Abstract
Accumulating evidence implicates small vessel cerebrovascular disease, visualized as white matter hyperintensities (WMH) on T2-weighted MRI, in the pathogenesis and diagnosis of Alzheimer's disease (AD). Cross-sectional volumetric measures of WMH, particularly in the parietal lobes, are associated with increased risk of AD. In the current study, we sought to determine whether the longitudinal regional progression of WMH predicts incident AD above-and-beyond traditional radiological markers of neurodegeneration (i.e., hippocampal atrophy, cortical thickness). Three hundred three non-demented older adults (mean age = 79.24±5.29) received high-resolution MRI at baseline and then again 4.6 years (SD=1.01) later. Over the follow-up interval 26 participants progressed to AD. Using structural equation modeling (SEM), we calculated latent difference scores of parietal/non-parietal WMH, hippocampus volumes, and cortical thickness values in AD-related regions. Within the SEM framework, we determined whether baseline or change scores or both predicted AD conversion, while controlling for several time-invariant relevant variables. Smaller baseline hippocampus volume, change in hippocampus volume (i.e., atrophy), higher baseline parietal lobe WMH, and increasing parietal lobe WMH volume but not WMH in other regions or measures of cortical thickness, independently predicted progression to AD. The findings provide strong evidence that regionally accumulating WMH, in addition to degenerative changes in the medial temporal lobe, predict AD onset in addition to hallmark neurodegenerative changes typically associated with AD.
1. Introduction
Alzheimer's disease (AD) is one of the most pernicious public health issues affecting older adults. There are currently no effective interventions that prevent the disease or fundamentally alter its clinical course. Alzheimer's disease has been described and defined historically as a mixed-pathological condition, comprising intercellular accumulation of fibrillar forms of the beta-amyloid protein and intracellular deposition of neurofibrillary tangles (Rothschild, 1934). Recent evidence, however, implicates small vessel cerebrovascular disease as an additional important feature of the disease, contributing at least additively, but possibly in a synergistic or primary manner, to disease pathogenesis (Brickman, 2013,Brickman, et al., 2009).
In addition to microhemorrhages and lacunar infarcts, small vessel cerebrovascular disease is best visualized as increased signal, or white matter hyperintensities (WMH), on T2-weighted magnetic resonance imaging (MRI). White matter hyperintensity volume is associated with risk for AD, the diagnosis of AD, and rate of cognitive decline among individuals with AD(Brickman, et al., 2008a,Brickman, et al., 2012,Luchsinger, et al., 2009,Meier, et al., 2012,Provenzano, et al., 2013). The regional distribution of WMH is also important, in terms of clinical outcome. In our previous work, increased parietal lobe distribution of WMH is specifically associated with risk of AD(Brickman, et al., 2012), whereas more anterior distribution appears to be non-specific and associated with mortality(Wiegman, et al., 2013). To examine the causal impact of regionally-distributed cerebrovascular disease and its specificity, we determined whether longitudinal progression of parietal lobe WMH predicts incident AD in addition to hippocampal atrophy and cortical thickness, measures of AD-related neurodegeneration(Whitwell, et al., 2008), in a large cohort of community-dwelling older adults. We hypothesized that both markers of AD-related neurodegeneration and progression of WMH in the parietal lobes would predict incident AD.
2. Material and methods
2.1.Participants
Participants came from the Washington Heights Inwood Columbia Aging Project (WHICAP), an ongoing longitudinal study of cognitive aging and dementia. Participants were initially recruited at two time points, in 1992 and 1999 (see (Tang, et al., 2001)), and are evaluated approximately every 24 months. Beginning in 2004, active participants (n=2776) who were non-demented at their preceding visit were invited to participate in an MRI study (see (Brickman, et al., 2008b)). Seven hundred sixty-nine participants underwent MRI scanning. They were about 1 year older, more likely to be women, and more likely to be African American than the 407 study members who were eligible for MRI scanning but refused participation (Brickman, et al., 2008b). Approximately 4.5 years following their initial scan, individuals who were non-demented at the time of their first MRI scan (n=717) were invited to return for a second MRI scan; 303 participants had available baseline and follow-up MRI data (see Table 1 for baseline characteristics). Individuals with follow-up MRI data were younger at baseline (79.27±5.29 vs. 80.64±5.66, t(715)=3.29, p=0.001), but were similar in terms of sex (χ2(1)=0.778, p=0.378) and race/ethnicity (χ2(3)=5.94, p=0.115) distributions, than individuals for whom a second MRI scan was not conducted. The study was approved by our Institutional Review Board and all participants gave written informed consent.
Table 1.
