Abstract
Previous research has indicated increased functional connectivity between subthalamic nucleus (STN) and sensorimotor cortex in off-medication Parkinson’s disease (PD) compared with control subjects. It is not clear if the increase in functional connectivity between STN and sensorimotor cortex occurs in de novo PD, which is prior to when patients begin dopamine therapy. Resting state functional magnetic resonance imaging was carried out in 20 de novo (drug-naïve) patients with PD (HY stage: I-II), 19 patients with moderate PD (HY stage: II-III), and 19 healthy controls. The functional connectivity analysis in de novo and moderate PD patients focused on the connectivity of the more affected STN and the sensorimotor cortex. Using resting state functional connectivity analysis, we provide new evidence that people with de novo PD and off-medicated moderate PD have increased functional connectivity between the more affected STN and different regions within the sensorimotor cortex. The overlapping sensorimotor cortex found in both de novo and moderate PD had functional connectivity values that correlated positively with the Unified Parkinson’s Disease Rating Scale part III. This key finding suggests that changes in functional connectivity between STN and sensorimotor cortex occur early in the disease following diagnosis and prior to dopamine therapy.
Introduction
The subthalamic nucleus (STN) is a structure within the basal ganglia that is located ventrally to the thalamus and plays a crucial role within the basal ganglia motor circuitry (DeLong and Wichmann, 2007,Hamani, et al., 2004). The STN has been linked to the pathophysiology of a variety of movement disorders, including Parkinson’s disease (PD) (DeLong and Wichmann, 2007). PD is a neurodegenerative syndrome whose hallmark symptoms include tremor, rigidity and bradykinesia, and is characterized by degeneration of dopaminergic nigrostriatal neurons that leads to dysfunction of the activity within the cortico-striatal-thalamic circuit (Herz, et al., 2013,Spraker, et al., 2010). Recently, studies using functional magnetic resonance imaging (fMRI) indicated that neuronal activity in PD is abnormal not only during task performance (Spraker et al., 2010) but also in the resting state of the brain. Specifically, regional homogeneity is decreased in several regions such as the putamen, thalamus, and supplementary motor area; and increased in the cerebellum and primary motor cortex (Wu et al., 2009). Moreover, alterations of the resting state functional connectivity between different motor regions (e.g. supplementary motor area and primary motor cortex) have been demonstrated (Wu et al., 2011). Therefore, resting state connectivity changes may help characterize the functional brain organization and reorganizarion in PD.
Deep brain stimulation (DBS) studies have found abnormal neuronal oscillations within STN (Cassidy, et al., 2002,Hammond, et al., 2007,Kuhn, et al., 2009,Levy, et al., 2002,Moran, et al., 2008). Electroencephalography studies of off-medicated PD patients with DBS electrodes placed in the STN have revealed an increased coherence between STN and cortical areas in alpha and beta frequencies when compared to the on-medicated state (Brown, 2003,Eusebio, et al., 2009,Fogelson, et al., 2006). Enhanced beta coherence between STN and M1 was found in PD patients selected for therapeutic STN stimulation tested off-medication, and coherence was suppressed by administration of levodopa (Hirschmann et al., 2013). Dopaminergic medication was found to modulate the beta network at rest by increasing the coherence between the STN and prefrontal cortex (Litvak et al., 2011). Early stage PD patients showed increased sensorimotor cortical power at beta frequency (13–30 Hz) both during rest and during isometric contraction as compared to healthy controls (Pollok et al., 2012).
Moreover, a resting state functional magnetic resonance imaging (RS-fMRI) study observed an increased functional connectivity between STN and areas within primary sensorimotor cortex (M1S1) in off-medicated PD patients as compared to healthy controls (Baudrexel, et al., 2011). Taken together, these findings demonstrate that PD is associated with alterations in functional connectivity and information transmission between M1S1 and STN.
A recent RS-fMRI study in de novo PD patients found reduced functional connectivity between the mesolimbic-striatal and cortico-striatal regions when seeds were placed within the caudate and putamen (Luo, et al., 2014). What remains unclear though, is if the changes in STN-M1S1 functional connectivity are present in people with PD before anti-parkinsonian medications are used (i.e. de novo PD group). Moreover, it is not clear how people with de novo PD would compare to those with moderate PD in the degree of changes in functional connectivity between STN-M1S1 and the specific location of the cortical regions within M1S1 where increased STN-M1S1 functional connectivity is observed. Finally, it remains to be determined whether there is a relation between STN-M1S1 functional connectivity and the severity of motor symptoms in PD.
In the current study we utilized RS-fMRI to compare STN-M1S1 functional connectivity between healthy controls, de novo PD, and moderate PD. We tested the hypothesis that STN-M1S1 functional connectivity would be increased in de novo PD as well as moderate PD as compared to controls, and that disease severity, as measured by the motor section of the United Parkinson’s Disease Rating Scale (UPDRS-III), would be related to STN-M1S1 functional connectivity in the de novo PD and moderate PD groups.
