using these substrates, we have performed pulldown assays to identify proteins that preferentially bind unmodified or ubiquitinated histones or nucleosomes ([22], L.L and T.Y., unpublished). For simplicity, we will use human H2A ubiquitinated at K119 as an example throughout the method description. However, the same method can be used to generate all types of Ub-histone mimics regardless of the particular ubiquitination site or histone type.
2. Generation of ubiquitinated histone mimics
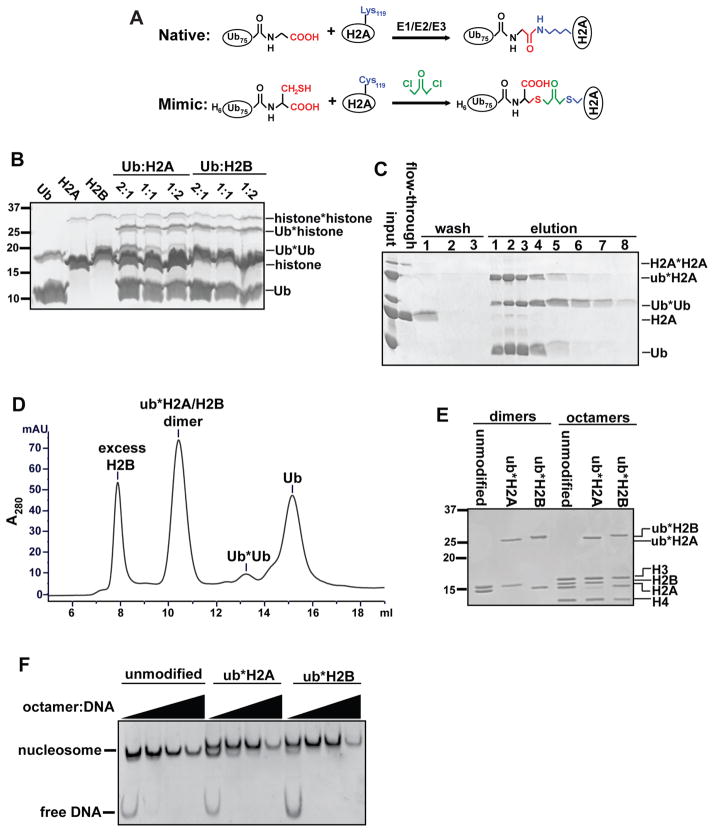
In the past decade, there have been significant advances in the development of strategies to generate diUb of specific linkage types [23]. Several mimics of the native isopeptide bond have been introduced that are resistant to DUB cleavage, such as oxime or triazole linkages [24–26]. The approach we used to generate nonhydrolyzable Ub-histone mimics was derived from a strategy previously employed to generate diUb [27] and ubiquitinated PCNA [28] analogs. It takes advantage of a highly reactive bifunctional thiol crosslinker, 1,3-dichloroacetone. Since neither Ub nor H2A contains naturally occurring cysteine residues, we can implement site-specific crosslinking by introducing cysteine at the C-terminus of Ub (G76C) and the native ubiquitination site of human H2A (K119C). Compared to a native Ub-protein isopeptide linkage, the crosslinked product contains an additional carboxylate group and is one C-C bond longer (Figure 1A). The crosslinked Ub-H2A mimic (ub*H2A, where the asterisk denotes the crosslink) was further assembled into H2A/H2B dimers, histone octamers and nucleosomes.
Figure 1.
Synthesis and purification of ubiquitinated histone mimics. (A) Schematic comparing native ubH2A and the crosslinked mimic. In the mimic, Gly76 of Ub and Lys-119 of H2A were mutated to cysteines. Crosslinking with 1,3-dicholoroacetone produces a dithioether linkage that is one C-C longer than an isopeptide linkage, contains an additional carboxylate, and is resistant to cleavage by DUBs (origninally published in [22]). (B) Pilot-scale crosslinking reactions were done by varying the ratio of Ub and histones. Products were separated by SDS-PAGE and visualized by Coomassie blue staining. (C) Crosslinked products were purified using Ni-NTA agarose. Eluates (containing Ub, Ub*Ub, and Ub*histones) were separated by SDS-PAGE and visualized by Coomassie blue staining. (D) HPLC chromatogram of purification of ub*H2A/H2B dimers. Dimers assembled as described in section 2.4 were injected on a Superdex 75 column. Absorbance at 280 nm was monitored over time and eluates were visualized by SDS-PAGE and Coomassie blue staining. ub*H2A/H2B eluted at ~10.4 ml. H2B, Ub*Ub, and Ub eluted at approximately 7.9, 13.2, and 15.1 ml, respectively. (E) 1 μg of purified, unmodified or Ub*histone-containing dimers and octamers were analyzed by SDS-PAGE and Coomassie blue staining. (F) Purified histone octamers containing unmodified histones, ub*H2A or ub*H2B mimics, were assembled into mononucleosomes with 183mer DNA containing a centrally-localized 601 sequence. Assembly was done by salt dilution [16] and the octamer:DNA ratio was titrated in order to achieve the optimal condition.