Sample characteristics
| Total sample (N=303) | pAD (N=26) | Non-demented (N=261) | ||
|---|---|---|---|---|
| Age, mean yrs (SD) | 79.24 (5.29) | 81.88 (5.74)1 | 79.00 (5.14) | |
| Sex | 69.0% Female | 84.6% Female | 67.4% Female | |
| Race/ethnicity | 37.3% Black | 26.9% Black | 38.3% Black | |
| 33.0% Hispanic | 61.5% Hispanic1 | 29.1% Hispanic | ||
| 29.7% White | 11.6% White | 32.6% White | ||
| Education, mean yrs (SD) | 11.14 (4.83) | 8.65 (5.18)1 | 11.69 (4.57) | |
| Recruitment year | 18.5% 1992 | 15.4% 1992 | 18.4% 1992 | |
| 81.5 % 1999 | 84.6% 1999 | 81.6% 1999 | ||
| Years between scans, mean (SD) | 4.61 (1.01) | 4.46 (0.76) | 4.63 (1.03) | |
| Years in follow-up, mean (SD) | 5.53 (1.66) | 5.85 (0.96) | 5.48 (1.71) | |
| ICV, mean cm3 (SD) | 1305.11 (154.67) | 1276.97 (138.72) | 1309.81 (154.98) | |
| APOE e4 allele | 26.8% Yes | 34.6% Yes | 25.3% Yes | |
| 73.2% No | 65.4% No | 74.7% No | ||
| Vascular risk summary score, mean (SD) number of items endorsed | 1.15 (0.88) | 1.04 (0.77) | 1.16 (0.89) | |
| WMH vol., mean cm3 (SD) | Frontal | 3.83 (5.87) | 4.08 (6.98) | 3.81 (5.76) |
| Temporal | 0.38 (0.56) | 0.42 (0.08) | 0.38 (0.58) | |
| Parietal | 2.69 (3.90) | 3.67 (4.71) | 2.60(3.82) | |
| Occipital | 0.80 (0.90) | 1.01 (0.98) | 0.77 (0.90) | |
Note. pAD=probable Alzheimer's disease, ICV=intracranial volume. Total sample also includes individuals who progressed to a dementia other than probable AD (N=16).
Significant difference between pAD and those who remained non-demented (p<.05)
2.2.Diagnostic procedures
Participants underwent in-person evaluation at each follow-up visit, including full medical and neurological examination and neuropsychological testing in English or Spanish. The neuropsychological battery included measures of memory, orientation, language, abstract reasoning, and visuospatial functioning(Stern, et al., 1992), which measured equivalent traits across the two language groups represented in the study population(Siedlecki, et al., 2010). The diagnosis of dementia was established via review of all available clinical information (not including radiological data), medical evaluation, and was based on standard research criteria(American Psychiatric Association, 1987). Following each clinical evaluation, a consensus conference, including at least one physician and one neuropsychologist, reviewed available data to assign a research diagnosis. First, a diagnosis of dementia was made(American Psychiatric Association, 1987) and then the etiology was determined based on research criteria for probable or possible AD(McKhann, et al., 1984), Lewy body dementia(McKeith, et al., 1999), vascular dementia(Roman, et al., 1993), and other dementias. In the case of vascular dementia, history of stroke and its contribution to the dementia syndrome was determined via medical history and evaluation. History of heart disease, clinical stroke, hypertension, and diabetes was ascertained by self-report, supplemented by physical examination. These four dichotomous variables were summed to create a single vascular risk summary score (Brickman, et al., 2008b).
Participants were classified as incident AD versus those who remained non-demented throughout the follow-up, based on whether they met diagnostic criteria for probable AD at any point following the initial MRI scan, over a 5.5-year follow-up period. Descriptive statistics for the two groups are displayed in Table 1. Cases were older, more likely to be Hispanic, and had lower educational levels than those who remained non-demented, but were similar in terms of sex, year of recruitment, time interval between MRI scans, number of years of follow-up after the initial MRI scan, total cranial volume, and APOE-ε4 allele status.
2.3.MRI protocol
Magnetic resonance imaging scan acquisition took place on the same 1.5 T Philips Intera scanner at the two time points, using the identical acquisition sequences (Brickman, et al., 2008b). T1-weighted (TR=20ms,TE=2.1ms, FOV 240cm, 256×160 matrix, 1.3mm slice thickness) and T2-weighted fluid attenuated inversion recovery (FLAIR; TR=11,000ms, TE=144.0ms, inversion time=2800, FOV 25cm, 2 nex, 256×192 matrix with 3mm slice thickness) images were acquired in the axial orientation. Regional WMH volumes were derived as described previously(Brickman, et al., 2009,Brickman, et al., 2012,Brickman, et al., 2011). Briefly, FLAIR images were skull stripped and a Gaussian curve was fit to map the voxel intensity values. Voxels falling above 3.0SD of the image mean were labeled as WMH. Labeled images were inspected visually and corrected in the case of labeling commission or omission errors. To derive WMH volumes in the frontal, temporal, parietal, and occipital lobes, a standardized atlas(Admiraal-Behloul, et al., 2004) was spatially normalized to each subject's labeled FLAIR image. Regional volumes were defined by the intersection of each atlas lobe with the labeled WMH voxels in that region; labeled voxel values were multiplied by voxel dimensions and summed to yield volumes in cm3. Because we were interested in testing the hypothesis that increasing parietal lobe WMH volume specifically contributes to conversion of AD, we combined WMH volumes in frontal, temporal, and occipital regions to create two regional WMH variables (i.e., parietal WMH versus all others), although secondary analyses considered volume measures in each lobe separately.