Materials and methods
Participants
We conducted a prospective case–controlled study of 58 participants: 20 de novo Parkinson’s disease (PD) patients (mean age: 59.7 ± 12.4 years), 19 moderate PD patients (mean age: 62.4 ± 8.1 years), and 19 healthy controls (mean age: 62.0 ± 9.7 years). De novo PD had never been treated with anti-parkinsonian medications, and did not have clinically significant cognitive impairment (all patients had ≥ 26 points on the Mini-Mental State Evaluation; Folstein et al., 1975). We defined anti-parkinsonian medication to include any drug designed to alter symptoms of PD or any drug posited to slow the progression of PD. All moderate PD patients were tested off anti-parkinsonian medication following overnight withdrawal. Patients who have been taking anti-parkinsonian medications over a minimum 6-month period were classified as moderate PD. Most moderate PD patients were treated with carbidopa/levodopa (dosage range: 25/250 mg 3–9 times a day), and/or rasagiline (dosage: 1 mg once a day), amantadine (dosage: 100 mg 1–4 times a day), pramipexole (0.5/1 mg 1–3 times a day). De novo PD and moderate PD patients were diagnosed by a movement disorders specialist and met the UK PD Society Brain Bank diagnostic criteria (Hughes, et al., 2001). As part of the protocol, the severity of their motor symptoms was evaluated using part III of the Unified PD Rating Scale (UPDRS) (Table 1) (Fahn, et al., 1987). In the moderate PD group, UPDRS-III was scored off-medication. Control participants were physically healthy and had no history of neurological disease or substance abuse. Healthy controls were age-and gender-matched with de novo and moderate PD patients. Age did not significantly differ between the three groups. Moderate PD presented with longer disease duration as compared to de novo PD (Table 1). All participants gave written informed consent consistent with the Declaration of Helsinki, which was approved by the local institutional review board.
Table 1.
Demographic and clinical charact eristics
| Variable | Controls | De novo PD | Mod PD |
|---|---|---|---|
| N | 19 | 20 | 19 |
| Age | 62.0 (9.7) | 59.7 (12.3) | 62.4 (8.1) |
| Gender (M/F) | 15/4 | 15/5 | 16/3 |
| Handedness (L/R) | 2/17 | 3/17 | 1/18 |
| Affected side (L/R) | n/a | 9/11 | 13/6 |
| HY stage | n/a | I-II | II-III |
| Disease duration | n/a | 7.1 (7.6) | 86.7 (54.9) |
| UPDRS-III | n/a | 18.6 (6.8) | 31.5 (9.9) |
PD, Parkinson’s disease; Mod, moderate; M, male; F, female; L, left; R, right; HY, Hoehn and Yahr; UPDRS-III, Part III of the Unified Parkinson’s Disease Rating Scale. Disease duration is measured in months, UPDRS-III is scored off-medication, and handedness is based on self-report. Mean (± SD) is given for age, disease duration, and UPDRS-III.
MRI data acquisition protocol
MR imaging was carried out on a 3.0-Tesla SignaHDx scanner (General Electric Healthcare, Waukesha, WI) using an 8-channel phased-array head coil for signal reception and whole-body coil for radio frequency transmission. During resting state scans, subjects were instructed to lie still, relax and keep their eyes focused on the word “RELAX” which was written in white on a black background on a monitor mounted at the level of their eyes (Van Dijk, et al., 2010). Functional MRI scans were acquired using a gradient echo EPI pulse sequence with the following parameters: TR = 2000 ms, TE = 25 ms, matrix size = 64 × 64, FOV = 200 × 200 mm, 40 slices, 3 mm slice thickness, resolution of 3.125 × 3.125 × 3.000 mm and a scan duration of 6.57 minutes. T1-weighted anatomical scans were acquired using a spoiled gradient-recalled acquisition (SPGR) with the following parameters: TR = 13.836 ms, TE = 4.3 ms, flip angle 25, matrix size = 512 × 512, FOV = 220 × 220 mm, 120 slices, 1.5 mm slice thickness, resolution of 0.430 × 0.430 × 1.500 mm.
Image processing
Image processing methods were adapted from prior resting state fMRI analyses (Jo, et al., 2010,Saad, et al., 2012) using Freesurfer (http://surfer.nmr.mgh.harvard.edu/) and AFNI (http://afni.nimh.nih.gov/afni). Prior to analysis, the first 4 volumes of each fMRI scan were discarded to account for saturation effects. The remaining 197 volumes (concatenated into a 4D file for each subject) were used in the subsequent resting state analysis. Processing consisted of the following steps: 1) Motion correction, intensity normalization and skull stripping of anatomical scans. 2) Removal of neck tissue and volumetric labeling of brain structures as gray matter (GM), white matter (WM) and cerebral spinal fluid (CSF). 3) Surface mapping and cortical parcellation of surface brain structures. 4) At this stage we used Freesurfer’s registration tool (bbregister) to register the resting state scans (197th timepoint – reference frame) with anatomical scans. 5) Preprocessing functional data continued using afni_proc.py. The following steps were included: a) despiking to remove extreme time series outliers, b) slice-timing correction for interleaved acquisition, and c) 3D rigid motion correction of resting state scan to the reference frame. Additionally, we detected specific time points with movements > 0.5 mm for exclusion at a later stage. 6) Time series were further processed using ANATICOR (AFNI) to extract spurious or nonspecific sources of variance from the BOLD data. These sources included the 6 motion parameters that describe rigid-body transformation, CSF signals, as well as local WM signals. Also, at this stage the average signal across all voxels in the brain (i.e. global signal) was regressed out (Fox, et al., 2009). 7) Bandpass filtering of the time series (0.008 Hz to 0.10 Hz) was performed in order to restrict the analysis to frequencies of interest corresponding to low frequency fluctuations in the BOLD signal. 8) Data were smoothed using 3dBlurtoFWHM to reach a final smoothing of 4mm FWHM blur level. This increases signal to noise ratio and improves sensitivity of results while taking into account inherent smoothing from the data acquisition so that images are not over-smoothed. 9) Anatomical and processed scans were left-right flipped for subset of patients (9 de novo PD and 13 moderate PD) such that motor symptoms were left-hemisphere dominant for all subjects. Control subjects were not flipped. 10) All anatomical images were warped into MNI space (2 × 2 x 2 mm). This step ensures a common space for the subsequent group analysis and allows warping parameters to be derived for each participant.