2.1 Expression and purification of recombinant proteins
We inserted a 6xHis tag at the N-terminus of Ub to facilitate purification of Ub-histone crosslinked products. To express His6-Ub(G76C), BL21(DE3) E. coli bearing the expression plasmid were grown to log phase and expression was induced with 0.4 mM IPTG at 37 °C for 3 h. Cell lysis (Protocol 9) and batch purification under native conditions using Ni-NTA agarose (Protocol 12) were performed according to the manufacturer’s protocol [29]. The only modification was that all buffers were supplemented with 5 mM β-mercaptoethanol (βME). Eluates were then dialyzed against 10 mM Tris, pH 8.0, 50 mM NaCl, 0.2 mM EDTA, 10 mM βME at 4 °C overnight. To remove minor impurities, dialyzed eluates were passed through Q Sepharose Fast Flow resin (GE Healthcare). His6-Ub(G76C) remained in the flow-through, which was dialyzed exhaustively against 1 mM HOAc before lyophilization. The use of 1 mM HOAc in the final step prior to lyophilization helps to prevent oxidation of cysteine thiols. Typically, 200 mg of purified proteins were obtained from 4-liter cultures.
Site-directed mutagenesis was used to introduce the K119C mutation in human H2A. Mutant histones were expressed and purified according to Dyer et al. [30] with the modification that they were dialyzed in 1 mM HOAc before lyophilization.
2.2 Crosslinking
Lyophilized His6-Ub(G76C) or H2A(K119C) was resuspended in 10 mM HOAc, 7 M urea at ~10 mg/ml, as the crosslinking reaction is more efficient when the reactants are at high concentrations. As a quality control step, Ellman’s reagent (5,5′-dithio-bis-(2-nitrobenzoic acid), also known as DTNB) was used to determine the percentage of cysteines in the reduced state for each batch of purified protein. Briefly, an aliquot of dissolved His6-Ub(G76C) or H2A(K119C) is diluted in stock buffer (100 mM NaPi, pH 7.5, 1 mM EDTA) to a final concentration of 25 μM. 1 ml of the diluted protein is then mixed with 0.5 ml of 0.4 mM DTNB at room temperature for 30 min. A yellow color will develop and absorbance at 412 nm is measured to determine the sulfhydryl concentration using an extinction coefficient of 14150 M−1cm−1. Subsequently, sulfhydryl concentrations are used in place of protein concentrations to reflect true concentrations of the reactants.
Pilot-scale crosslinking reactions were performed with varying Ub:histone ratios to determine the optimal condition with each protein preparation (Figure 1B). We found that a Ub:histone molar ratio of 1:2 usually gave the highest yield. A typical crosslinking reaction is performed as follows:
Mix dissolved His6-Ub(G76C) and H2A(K119C) at 1:2 molar ratio (i.e., in terms of reduced-cysteine concentrations) in 50 mM sodium tetraborate, pH 8.5, 6 M urea.
Add 1 M TCEP (tris(2-carboxyethyl)phosphine) stock (adjusted to neutral pH) to reach 5 mM final concentration and incubate at room temperature for 30 min.
Freshly prepare 0.1 M 1,3-dichloroacetone in N,N′-dimethylformamide. Add an amount of crosslinker equal to one-half of the total sulfhydryl groups in the reaction. After incubation on ice for 30 min, the reaction is stopped with the addition of 5 mM βME.
The resulting products contain a mixture of unreacted Ub and H2A, Ub*Ub, ub*H2A and H2A*H2A; these are separated in the subsequent purification steps.