Hippocampus volume and total intracranial volumes were derived with FreeSurfer version 5.1 (http://surfer.nmr.mgh.harvard.edu/) applied to the T1-weighetd MRI scans. FreeSurfer was also used to derive cortical thickness values. A single “AD signature” measurement was derived for each subject by averaging cortical thickness values across hemispheres in regions that have been shown previously to reflect AD-associated neurodegeneration (Dickerson, et al., 2009). The 9 regions previously implicated by Dickerson and colleagues (Dickerson, et al., 2009) and the FreeSurfer regions-of-interest we chose to represent them (in parentheses) include: rostral medial temporal lobe (entorhinal cortex and parahippocampus), angular gyrus (inferior parietal lobe), inferior frontal lobe (pars opercularis, pars orbitalis, and pars triangularis), inferior temporal lobe (inferior temporal lobe), temporal pole (temporal pole), precuneus (precuneus), supramarginal gyrus (supramarginal gyrus), superior parietal lobe (superior parietal lobe), and superior frontal lobe (superior frontal lobe). All images were visually inspected for accuracy. Identical procedures were used for quantitation of WMH and volumetry at the two MRI visits.
2.4. Statistical Analysis
We used structural equation modeling (SEM) to examine the impact of baseline and change in regional WMH volume and hippocampal volume on the development of AD, while controlling for potential confounding variables.
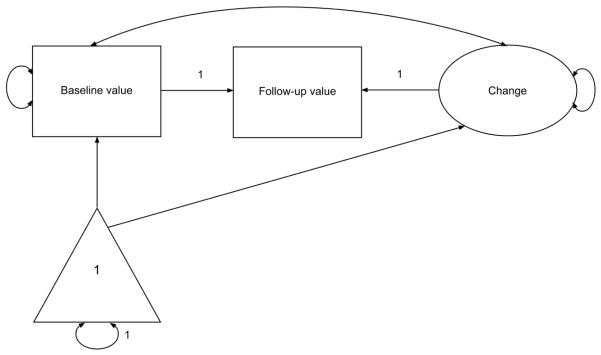
Structural equation modeling (SEM) was conducted in Mplus version 7(Muthén and Muthén, 1998–2012). Maximum likelihood estimation was used in the univariate models that characterized changes in individual MRI variables. Robust weighted least squares estimation was used in the conditional model that examined conversion to AD. Latent difference score (LDS) models were used to characterize change in the MRI variables (regional WMH, hippocampal volume, and cortical thickness) (McArdle and Nesselroade, 1994). Rather than calculating difference scores from the raw data, the LDS approach defines a latent variable as the portion of the follow-up value that is not identical to the initial value. In addition, features of change that are of interest (e.g., mean change, inter-individual variability in change, relationship between initial value and change) can be modeled as explicit parameters (McArdle, 2009). Modeling proceeded in two broad stages. First, separate LDS models were constructed for the three MRI variables of interest: parietal WMH, other WMH, hippocampal volume, and cortical thickness. A schematic of a univariate LDS model is shown in Figure 1. Results from these models represent the uncorrected initial value and magnitude of change in each variable between time 1 and time 2. Next, the three LDS models were combined, and covariates were added to the model. Covariates were chosen based on previous literature as well as the presence of bivariate relationships with the outcomes of interest. The following time-invariant covariates were included in the conditional LDS model: age, sex, race and ethnicity, education, recruitment cohort, intracranial volume, and presence of an APOE ε4 allele. The relationship between conversion to AD and the MRI variables was evaluated simultaneously via logistic regression in which AD status was the dichotomous dependent variable, and independent variables included initial values of the three MRI variables, the three latent variables representing change in these MRI variables, and the seven covariates listed above.
Figure 1.
Unconditional latent difference score (LDS) model. Squares represent measured variables at the two time points. The circle represents the latent change variable. The triangle represents a fixed constant of 1.0 for every individual. Paths from this constant reflect mean or intercept.