Functional connectivity: seed selection
To illustrate the robustness of seed-based resting state functional connectivity analysis and as a proof of principle, an initial control seed was placed within the posterior cingulate. Placing a seed within this region should result in a significant correlations between the resting state BOLD signal of the posterior cingulate cortex and various brain regions within the prefrontal, temporal and parietal cortices, known to be part of the default mode network (DMN) (Raichle, et al., 2001,Raichle and Snyder, 2007). The outcome of this analysis is provided in the Supplementary Material.
For the primary analysis, a seed was placed unilaterally in the STN. For controls, the seed was placed in the left STN. The brains of those patients having their left body-side more affected were left-right flipped. Thus, the seed was placed in the more affected hemisphere in all PD patients. The more affected STN corresponded to body-side with the lower UPDRS score. All patients presented side dominance of motor symptoms. The seed region was based on the MNI formatted STN mask included in the validated Basal Ganglia Human Area Template (Prodoehl, et al., 2008). This template has been validated, such that the results are similar when drawing the STN in EPI compared with standardized space. For each individual, an inverse transform derived from the anatomical scan was applied to the mask in order to convert it from MNI into subject/native space. The mean signal of non-zero voxels within the STN mask was quantified as the seed.
PD is typically marked by side predominance. In order to investigate to which extent alterations in functional connectivity are restricted to the more affected side, a control analysis was performed, assessing resting state functional connectivity between the less affected STN and the sensorimotor cortex (see Supplementary Table 1). Differences in functional connectivity at rest between the two sides (left vs. right STN) were expected in PD patients but not in controls.
Functional connectivity analysis
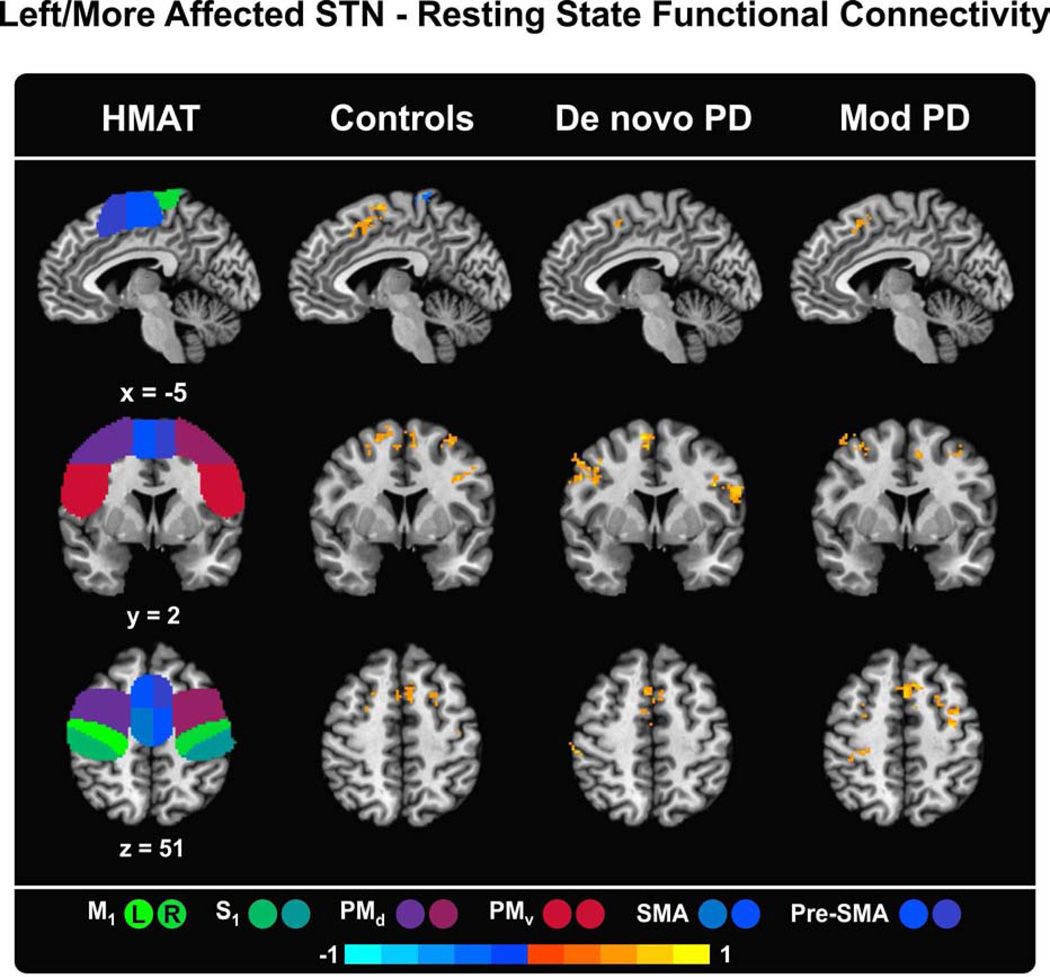
Pearson correlations were computed between the seed and other voxels in the brain while excluding time points detected in stage 5c of the methods section, (i.e. movements > 0.5 mm). The correlation maps were then converted to a Z-score via a Fisher tanh−1 transform in order to perform a group statistical analysis. Each Z-score map was transformed into MNI space via transforms derived from warping the anatomical scans. Finally, a two-sample t-test was used to compare functional connectivity maps between healthy controls and each patient group, as well as between patient groups, with age as a covariate. The analysis was restricted to the motor areas included within the Human Motor Area Template (HMAT). AFNI’s 3dClustSim program was used to generate a P-value and extent threshold equivalent to a Familywise Error Rate (FWER). The group-level analysis threshold was set at P < 0.05 (corrected) based on the 3dClustSim output (minimum cluster size of 59 voxels). Figure 1 depicts the functional connectivity of the left STN in controls/more affected STN in PD patients with the sensorimotor cortex. Supplementary Figure 1 presents connectivity for the right STN in controls and the less affected STN in PD patients with the sensorimotor cortex, whereas Supplementary Figure 2 presents the between-group differences.