2.3 Purification of the Ub-histone mimic
Unreacted histones are removed by nickel affinity purification. The crosslinking reaction was diluted 1:10 in denaturing binding buffer (50 mM NaPi, pH 8, 300 mM NaCl, 6 M urea, 10 mM imidazole, 5 mM βME) and incubated with Ni-NTA agarose at 4 °C for 1 h. After extensive washes with the binding buffer, bound proteins were eluted with the binding buffer supplemented with 250 mM imidazole (Figure 1C). Eluates will contain Ub, Ub*Ub and ub*H2A. The presence of Ub and Ub*Ub will not interfere with subsequent refolding of histone dimers or octamers. The mixture can be dialyzed into water and lyophilized for long-term storage. Alternatively, it can be directly used in the subsequent refolding steps without change of buffer.
2.4 Reconstitution of histone dimers or octamers containing Ub-histone mimics
Reconstitution of histone dimers or octamers was done as described by Dyer et al. [30]. Lyophilized proteins were resuspended in unfolding buffer (6 M guanidinium hydrochloride, 20 mM Tris, pH 7.5, 5 mM DTT) and allowed to unfold for at least 30 min prior to determining protein concentration by measuring the absorbance at 276 nm. As the eluates from the nickel affinity purification contain a mixture of Ub-containing species, the concentration of ub*H2A was estimated by SDS-PAGE and Coomassie staining. For dimer reconstitution, ub*H2A and H2B were mixed at equal molar ratio. Slight excess of H2B can be used to ensure all ub*H2A is incorporated into the dimer. For octamer reconstitution, ub*H2A, H2B, H3 and H4 were mixed at equal molar ratio. Total protein concentration should be ~ 2 mg/ml. These mixtures were then dialyzed into refolding buffer (10 mM Tris, pH 7.5, 2 M NaCl, 1 mM EDTA, 5 mM βME).
After refolding, histone dimers or octamers were purified by gel filtration on a Superdex 75 or Superdex 200 column, respectively [30]. Typically, excess histones will elute as aggregates in the void volume, whereas the much smaller Ub and Ub*Ub will elute later (Figure 1D). Purified dimers or octamers are concentrated to ~ 3.5 mg/ml with Amicon Ultra Centrifugal filters. Octamers were supplemented with glycerol to 20% v/v and stored at −80 °C in small aliquots. Examples of the final purified products are shown in Figure 1E.
2.5 Assembly of mono-nucleosomes containing Ub-histone mimics
Histone octamers containing ub*H2A (at K119) or ub*H2B (at K120) can be readily assembled into mono-nucleosomes by salt dilution [16] or salt dialysis [30]. In comparison with unmodified nucleosomes, Ub*histone-containing nucleosomes migrate slower on a native polyacrylamide gel due to the presence of two Ub moieties in each nucleosome (Figure 1F).
3. Pulldown assays with Ub-histone mimics
To identify effectors of ubiquitinated histones, we expressed recombinant Flag-tagged H2A or H2B and assembled dimers with unmodified histones or Ub-histone mimics. The dimers or Flag-Ub were immobilized on anti-Flag affinity resin and incubated with nuclear extract to identify proteins that interact differentially with unmodified and ubiquitinated histones.
Flag-tagged histone dimers were prepared as described in section 2.4. These include: Flag-H2A/H2B, H2A/Flag-H2B, ub*H2A/Flag-H2B, and Flag-H2A/ub*H2B. Typically, 20 μg histone dimers or 7 μg Flag-Ub (BostonBiochem) in refolding buffer was diluted to 150 μl to adjust salt concentration to 300 mM NaCl and then incubated with 20 μl anti-Flag M2 affinity gel (Sigma A2220) at 4 °C for at least 2 h. Unbound proteins were removed and the agarose beads were washed twice with high-salt binding buffer (10 mM Hepes, pH 7.9, 470 mM NaCl, 10 mM KCl, 1.5 mM MgCl2, 0.2% Triton X-100, 10% glycerol).
We employed stringent conditions (470 mM NaCl) in the pulldown assays to minimize non-specific interactions with the highly charged histones. HeLa nuclear extract was prepared from HeLa S3 cells as previously described [31]. It was then diluted to 2.5 mg/ml with high-salt binding buffer. Additional NaCl was added to reach the final concentration of 470 mM. To remove contaminating proteases, DNA, and RNA, nuclear extract was routinely supplemented with 0.5 mM PMSF, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 20 μg/ml DNase I and 20 μg/ml RNase A, and then incubated at room temperature for 30 min and centrifuged at 18,000 × g for 30 min at 4 °C to remove precipitates.