3. Results
Results of the univariate LDS models for the three MRI variables are shown in Table 2. The presented mean values represent the sample's average initial values and average change values; that is, at baseline, participants' WMH volumes were 2.79cm3 and 5.12 cm3 in the parietal lobes and other lobes, respectively, hippocampal volumes were 6.88 cm3, and cortical thickness was 2.58mm. The reported variances represent inter-individual differences in initial values and change. Between time 1 and time 2, WMH in parietal regions increased 0.29cm3 in volume. White matter hyperintensities in other lobes increased 0.65cm3, hippocampal volumes decreased 0.50cm3, and cortical thickness decreased 0.02mm. Baseline parietal lobe WMH, increasing parietal lobe WMH, baseline hippocampal volume, and decreasing hippocampal volume independently predicted progression to AD (Table 3); individuals who progressed to AD accumulated 0.26cm3 more parietal WMH and lost 0.68cm3 more hippocampus volume than those who did not over the 5-year period. These predictors explained 43.8% of the variance in AD conversion. Neither baseline nor change in cortical thickness predicted progression to AD. None of the covariates in the model, including age, race/ethnicity, education, sex, cohort, and APOE ε4, reliably predicted conversion to AD independent of the MRI variables. Examination of whether change in each of the MRI variables was associated with their initial levels showed that higher parietal WMH volume was associated with more rapid increases in parietal WMH (B=0.05; SE=0.02; z=2.19; p=0.05), higher WMH volume in other lobes was associated with more rapid increases in WMH in those regions (B=0.09; SE=0.03; z=3.59; p<0.001), and higher cortical thickness was associated with less cortical thickness atrophy (B=0.78; SE=0.35; z=2.19; p=0.028). This model fit well: χ2(57)=887.70; p<.001; RMSEA=0.06 (90% confidence interval: 0.05 – 0.07); CFI=0.91; TLI=0.89. The model was re-run to include conversion to possible AD as an outcome, rather than restricting the sample to only those meeting criteria for probable AD or remaining non-demented. Thirteen additional participants were added, which included individuals with AD and stroke (n=7), AD and concomitant Parkinson's disease (n=1), and AD with other concomitant disease (n=5). Three participants who had dementia due to other causes were excluded. Results of the analysis were similar to those obtained from the model that was restricted to conversion to probable AD only in that an increase in parietal WMH independently predicted progression to possible AD (B=0.33; SE=0.14; z=2.44; p=0.02). Initial hippocampal volume (B=−0.40; SE=0.09; z=-4.24; p<.001) and hippocampal atrophy (B=−0.79; SE=0.22; z=−3.58; p<.001) were also associated with progression. In contrast, neither Initial value (B=−0.03; SE=0.02; z=−1.42; p=0.16) nor change in WMH (B=0.07; SE=0.05; z=1.48; p=0.14) in other regions independently predicted progression. Similarly, the model was re-run after replacing the “all other” WMH volume with WMH volumes from each of the three other lobar regions (i.e., frontal, temporal, and occipital). Results remained similar in that increasing parietal lobe WMH predicted conversion to AD though at a trend level (p=0.06); neither baseline nor change in WMH volume in any of the other lobes predicted incident AD. We also re-ran the analysis contrasting frontal lobe WMH to all other lobes because of previous reports in the literature that implicate frontal lobe WMH in AD. In that analysis, progression of frontal lobe WMH did not emerge as a predictor of progression to AD (p>0.50), but progression of WMH in all other lobes did (p=0.01), likely because that measure comprised the parietal lobe WMH value. Of note, parietal lobe WMH volume did not correlate reliably with either hippocampal volume (p=0.22) or with a measure of cortical thickness in the parietal lobe (p=0.27).
Table 2.
Results from the unconditional latent difference score models (unstandardized logits). The “initial value (mean)” effect refers to the baseline value MRI value. For example, mean WMH in the parietal lobe at baseline across all subjects was 2.79cm3 with a standard error of 0.23. The z-value represents the mean value transformed to a z-distribution and the inferential statistical test determines whether the mean value differs significantly from 0. The “initial value (variance)” effect reflects individual differences (i.e., random effects) or the amount of variability in the measurement; the inferential statistical test determines whether there is a significant amount of variability (i.e., not 0) in the measurement. The “change (mean)” effect refers to the average amount of decrease (or increase) in the value. For example, parietal lobe WMH volume increased 0.29cm3 over the follow-up interval, which was significantly greater than 0. Finally, the “change (variance)” effect refers to the amount of individual differences (i.e., random effects) in change over time; the significant change (variance) effects indicate that there are multiple trajectories of changes in these variables across the follow-up interval. Note that this table presents results for the entire sample together, without consideration of the AD progression status.
| Model 1: Parietal WMH (cm3) | Model 2: Other WMH (cm3) | Model 3: Hippocampal volume (cm3) | Model 4: Cortical thickness (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| estimate | SE | z | estimate | SE | z | estimate | SE | z | estimate | SE | z | |
| Initial value (mean) | 2.79 | 0.23 | 12.02** | 5.12 | 0.38 | 13.35** | 6.88 | 0.05 | 133.95** | 2.58 | 0.007 | 348.40** |
| Initial value (variance) | 16.31 | 1.33 | 12.31** | 44.56 | 3.62 | 12.31** | 0.71 | 0.06 | 11.62** | 0.02 | 0.001 | 12.16** |
| Change (mean) | 0.29 | 0.09 | 3.34* | 0.65 | 0.16 | 4.04** | −0.50 | 0.03 | −15.18** | −0.07 | 0.008 | −8.08** |
| Change (variance) | 2.27 | 0.18 | 12.31** | 7.84 | 0.64 | 12.31** | 0.30 | 0.03 | 11.60** | 0.02 | 0.002 | 12.11** |
Note. WMH=white matter hyperintensity volume, SE=standard error.
p<.05,
<.001
Table 3.