Figure 1.
Functional connectivity maps of the left STN in controls/more affected STN in PD patients and the regions of the Human Motor Area Template (HMAT). The HMAT mask used in the analysis includes the following structures: primary motor cortex (M1), sensory cortex (S1), dorsal premotor cortex (PMd), ventral premotor cortex (PMv), supplementary motor area (SMA), and pre-supplementary motor area (Pre-SMA). Results are given in MNI space, thresholded at P < 0.05 (corrected using 3dClustSim in AFNI) and presented on sagittal, coronal and axial slices of the Collin 27 average brain. The color scale provides information about the strength and sign of the correlation. L = left, R = right.
M1S1 cluster extent analysis
The number of voxels that were positive in the M1S1 cluster was examined for each participant. This analysis was conducted to determine if the cluster extent changed between groups. The Z values were thresholded such that only positive values were included, and the number of voxels that were positive was counted within the M1S1 cluster that was determined from the voxel-wise functional connectivity analysis. The region examined was the combined M1S1 region found across de novo PD and moderate PD. A one-way analysis of variance (ANOVA) was used in conjunction with Tukey’s post-hoc test to assess differences in the number of voxels between controls, de novo PD, and moderate PD.
STN – sensorimotor cortex functional connectivity and UPDRS-III correlation analysis
SPSS software (http://www-01.ibm.com/software/analytics/spss/) was used to perform Spearman’s rank correlation analysis. This non-parametric test was used to determine if the strength of functional connectivity results (Z values) correlated with the total UPDRS-III (motor section), or sub-score measurements of bradykinesia, tremor, rigidity or axial function. For this correlation, the region of overlap between the functional connectivity maps pertaining to the de novo PD-controls and moderate PD-controls comparisons was selected. P-values were corrected in Matlab (http://www.mathworks.com/matlabcentral/fileexchange/27418-benjamini-hochbergyekutieli-procedure-for-controlling-false-discovery-rate/content/fdr_bh.m) at a false discovery rate (FDR) of 0.05 using the Benjamini-Hochberg-Yekutieli method.
Results
Posterior cingulate functional connectivity
The purpose of this analysis was to show the integrity of the data and analysis pipeline. As expected, placing a control seed within the posterior cingulate resulted in increased functional connectivity between the posterior cingulate cortex and other brain regions known to compose within the default mode network (DMN). In all three groups (healthy controls, de novo PD, and moderate PD), a classical DMN network was obtained, with the posterior cingulate cortex being positively correlated with the medial temporal cortex (bilaterally), ventromedial prefrontal cortex, precuneus and inferior parietal lobule (Supplementary Material – Figure 3). Between-group comparisons revealed no significant differences between the DMN of de novo PD and controls. Several differences were found when comparing moderate PD and controls. While controls presented increased connectivity with the DMN of the left middle fronto-orbital gyrus as compared to moderate PD, moderate PD presented increased connectivity with the DMN of the right (less affected) motor cortex as compared to controls. Differences in DMN connectivity were also found when directly comparing the two PD groups. De novo PD were found to have increased connectivity with the DMN of the left superior frontal gyrus as compared to moderate PD, whereas moderate PD was found to have increased connectivity with the DMN of the same left superior frontal gyrus (however a more rostral region) as compared to de novo PD.
Left STN – sensorimotor cortex functional connectivity in healthy controls
In healthy controls, the left STN showed significant (P < 0.05, corrected using 3dClustSim) bilateral increased functional connectivity at rest with the ventral (PMv) and dorsal (PMd) premotor cortices, supplementary (SMA) and pre-supplementary (Pre-SMA) motor areas as well as the lateral section of primary motor cortices (M1) (Table 2). A significant decreased functional correlation was found between the left STN and the more medial section of the right primary motor cortex (M1). The cluster extended into the adjacent left motor cortex. The motor regions significantly interconnected with the left STN are shown in Figure 1.
Table 2.
Left/more affected STN - sensorimotor cortex resting-state functional connectivity results.