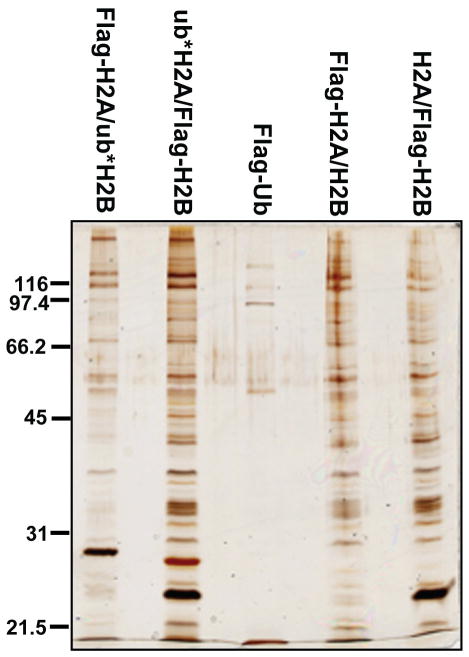
Typically, 500 μg nuclear extract was added to each pulldown reaction; tubes were incubated for 3 h at 4 °C with rotation. Unbound proteins were removed and the agarose beads were washed 3-times with 200 μl high-salt binding buffer. Bound proteins were eluted by incubation with 3xFlag peptide (Sigma) at 0.2 mg/ml in binding buffer at 4 °C for 30 min. The elution step was repeated and eluates were pooled. Fractions of the eluates (5–10%) were analyzed by SDS-PAGE and silver staining (Figure 2). The rest of the eluates were subject to LC-MS/MS to identify the bound proteins.
Figure 2.
A silver-stained gel of proteins bound to histone dimers containing unmodified histones, ub*H2A or ub*H2B mimics. Pulldown performed with Flag-Ub serves as a control.
4. Discussion
Most histone PTMs are on the N-terminal tails of the core histones. In these cases, tail peptides that bear the modification of interest are good surrogates and can be used as baits to identify modification-binding proteins. In contrast, mediators of Ub-dependent signaling often recognize their substrates through multiple interactions with both Ub and the substrate protein [32]. Because histone ubiquitination at different positions has different functional consequences, an effective surrogate must mimic the structural context of the Ub-histone modification. We have used ub*H2A (at K119) and ub*H2B (at K120) successfully to reconstitute histone dimers, octamers and nucleosomes, indicating that the Ub crosslinked to cysteine at these positions does not interfere with assembly. We envision that these Ub-containing dimers and nucleosomes will be broadly useful in structural and functional studies of histone ubiquitination.
Recently, a pulldown study was carried out with nucleosome arrays assembled with chemically synthesized ubH2B, which contains the native isopeptide linkage [33]. In comparison with these semi-chemical synthesis strategies, our crosslinking-based method is much simpler. It is possible that the crosslink, being one C-C bond longer and containing an additional carboxylate, will interfere with the binding of some effector proteins. On the other hand, the crosslink is not susceptible to cleavage by DUBs present in cell or nuclear extract. We have developed a second-generation nonhydrolyzable mimic where the additional carboxylate was eliminated by intein-mediated conjugation of 2-aminoethanethiol to the C-terminus of His6-Ub(1–75) (M.F. and T.Y., unpublished results). However, the yield of the second-generation mimic is significantly lower. We have not observed any differences between the mimics with or without the carboxylate in a variety of experiments.
We conducted our pulldown experiments under high salt conditions (470 mM NaCl) to prevent non-specific electrostatic interactions that otherwise may occur between the highly charged histones and other proteins. Under this condition, it is possible that weak interactions that naturally occur in the cell would not be preserved. It is also important to consider that bound proteins may be associated indirectly. Further studies using purified recombinant proteins are necessary to confirm direct interactions with unmodified or ubiquitinated histones. We have performed these studies with a number of proteins identified in our pulldown assays, including Usp15 and SART3, and found that their binding to histone dimers is direct [22].
Many sites of mono-ubiquitination have been identified on H2A, H2B, H3, and H4 in mammalian cells (Table 1). Compared to the extensive studies on monoubiquitination of H2A at K119 and H2B at K120, little is known about the effectors of histone ubiquitination at other lysine residues. In addition, the conjugation of an Ub-like protein, Ub-related modifier (SUMO), onto histones has been described as well [34, 35]. Like Ub, SUMO does not encode any naturally occurring cysteine residues. In principle, our technique to generate Ub*histones could also be applied to prepare nonhydrolyzable SUMO*histone mimics. Generation of a variety of ubiquitinated or sumoylated histones could prove to be useful tools to identify downstream effectors and to provide new mechanistic insights into the functions of these modifications.