Predictors of conversion to probable Alzheimer's disease (unstandardized values). The Estimate values are log odds for the regression analysis. For example, individuals who progressed to AD had 0.08 cm3 greater WMH volume in the parietal lobes and accumulated 0.26cm3 more parietal lobe WMH volume than individuals who remained non-demented.
| Estimate | SE | z | p | |
|---|---|---|---|---|
| Baseline parietal WMH | 0.08 | 0.04 | 2.14 | 0.03 |
| Baseline other WMH | −0.04 | 0.02 | −1.80 | 0.07 |
| Baseline hippocampal volume | −0.30 | 0.09 | −3.37 | 0.001 |
| Baseline cortical thickness | −0.27 | 0.50 | −0.53 | 0.59 |
| Change in parietal WMH | 0.26 | 0.12 | 2.15 | 0.03 |
| Change in other WMH | 0.08 | 0.04 | 1.86 | 0.06 |
| Change in hippocampal volume | −0.68 | 0.19 | −3.61 | <0.001 |
| Change in cortical thickness | −0.50 | 0.78 | −0.64 | 0.52 |
| Age | 0.02 | 0.02 | 0.93 | 0.35 |
| Education | −0.07 | 0.04 | −1.91 | 0.06 |
| Black | 0.24 | 0.40 | 0.61 | 0.54 |
| Hispanic | 0.36 | 0.50 | 0.72 | 0.47 |
| Recruitment year | 0.24 | 0.31 | 0.79 | 0.43 |
| Sex | 0.01 | 0.27 | 0.03 | 0.97 |
| ICV | −0.001 | 0.001 | −0.83 | 0.41 |
| APOE e4 allele | 0.31 | 0.26 | 1.18 | 0.24 |
Note. SE=standard error, WMH=white matter hyperintensity volume, ICV=intracranial volume.
Discussion
Increasing parietal lobe WMH, specifically, predicted progression to AD in this large cohort of older, community-dwelling adults. Together with our earlier observations(Brickman, et al., 2008a,Brickman, et al., 2012), these findings establish a strong connection between the accumulation of regionally-distributed WMH and progression to AD. We also confirmed that hippocampus atrophy, but not cortical thickness or change in cortical thickness, is independently associated with progression to AD. To the extent that hippocampus atrophy reflects neurodegenerative changes related to tau pathology and WMH reflects small vessel cerebrovascular disease, our findings raise the possibility that accumulating small vessel cerebrovascular disease plays an important role in AD pathogenesis, disease expression, and/or is a marker of degenerative changes that precedes frank diagnosis. Indeed, WMH are thought to reflect arteriolosclerosis and increased myelin pallor (Erten-Lyons, et al., 2013) and atherosclerotic changes are highly associated with AD pathology specifically (Yarchoan, et al., 2012).
There have been several previous cross-sectional reports showing elevated WMH among individuals with and at risk for AD(Brickman, 2013,Brickman, et al., 2009,Hogervorst, et al., 2002,Makedonov, et al., 2013, Provenzano, et al., 2013, Yoshita, et al., 2006), though with some inconsistencies regarding the regional distribution with some studies showing a greater propensity in the frontal lobes (e.g., (Capizzano, et al., 2004, Polvikoski, et al., 2010)). However, examination of the longitudinal progression of WMH and its temporal relationship to clinical outcomes has been relatively understudied. The expansion of WMH severity over a 4-year period was associated with worsening cognitive functioning in a sample of 150 older adults with AD, mild cognitive impairment, or without neurological disease(Maillard, et al., 2012) and acceleration of WMH accumulation has been observed to precede in presymptomatic phase that leads to mild cognitive impairment (MCI) (Silbert, et al., 2012). In the Alzheimer's Disease Neuroimaging Initiative dataset, WMH volume at baseline and follow-up were associated with cognitive decline after a 1-year interval (Carmichael, et al., 2010) and WMH volume increased over time to a greater extent among individuals with MCI and AD than among controls (Lo and Jagust, 2012). Cognitive decline is indeed a core element of the clinical syndrome of AD, but the clinical diagnosis of AD requires cognitive impairment that is severe enough to have an impact on activities of daily living. Our results establish that increasing WMH, in addition to neurodegenerative changes that manifest as hippocampal atrophy, are associated with the progression to AD. Our study is also unique in that we used multi-modal neuroimaging to establish the combined and independent effects of markers of regionally distributed cerebrovascular disease and neurodegeneration to predict outcome. It is interesting to note that factors such as race/ethnicity, education, and age differed systematically between individuals with probable AD and those who remained non-demented, as expected. However, these variables did not predict the progression to AD when considered in the context of the brain imaging variables, which suggests that the association between these demographic variables and risk for AD is mediated partially by differences in hippocampus and WMH volumes.