| Comparison | Brain region(s) | Side | Size (mm3) | MNI coordinates (peak) |
t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Mean resting-state functional connectivity | |||||||
| Controls | PMv, M1 | R | 2008 | −44 | −14 | 28 | 6.07 |
| Pre-SMA, SMA | R | 1872 | −6 | 6 | 56 | 5.22 | |
| SMA, PMd | L | 1352 | 22 | 12 | 56 | 5.04 | |
| PMd | R | 952 | −32 | 2 | 60 | 6.29 | |
| M1 | R | 936 | −2 | −40 | 72 | −4.82 | |
| PMv | L | 656 | 46 | 12 | 32 | 4.83 | |
| PMv | R | 496 | −46 | 16 | 28 | 4.49 | |
| De novo PD | PMv, PMd | L | 2944 | 52 | 6 | 42 | 5.71 |
| SMA, Pre-SMA | L | 1600 | 10 | 0 | 62 | 5.01 | |
| PMv | R | 1008 | −54 | 6 | 16 | 4.87 | |
| S1 | L | 960 | 54 | −32 | 58 | 5.33 | |
| Mod PD | Pre-SMA | L | 1136 | 4 | 20 | 56 | 4.96 |
| PMd | L | 888 | 44 | −2 | 62 | 4.53 | |
| S1, M1 | L | 512 | 38 | −32 | 54 | 4.08 | |
| PMd | R | 472 | −28 | 8 | 48 | 4.60 | |
| Between-group differences | |||||||
| De novo PD > Controls | M1, S1 | L | 1128 | 46 | −24 | 66 | −4.28 |
| Mod PD > Controls | M1, S1 | L | 632 | 26 | −30 | 52 | −3.75 |
| De novo PD > Mod PD | PMv | L | 544 | 44 | 4 | 34 | 3.58 |
Significance set at P < 0.05 (corrected using 3dClustSim in AFNI). Results are given in MNI space. PD, Parkinson’s disease; Mod, moderate; Corr, correlation; M1, primary motor cortex; S1, sensorimotor cortex; PMd, dorsal premotor cortex; PMv, ventral premotor cortex; SMA, supplementary motor area; Pre-SMA, pre-supplementary motor area; L, left; R, right.
More affected STN – sensorimotor cortex functional connectivity in PD patients
In de novo PD patients, the more affected STN significantly correlated with the PMv, (bilaterally), left PMd, and both supplementary and pre-supplementary motor areas (bilaterally) (Figure 1, Table 2). An additional cluster of increased positive connectivity was found in the left sensorimotor cortex. There was no cortical motor region that negatively correlated with STN in de novo PD patients.
As for moderate PD patients, the more affected STN was found to be positively interconnected with the pre-supplementary motor area (Pre-SMA) and dorsal premotor cortex (PMd) (bilaterally). As shown in Figure 1, activity at rest of the left sensorimotor cortex (M1S1) also correlated with the more affected STN. Similar to the de novo PD group, the more affected STN did not negatively correlate with any of the cortical motor regions.
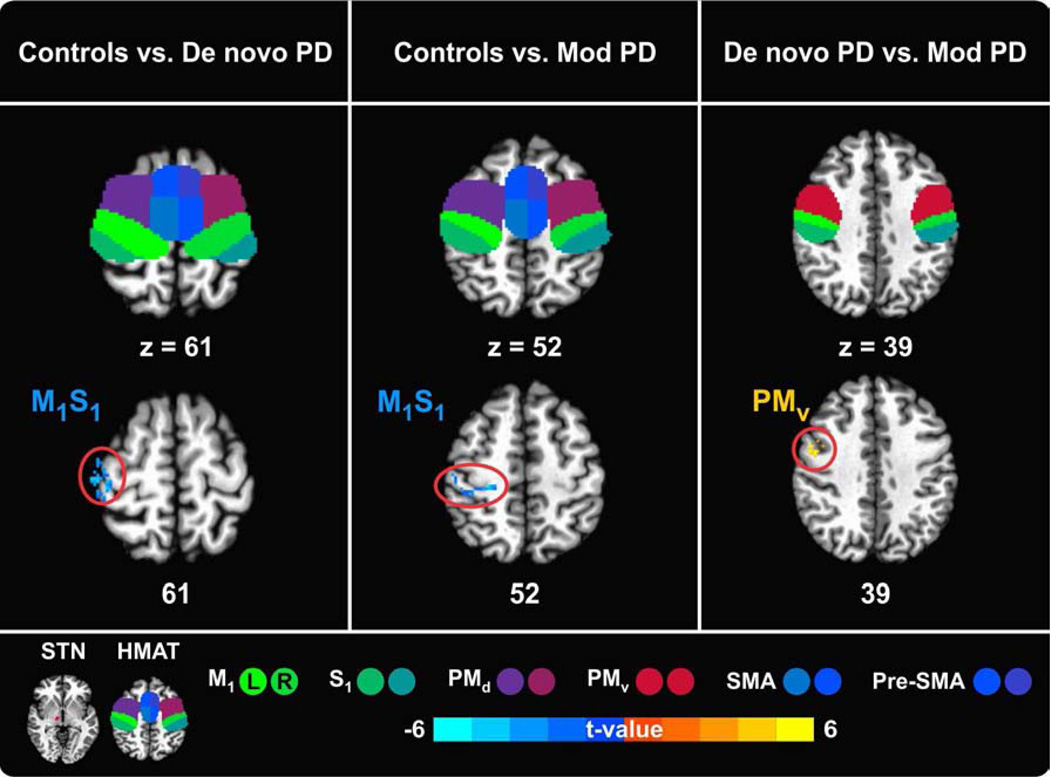
Between-group differences in STN – sensorimotor cortex functional connectivity
A comparison of the connectivity maps associated with the STN seed region between patients and controls, revealed similar connectivity patterns in the two PD groups. In both de novo and moderate PD the sensorimotor cortex (M1S1) was significantly more interconnected with STN in the more affected hemisphere relative to controls (Figure 2, Table 2). Although both patient groups exhibited increased functional connectivity of the M1S1 with the more affected STN, the location of the significant cluster differed between groups. In de novo PD, the more superior regions of M1S1 (z = 61) significantly correlated with STN, whereas in moderate PD, STN correlated with the more inferior regions of M1S1 (z = 52).
Figure 2.