Highlights.
We have developed a strategy to generate nonhydrolyzable ubiquitin-histone mimics.
Ubiquitin-histone mimics were assembled into dimers, octamers, and nucleosomes.
These mimics can be used to capture and identify effector proteins of the PTM.
Acknowledgments
We thank Benjamin Schmitt for performing some of the crosslinking experiments. We thank the Protein Expression and Purification Facility at CSU for providing reagents for this work. We are also grateful to Karolin Luger and members of her lab who shared their expertise with us during the development of this technique. This work was supported in part by National Institute of Health grant R01GM098401 (to T.Y.) and National Science Foundation award 1158323 (to T.Y.). T.Y. is a Boettcher Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kouzarides T. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Patel DJ, Wang Z. Annu Rev Biochem. 2013;82:81–118. doi: 10.1146/annurev-biochem-072711-165700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan DW, Wang Y, Wu M, Wong J, Qin J, Zhao Y. Proteomics. 2009;9:2343–2354. doi: 10.1002/pmic.200800600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothbart SB, Krajewski K, Strahl BD, Fuchs SM. Methods Enzymol. 2012;512:107–135. doi: 10.1016/B978-0-12-391940-3.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wysocka J. Methods. 2006;40:339–343. doi: 10.1016/j.ymeth.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian MX, Pang Y, Liu CH, Haratake K, Du BY, Ji DY, Wang GF, Zhu QQ, Song W, Yu Y, Zhang XX, Huang HT, Miao S, Chen LB, Zhang ZH, Liang YN, Liu S, Cha H, Yang D, Zhai Y, Komatsu T, Tsuruta F, Li H, Cao C, Li W, Li GH, Cheng Y, Chiba T, Wang L, Goldberg AL, Shen Y, Qiu XB. Cell. 2013;153:1012–1024. doi: 10.1016/j.cell.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osley MA. Brief Funct Genomic Proteomic. 2006;5:179–189. doi: 10.1093/bfgp/ell022. [DOI] [PubMed] [Google Scholar]
- 8.Weake VM, Workman JL. Mol Cell. 2008;29:653–663. doi: 10.1016/j.molcel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Wright DE, Wang CY, Kao CF. Frontiers in bioscience. 2012;17:1051–1078. doi: 10.2741/3973. [DOI] [PubMed] [Google Scholar]
- 10.Pinder JB, Attwood KM, Dellaire G. Frontiers in genetics. 2013;4:45. doi: 10.3389/fgene.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trujillo KM, Osley MA. Mol Cell. 2012;48:734–746. doi: 10.1016/j.molcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Roeder RG. Methods. 2011;54:331–338. doi: 10.1016/j.ymeth.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KK, Florens L, Swanson SK, Washburn MP, Workman JL. Mol Cell Biol. 2005;25:1173–1182. doi: 10.1128/MCB.25.3.1173-1182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joo HY, Zhai L, Yang C, Nie S, Erdjument-Bromage H, Tempst P, Chang C, Wang H. Nature. 2007;449:1068–1072. doi: 10.1038/nature06256. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Zee BM, Wang Y, Garcia BA, Dou Y. Mol Cell. 2011;43:132–144. doi: 10.1016/j.molcel.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGinty RK, Kim J, Chatterjee C, Roeder RG, Muir TW. Nature. 2008;453:812–816. doi: 10.1038/nature06906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fierz B, Kilic S, Hieb AR, Luger K, Muir TW. J Am Chem Soc. 2012;134:19548–19551. doi: 10.1021/ja308908p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar KS, Spasser L, Ohayon S, Erlich LA, Brik A. Bioconjugate chemistry. 2011;22:137–143. doi: 10.1021/bc1004735. [DOI] [PubMed] [Google Scholar]