The regional specificity of the findings points to a unique role of parietal lobe WMH in disease onset. The parietal lobes have been implicated in AD previously. Metabolic neuroimaging studies consistently suggest a diminution in parietal regions (Ishii, et al., 2005,Jagust, et al., 2002), and the earliest deposition of amyloid plaque pathology occurs in posterior association areas(Braak and Braak, 1991,Braak and Braak, 1996). The regional selectivity we observed suggests that the pathological features of WMH may vary across lobar regions in the context of AD, reflecting ischemic disease with other pathological markers(Brickman, 2013,Brickman, et al., 2009). For example, amyloid angiopathy has a propensity for posterior brain regions in AD(Vinters and Gilbert, 1983). The parietal lobes and entorhinal cortex, where early dysfunction is apparent in AD(Small, et al., 2011), are strongly interconnected, so damage to parietal lobes may reflect Wallerian-type degeneration(Khan, et al., 2014). Shared vascularization between medial temporal and parietal regions may implicate a primary hemodynamic role in AD-associated regional dysfunction and damage, suggesting a single driver of both neurodegeneration and WMH accumulation. APOE-ε4, the strongest genetic risk factor for late onset AD, is differentially associated with increased parietal lobe WMH in two cohorts, suggesting either a shared genetic risk for regionally distributed WMH and AD or a causal path between APOE-ε4 and AD, mediated through regional WMH (Brickman, et al., submitted). In the current study, parietal lobe WMH volume did not correlate hippocampal volume or with cortical thickness in the parietal lobe, which suggests the parietal WMH measurement does not necessarily reflect neurodegeneration per se nor is it secondary to atrophic changes. A complementary interpretation is that WMH distributed in parietal lobes may have more clinical relevance with regard to the expression of the disease than WMH distributed in other regions; that is, the neurobiological underpinnings of WMH may be similar across regions but damage to parietal lobes specifically may impact the cognitive and functional outcomes that define the disease clinically to a greater extent than damage to other regions.
It is important to compare the current work with our previous study in this population, which showed that baseline measurements of parietal WMH but not hippocampal volume predicted incident AD(Brickman, et al., 2012). In the current study, we replicated the observation that parietal lobe WMH burden selectively predicts incident AD and extend the findings to show that progressing WMH over time in the parietal lobes specifically predicts incident AD. We interpret the findings as evidence that increased parietal lobe WMH volume is a harbinger for future development of AD. However, unlike in our previous report, baseline measures of hippocampal volume did predict incident AD. Here, we used FreeSurfer to derive hippocampal volumes, which is more reliable than the manual derivation approach we used previously. We attribute the difference in observations to the increased precision of measurement. The fundamental observation that parietal lobe WMH predict incident AD independent of estimates of hippocampal atrophy holds true and the current study expands the work notably by considering longitudinal data.
Given the link between WMH and AD, obvious targets for therapeutic or preventative strategies should include WMH antecedents. Traditional vascular risk factors, such as diabetes, heart disease, obesity, inflammation, and particularly hypertension are associated with WMH severity (Brickman, et al., 2008b,Dufouil, et al., 2001). We found a relationship between fluctuating blood pressure over time and increased WMH burden, suggesting a role for hemodynamic or autoregulatory failure (Brickman, et al., 2010). White matter hyperintensities progression tends to be quite gradual, reflecting an accumulation of small vessel changes to the brain. Thus, preventative strategies will likely be most effective if commenced during mid-life or earlier and treatment strategies could focus on aggressive management of vascular risk factors, even if subclinical, though results from the current study would also suggest that reducing WMH accumulation over a 5-year period in late life might also diminish AD risk.
Our study has a number of strengths, which include the large sample of diverse community-dwelling older adults, use of quantitative longitudinal multimodal neuroimaging, highly standardized subject evaluation, and sophisticated analytic approach. Although we established that progressing regionally-distributed WMH predicts onset of AD, we were unable to examine directly causal relationships between WMH and the beta amyloid and tau pathology that define the disease pathologically. It is possible that WMH provide an additive “second hit,” which lowers the threshold for disease onset. It is also possible that the WMH interacts with other AD biological markers more fundamentally. White matter hyperintensities are typically considered to be a non-specific marker of cerebrovascular diseases associated with aging and not AD specifically, but beta amyloid and tau pathology can be described similarly; both are common in aging and certainly occur among older adults without any clinical evidence of neurodegenerative disease (Aizenstein, et al., 2008,Lockhart, et al., 2007,Reiman, et al., 2009).