Between-group differences in left/more affected STN – sensorimotor cortex functional connectivity. The sensorimotor cortex is represented by the regions of the Human Motor Area Template (HMAT): primary motor cortex (M1), sensory cortex (S1), dorsal premotor cortex (PMd), ventral premotor cortex (PMv), supplementary motor area (SMA), and pre-supplementary motor area (Pre-SMA). Results are in MNI space, thresholded at P < 0.05 (corrected using 3dClustSim in AFNI) and presented on axial slices of the Collin 27 average brain. The color scale provides information about the strength of between-group differences (expressed as t-value). L = left, R = right.
The direct comparison of the two PD groups revealed that compared to moderate PD, de novo PD patients have a larger cluster extent of increased functional connectivity of their more affected STN and left ventral premotor cortex (PMv). There was no region where moderate PD patients had more functional connectivity with the more affected STN than de novo PD patients.
M1S1 cluster extent analysis
To further explore whether the cluster extent of the M1S1 differed between groups, we combined the M1S1 clusters from de novo PD and moderate PD connectivity maps (Figure 2), and counted the number of positive Z value voxels in each subject. A one-way analysis of variance (ANOVA) indicated a significant group effect (F = 15.1; p < 0.001). Tukey’s post-hoc tests showed that in both de novo and moderate PD patients the cluster extent of M1S1 was significantly larger as compared to controls (P-values < 0.01). However, the cluster extent of M1S1 did not differ between the de novo PD and moderate PD groups (P = 0.6).
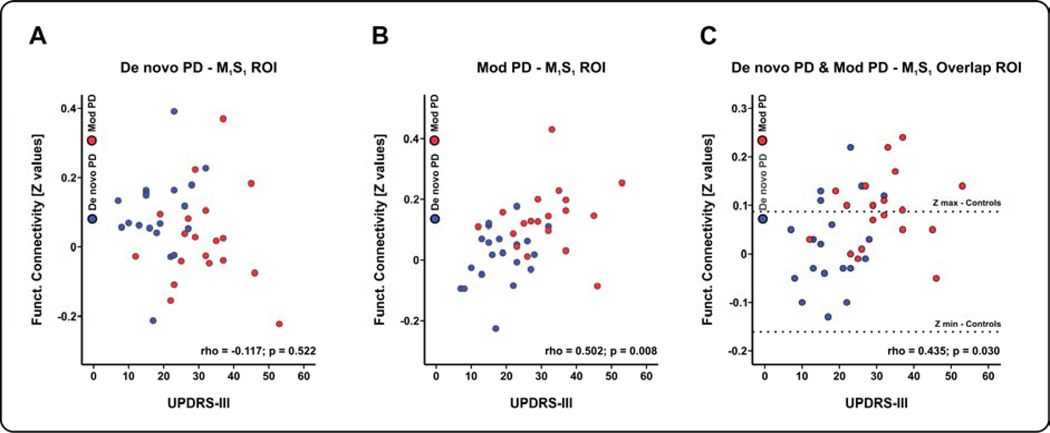
STN – sensorimotor cortex functional connectivity and UPDRS-III correlation analysis
Finally, the region where the two significant M1S1 clusters resulting from the between-group analysis overlapped was used as a mask to extract a mean Z value for each de novo and moderate PD patient. Similarly, Z values were extracted from the M1S1 clusters resulting from the de novo PD-controls/moderate PD-controls comparisons. These Z values along with the motor scores of the Unified Parkinson’s Disease Rating Scale (UPDRS-III) were submitted to a correlation analysis (i.e. Spearman rank correlation). As shown in Figure 3C, the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS-III) correlated with STN-M1S1 functional connectivity overlap in PD patients (Spearman’s Rho = 0.435, p = 0.030, FDR corrected), but also with the M1S1 cluster from the moderate PD-controls comparison (Spearman’s Rho = 0.502, p = 0.008, FDR corrected) (Figure 3B). There was not a significant correlation between the Z values extracted from the M1S1 cluster in de novo PD-controls comparison and the mean UPDRS-III (Spearman’s Rho = −0.117, p = 0.522, FDR corrected) (Figure 3A). Bradykinesia, rigidity, tremor and axial function subscores did not correlate with the M1S1 increased functional connectivity (p values > 0.05, FDR corrected). When examining the correlation between STN-M1S1 functional connectivity and the UPDRS-III within each of the three groups, we did not observe a significant correlation. This suggests that to obtain a significant correlation that a broad range of severity is needed as observed when combining the de novo and moderate PD groups into the correlation analysis.
Figure 3.
(A) Correlation of the UPDRS-III score and the Z functional connectivity values pertaining to the M1S1 cluster resulting from the de novo PD vs. controls comparison. (B) Similar correlation analysis between the UPDRS-III score and the Z functional connectivity values pertaining to the M1S1 cluster resulting from the moderate PD vs. controls comparison. (C) Correlation analysis between the UPDRS-III score and the Z functional connectivity values pertaining to the M1S1 region of overlap. Dotted lines in section C represent the Z values range in controls.
Discussion
The current study found that both the de novo PD as well as off-medicated moderate PD groups had increased functional connectivity between the more affected STN and M1S1 compared with matched control subjects. The overlapping M1S1 region in de novo PD and moderate PD had functional connectivity values that correlated positively with the UPDRS motor section. These findings provide new evidence that de novo PD have increased functional connectivity between STN-M1S1 and the severity of motor symptoms in PD relate to the changes in STN-M1S1 functional connectivity.