- 19.Haj-Yahya M, Eltarteer N, Ohayon S, Shema E, Kotler E, Oren M, Brik A. Angew Chem Int Ed Engl. 2012;51:11535–11539. doi: 10.1002/anie.201205771. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee C, McGinty RK, Fierz B, Muir TW. Nat Chem Biol. 2010;6:267–269. doi: 10.1038/nchembio.315. [DOI] [PubMed] [Google Scholar]
- 21.Fierz B, Chatterjee C, McGinty RK, Bar-Dagan M, Raleigh DP, Muir TW. Nat Chem Biol. 2010;7:113–119. doi: 10.1038/nchembio.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long L, Thelen JP, Furgason M, Haj-Yahya M, Brik A, Cheng D, Peng J, Yao T. J Biol Chem. 2014;289:8916–8930. doi: 10.1074/jbc.M114.551754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spasser L, Brik A. Angew Chem Int Ed Engl. 2012;51:6840–6862. doi: 10.1002/anie.201200020. [DOI] [PubMed] [Google Scholar]
- 24.Shanmugham A, Fish A, Luna-Vargas MP, Faesen AC, El Oualid F, Sixma TK, Ovaa H. J Am Chem Soc. 2010;132:8834–8835. doi: 10.1021/ja101803s. [DOI] [PubMed] [Google Scholar]
- 25.Weikart ND, Mootz HD. Chembiochem. 2010;11:774–777. doi: 10.1002/cbic.200900738. [DOI] [PubMed] [Google Scholar]
- 26.Eger S, Scheffner M, Marx A, Rubini M. Methods in molecular biology. 2012;832:589–596. doi: 10.1007/978-1-61779-474-2_41. [DOI] [PubMed] [Google Scholar]
- 27.Yin L, Krantz B, Russell NS, Deshpande S, Wilkinson KD. Biochemistry. 2000;39:10001–10010. doi: 10.1021/bi0007019. [DOI] [PubMed] [Google Scholar]
- 28.Carlile CM, Pickart CM, Matunis MJ, Cohen RE. J Biol Chem. 2009;284:29326–29334. doi: 10.1074/jbc.M109.043885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.QIAGEN. The QIAexpressionist. 5. 2003. [Google Scholar]
- 30.Dyer PN, Edayathumangalam RS, White CL, Bao Y, Chakravarthy S, Muthurajan UM, Luger K. Methods Enzymol. 2004;375:23–44. doi: 10.1016/s0076-6879(03)75002-2. [DOI] [PubMed] [Google Scholar]
- 31.Abmayr SM, Yao T, Parmely T, Workman JL. In: Current protocols in pharmacology. Unit12. Enna SJ, editor. Chapter 12. 2006. p. 13. [DOI] [PubMed] [Google Scholar]
- 32.Panier S, Ichijima Y, Fradet-Turcotte A, Leung CC, Kaustov L, Arrowsmith CH, Durocher D. Mol Cell. 2012;47:383–395. doi: 10.1016/j.molcel.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 33.Shema-Yaacoby E, Nikolov M, Haj-Yahya M, Siman P, Allemand E, Yamaguchi Y, Muchardt C, Urlaub H, Brik A, Oren M, Fischle W. Cell reports. 2013;4:601–608. doi: 10.1016/j.celrep.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Nathan D, Ingvarsdottir K, Sterner DE, Bylebyl GR, Dokmanovic M, Dorsey JA, Whelan KA, Krsmanovic M, Lane WS, Meluh PB, Johnson ES, Berger SL. Genes Dev. 2006;20:966–976. doi: 10.1101/gad.1404206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiio Y, Eisenman RN. Proc Natl Acad Sci U S A. 2003;100:13225–13230. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gatti M, Pinato S, Maspero E, Soffientini P, Polo S, Penengo L. Cell Cycle. 2012;11:2538–2544. doi: 10.4161/cc.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattiroli F, Vissers JH, van Dijk WJ, Ikpa P, Citterio E, Vermeulen W, Marteijn JA, Sixma TK. Cell. 2012;150:1182–1195. doi: 10.1016/j.cell.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama A, Yamaguchi L, Sharif J, Johmura Y, Kawamura T, Nakanishi K, Shimamura S, Arita K, Kodama T, Ishikawa F, Koseki H, Nakanishi M. Nature. 2013;502:249–253. doi: 10.1038/nature12488. [DOI] [PubMed] [Google Scholar]
- 39.Kim K, Lee B, Kim J, Choi J, Kim JM, Xiong Y, Roeder RG, An W. Cell reports. 2013;5:1690–1703. doi: 10.1016/j.celrep.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan Q, Dutt S, Xu R, Graves K, Juszczynski P, Manis JP, Shipp MA. Mol Cell. 2009;36:110–120. doi: 10.1016/j.molcel.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]