Diagnostic criteria and proposed pathogenic models of AD should be more inclusive to reflect the prominent role of small vessel cerebrovascular disease considering and future intervention or prevention trials should consider targeting the vascular system.
Acknowledgments
This work was supported by NIH grants AG034189 and AG037212
References
- Admiraal-Behloul F, Olofesen H, Van den Heuvel DM, Schmitz N, Reiber JH, Van Buchem MA. Fully automated lobe delineation for regional white matter lesion load quantification in a large scale study. Proceedings International Society for Magnetic Resonance in medicine. 2004:138. [Google Scholar]
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of neurology. 2008;65(11):1509–17. doi: 10.1001/archneur.65.11.1509. doi:10.1001/archneur.65.11.1509 65/11/1509 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd ed American Psychiatric Press; Washington, DC: 1987. [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Brickman AM. Contemplating Alzheimer's disease and the contribution of white matter hyperintensities. Curr Neurol Neurosci Rep. 2013;13(12):415. doi: 10.1007/s11910-013-0415-7. doi:10.1007/s11910-013-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, Brandt J, Blacker D, Stern Y. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Archives of neurology. 2008a;65(9):1202–8. doi: 10.1001/archneur.65.9.1202. doi:10.1001/archneur.65.9.1202 65/9/1202 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Alzheimer's disease: do white matter hyperintensities matter? Dialogues in clinical neuroscience. 2009;11(2):181–90. doi: 10.31887/DCNS.2009.11.2/ambrickman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, Stern Y, Brown TR, Luchsinger JA, Mayeux R. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Archives of neurology. 2012;69(12):1621–7. doi: 10.1001/archneurol.2012.1527. doi:10.1001/archneurol.2012.1527 1356498 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Archives of neurology. 2010;67(5):564–9. doi: 10.1001/archneurol.2010.70. doi:10.1001/archneurol.2010.70 67/5/564 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Archives of neurology. 2008b;65(8):1053–61. doi: 10.1001/archneur.65.8.1053. doi:10.1001/archneur.65.8.1053 65/8/1053 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Stern Y, Provenzano FA, Narkhede A, Razlighi Q, Collins-Praino L, Artero S, Akbaraly T, Ritchie K, Mayeux R, Portet F. Does APOE-ε4 confer risk for Alzheimer's disease through regionally distributed white matter hyperintensities? submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Sneed JR, Provenzano FA, Garcon E, Johnert L, Muraskin J, Yeung LK, Zimmerman ME, Roose SP. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Research: Neuroimaging. 2011;193(2):101–6. doi: 10.1016/j.pscychresns.2011.03.007. doi:S0925-4927(11)00116-8 [pii] 10.1016/j.pscychresns.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capizzano AA, Ación L, Bekinschtein T, Furman M, Gomila H, Martínez A, Mizrahi R, Starkstein SE. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75(6):822–7. doi: 10.1136/jnnp.2003.019273. doi:10.1136/jnnp.2003.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, et al. LOngitudinal changes in white matter disease and cognition in the first year of the alzheimer disease neuroimaging initiative. Archives of neurology. 2010;67(11):1370–8. doi: 10.1001/archneurol.2010.284. doi:10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The Cortical Signature of Alzheimer's Disease: Regionally Specific Cortical Thinning Relates to Symptom Severity in Very Mild to Mild AD Dementia and is Detectable in Asymptomatic Amyloid-Positive Individuals. Cerebral Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. doi:10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, de Kersaint–Gilly A, Besançon V, Levy C, Auffray E, Brunnereau L, Alpérovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities: The EVA MRI Cohort. Neurology. 2001;56(7):921–6. doi: 10.1212/wnl.56.7.921. doi:10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- Erten-Lyons D, Woltjer R, Kaye J, Mattek N, Dodge HH, Green S, Tran H, Howieson DB, Wild K, Silbert LC. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology. 2013;81(11):977–83. doi: 10.1212/WNL.0b013e3182a43e45. doi:10.1212/WNL.0b013e3182a43e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E, Ribeiro HM, Molyneux A, Budge M, Smith AD. Plasma homocysteine levels, cerebrovascular risk factors, and cerebral white matter changes (leukoaraiosis) in patients with Alzheimer disease. Archives of neurology. 2002;59(5):787–93. doi: 10.1001/archneur.59.5.787. doi:noc10273 [pii] [DOI] [PubMed] [Google Scholar]