Electrophysiological studies have shown that PD patients have increased burst and oscillatory activity in the STN (Kuhn, et al., 2009,Moran, et al., 2008). The STN is frequently targeted for deep brain stimulation surgery in an effort to disrupt abnormal firing patterns and to improve cardinal motor symptoms. The mediation of these symptoms is likely related to changing the abnormal firing patterns between STN and sensorimotor cortex. Prior studies using virus tracing demonstrate both direct and indirect connections between motor cortex and STN (Kelly and Strick, 2004). RS-fMRI data provide clear evidence in support of functional connectivity between STN and the sensorimotor cortex in humans. The functional coupling between the more affected STN and the sensorimotor cortex in de novo PD and off-medicated, moderate PD is significantly increased as compared to healthy controls. It is important to note that the less affected hemisphere STN-M1S1 did not differ between PD and controls. This observed abnormal functional connectivity in the more affected hemisphere is supported by the findings of a PET study (Payoux, et al., 2004), which evaluated regional cerebral blood flow (rCBF) in 7 off-medicated PD subjects with unilateral (left hemisphere) STN DBS. During high frequency stimulation of the left STN, there was a significant reduction in rCBF in the lateral portions of left sensorimotor cortex when compared to the off-stimulation condition, and these changes were associated with improved motor symptoms. This finding suggests that off-medicated PD subjects typically have increased rCBF in the more affected sensorimotor cortex as compared to healthy controls. The findings are also supported by a RS-fMRI study which also observed an increased functional connectivity between STN and M1S1 in the off-medicated PD state (Baudrexel, et al., 2011), with 21 of 31 patients manifesting the left hemisphere as the more affected side. In the current study, we extend the findings of Baudrexel and colleagues of an increased functional connectivity between more affected STN and M1S1 to de novo PD (individuals who have never taken any type of anti-parkinsonian medication). It was also found that compared to control subjects, STN-M1S1 functional connectivity was increased for off-medicated moderate stage PD whose symptoms are more severe based on UPDRS-III. These findings collectively suggest that the increased functional connectivity between the more affected STN and areas of sensorimotor cortex occurs throughout various stages of disease progression, and includes subjects with no prior medication exposure.
Although both groups of PD subjects exhibited increased functional connectivity between the more affected STN and M1S1, the location within M1S1 differed between de novo PD and moderate PD. It was observed in de novo PD that the location for the functional connectivity region was located in the superior regions of M1S1 (z = 61). Meanwhile moderate PD subjects had a location in inferior regions of M1S1 (z = 52). The shift in location for STN-M1S1 between de novo PD and moderate PD suggests that perhaps disease progression or medication effects may have been a factor in the changes manifested within the more affected sensorimotor cortex. This hypothesis garners even more support when factoring in data from the study by Baudrexel and colleagues (2011). PD subjects, whose motor symptoms were slightly more severe than that of our de novo PD group, and less severe than our moderate PD group, had an increased functional connectivity in the more affected M1 at z = 60 and S1 at z = 58. We considered the possibility that the location does not shift but that the cluster extent of the STN-M1S1 functional connectivity grows larger. Our analysis of the size of the cluster in M1S1 suggests this is not the case. Longitudinal studies of disease progression would be needed to examine this hypothesis and further determine if the location shifts with progression of PD.
A prior study by Helmich and colleagues (2010) found a shift in cortico-striatal connections in PD compared to healthy controls. There was a reduced functional connectivity between the posterior putamen and cortical areas, and an increased functional connectivity between the anterior putamen and cortical areas. The authors interpreted these findings as a shift related compensation from the more affected to the least affected part of the putamen. The observation in this study of a shift in the region of increased functional connectivity between STN-M1S1 could also be related to a form of compensation. Together, these results support the hypothesis that the location of increased functional connectivity in the more affected M1S1 may change with the disease state, compensation, or medication effects.
Moreover, differences between de novo PD and moderate PD in functional connectivity of the more affected STN and ventral premotor cortex suggest specific changes within higher order regions of the resting state sensorimotor cortex which may help understand disease progression. An abnormal/increased BOLD signal at rest within the PMv of de novo PD as compared to moderate PD may be related to the early motor symptoms of PD such as difficulties in precision grasping.
Kwak and colleagues (Kwak, et al., 2010) employed a similar seed based approach to examine the effects of dopaminergic medication on resting state connectivity in patients with mild-moderate PD. However, they expanded the striatal regions examined to 6 striatal seed regions to be consistent with previous work in healthy controls (Di Martino, et al., 2008). Seeds were placed in the inferior ventral striatum, superior ventral striatum, dorsal caudate, dorsal caudal putamen, and ventral rostral putamen to probe connectivity differences between healthy controls and patients with PD, and to compare differences in the on medication compared to the off medication state in patients. Connectivity maps in the patients were similar to controls. Consistent with the results of Helmich and colleagues (2010), when comparing patients in the off medication state to healthy controls there was increased cortico-striatal connectivity. This increased connectivity was more significant in the two dorsal putamen seeds. The increased connectivity however was affected by medication such that in the on medication state, there was decreased cortico-striatal connectivity in patients compared to controls. There were no regions that showed increased connectivity in the on medication condition compared to the off medication state.
Examining patients in different stages of the disease may provide insight into how changes in functional connectivity evolve. In a study of de novo PD, Luo and colleagues (2014) examined resting state striatal functional connectivity changes in early stage drug naïve patients with PD using seeds in bilateral anterior and posterior putamen and caudate. Compared to healthy controls, there was reduced connectivity in mesolimbic-striatal and cortico-striatal loops in PD. Although the caudate connectivity pattern was relatively spared, posterior putaminal connectivity changes were pronounced and extended to include the sensorimotor cortex. Comparing patients who had a right side onset of symptoms to those with a left side onset also showed decreased functional connectivity with the sensorimotor cortex only with the more affected posterior putamen. Despite using similar methods and seed regions to Helmich and colleagues (2010), the patients in this study by Luo et al. (2014) did not exhibit any increased functional connectivity in cortico-striatal loops. The primary difference between studies was patient selection. Patients in the Luo et al. (2014) study were early stage drug naïve patients while patients in the Helmich et al. (2010) study were a mix of drug naïve early stage PD and patients with more moderate stage PD already taking anti-parkinsonian medication. This suggests the possibility that changes in the striatum in the early stages of the disease process in PD are limited to the posterior putamen with relative sparing of the anterior putamen and no evidence of compensatory changes in connectivity with the sensorimotor cortex. If this evolution of connectivity changes is true, examining patients with significantly more advanced PD should reveal a pattern of more distributed striatal involvement and emergence of increasing functional connectivity in striatal-cortical pathways.