- Ishii K, Sasaki H, Kono AK, Miyamoto N, Fukuda T, Mori E. Comparison of gray matter and metabolic reduction in mild Alzheimer's disease using FDG-PET and voxel-based morphometric MR studies. Eur J Nucl Med Mol Imaging. 2005;32(8):959–63. doi: 10.1007/s00259-004-1740-5. doi:10.1007/s00259-004-1740-5. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Eberling JL, Wu CC, Finkbeiner A, Mungas D, Valk PE, Haan MN. Brain function and cognition in a community sample of elderly Latinos. Neurology. 2002;59(3):378–83. doi: 10.1212/wnl.59.3.378. [DOI] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nat Neurosci. 2014;17(2):304–11. doi: 10.1038/nn.3606. doi:10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo RY, Jagust WJ. Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79(13):1349–55. doi: 10.1212/WNL.0b013e31826c1b9d. doi:10.1212/WNL.0b013e31826c1b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, Libri V, Leppert D, Beach TG. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain. 2007;130(Pt 10):2607–15. doi: 10.1093/brain/awm191. doi:awm191 [pii] 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Brickman AM, Reitz C, Cho SJ, Schupf N, Manly JJ, Tang MX, Small SA, Mayeux R, DeCarli C, Brown TR. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology. 2009;73(6):450–6. doi: 10.1212/WNL.0b013e3181b1636a. doi:10.1212/WNL.0b013e3181b1636a 73/6/450 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79(5):442–8. doi: 10.1212/WNL.0b013e3182617136. doi:10.1212/WNL.0b013e3182617136 WNL.0b013e3182617136 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makedonov I, Black SE, MacIntosh BJ. Cerebral small vessel disease in aging and Alzheimer's disease: a comparative study using MRI and SPECT. Eur J Neurol. 2013;20(2):243–50. doi: 10.1111/j.1468-1331.2012.03785.x. doi:10.1111/j.1468-1331.2012.03785.x. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. doi:10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Nesselroade JR. Structuring data to study development and change. In: Cohen SH, Reese HW, editors. Life-Span Developmental Psychology: Methodological Innovations. Erlbaum; Hillsdale, NJ: 1994. pp. 223–67. [Google Scholar]
- McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53(5):902–5. doi: 10.1212/wnl.53.5.902. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meier IB, Manly JJ, Provenzano FA, Louie KS, Wasserman BT, Griffith EY, Hector JT, Allocco E, Brickman AM. White matter predictors of cognitive functioning in older adults. J Int Neuropsychol Soc. 2012;18(3):414–27. doi: 10.1017/S1355617712000227. doi:10.1017/S1355617712000227 S1355617712000227 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Muthén & Muthén. Los Angeles, CA: 1998–2012. MPlus User's Guide. Seventh Edition. [Google Scholar]
- Polvikoski TM, van Straaten ECW, Barkhof F, Sulkava R, Aronen HJ, Niinistö L, Oinas M, Scheltens P, Erkinjuntti T, Kalaria RN. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75(23):2071–8. doi: 10.1212/WNL.0b013e318200d6f9. doi:10.1212/WNL.0b013e318200d6f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM. White Matter Hyperintensities and Cerebral Amyloidosis: Necessary and Sufficient for Clinical Expression of Alzheimer Disease. JAMA Neurol. 2013:1–7. doi: 10.1001/jamaneurol.2013.1321. doi:10.1001/jamaneurol.2013.1321 1653648 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6820–5. doi: 10.1073/pnas.0900345106. doi:0900345106 [pii] 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Rothschild D. Alzheimer's disease: A clinicopathologic study of five cases. American Journal of Psychiatry. 1934;91(3):486–519. [Google Scholar]
- Siedlecki KL, Manly JJ, Brickman AM, Schupf N, Tang MX, Stern Y. Do neuropsychological tests have the same meaning in Spanish speakers as they do in English speakers? Neuropsychology. 2010;24(3):402–11. doi: 10.1037/a0017515. doi:10.1037/a0017515 2010-07896-012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert LC, Dodge HH, Perkins LG, Sherbakov L, Lahna D, Erten-Lyons D, Woltjer R, Shinto L, Kaye JA. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology. 2012;79(8):741–7. doi: 10.1212/WNL.0b013e3182661f2b. doi:10.1212/WNL.0b013e3182661f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature reviews. 2011;12(10):585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, Mayeux R. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Archives of neurology. 1992;49(5):453–60. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of Alzheimer's disease in African-Americans, Caribbean Hispanics and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke. 1983;14(6):924–8. doi: 10.1161/01.str.14.6.924. doi:10.1161/01.str.14.6.924. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, Weigand SD, Vemuri P, Senjem ML, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW, Jack CR., Jr. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71(10):743–9. doi: 10.1212/01.wnl.0000324924.91351.7d. doi:10.1212/01.wnl.0000324924.91351.7d 71/10/743 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegman AF, Meier IB, Provenzano FA, Schupf N, Manly JJ, Stern Y, Luchsinger JA, Brickman AM. Regional white matter hyperintensity volume and cognition predict death in a multiethnic community cohort of older adults. Journal of the American Geriatrics Society. 2013;61(12):2246–8. doi: 10.1111/jgs.12568. doi:10.1111/jgs.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM-Y, Trojanowski JQ, Arnold SE. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135(12):3749–56. doi: 10.1093/brain/aws271. doi:10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–8. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]