In conclusion, we provide evidence that both de novo and off-medicated moderate PD have increased functional connectivity between the more affected STN and M1S1. The M1S1 overlapping region found in de novo PD and moderate PD had functional connectivity values that correlated positively with disease severity. The location of the M1S1 region shifted for de novo PD and moderate PD subjects, but the cluster extent did not change. These key findings demonstrate that functional connectivity between STN and M1S1 may be subject to plastic changes that occur early in the disease and future studies of disease progression will allow further study into this issue.
Supplementary Material
Supplementary Material – Figure 1. Functional connectivity maps of the right STN in controls/less affected STN in PD patients and the regions of the Human Motor Area Template (HMAT). The HMAT mask used in the analysis includes the following structures: primary motor cortex (M1), sensory cortex (S1), dorsal premotor cortex (PMd), ventral premotor cortex (PMv), supplementary motor area (SMA), and pre-supplementary motor area (Pre-SMA). Results are given in MNI space, thresholded at P < 0.05 (corrected using 3dClustSim in AFNI) and presented on sagittal, coronal and axial slices of the Collin 27 average brain. The color scale provides information about the strength and sign of the correlation. L = left, R = right.
Supplementary Material – Figure 2. Between-group differences in right/less affected STN – sensorimotor cortex functional connectivity. The sensorimotor cortex is represented by the regions of the Human Motor Area Template (HMAT): primary motor cortex (M1), sensory cortex (S1), dorsal premotor cortex (PMd), ventral premotor cortex (PMv), supplementary motor area (SMA), and pre-supplementary motor area (Pre-SMA). Results are in MNI space, thresholded at P < 0.05 (corrected using 3dClustSim in AFNI) and presented on axial slices of the Collin 27 average brain. The color scale provides information about the strength of between-group differences (expressed as t-value). L = left, R = right.
Supplementary Material – Figure 3. Default mode network (DMN) in controls, de novo PD and moderate PD as found using a control seed placed within the posterior cingulate cortex. Results are shown in MNI space, thresholded at P < 0.05 (corrected using 3dClustSim in AFNI), and overlaid on the Collin 27 average brain. The color scale provides information about the strength and sign of the correlation. L = left, R = right.
De novo PD have increased functional connectivity between STN and M1S1
Moderate PD have increased functional connectivity between STN and M1S1
Location of STN and M1S1 connectivity differed for de novo and moderate PD
Severity of PD scales positively with functional connectivity between STN and M1S1
Acknowledgments
We thank the patients and the healthy controls for their time and commitment to this research.
Funding
This work was supported by NINDS R01 NS052318 and R01 NS075012 and a grant from the Michael J. Fox Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Materials
Yes
Disclosure
Ajay Kurani has no conflicts of interest to report.
Dr. Rachael Seidler receives grant support from NASA and is a consultant for the University of Florida.
Dr. Roxana Burciu has no conflicts of interest to report.
Dr. Cynthia Comella receives research support from Allergan, Dystonia Coalition, Dystonia Medical Research Foundation, Ipsen Limited, Merz Pharmaceuticals, NIH and Parkinson Disease Foundation. She receives compensation/honoraria for services as a consultant or an advisory committee member for Allergan, Impax Pharmaceuticals, Ipsen Biopharmaceuticals, Medtronic Inc, Merz Pharmaceuticals, US World Meds, and Teva Neurosciences. She receives royalties from Cambridge, Humana Press, and Wolters Kluwer.
Dr. Daniel Corcos receives grant support from NIH, and receives lecture and reviewer fees from NIH.
Dr. Michael Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >36 months has received no support from industry including travel. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, and Cambridge (movement disorders books). Dr. Okun has participated in CME activities on movement disorders sponsored by the USF CME office, PeerView, Prime, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
Dr. Colum Mackinnon receives grant support from NIH.
Dr. David Vaillancourt receives grant support from NIH, Bachmann-Strauss Foundation, and onsults for projects at UT Southwestern Medical Center, University of Illinois at Chicago, and Great Lakes NeuroTechnologies. He is co-founder of Neuroimaging Solutions, LLC.
References
- Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, Steinmetz H, Deichmann R, Roeper J, Hilker R. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. NeuroImage. 2011;55(4):1728–1738. doi: 10.1016/j.neuroimage.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2003;18(4):357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain : a journal of neurology. 2002;125(6):1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cerebral cortex. 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Pogosyan A, Wang S, Averbeck B, Gaynor LD, Cantiniaux S, Witjas T, Limousin P, Azulay JP, Brown P. Resonance in subthalamo-cortical circuits in Parkinson's disease. Brain : a journal of neurology. 2009;132(8):2139–2150. doi: 10.1093/brain/awp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Members of the UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. New Jersey: MacMillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson's disease. Cerebral cortex. 2006;16(1):64–75. doi: 10.1093/cercor/bhi084. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127(1):4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends in neurosciences. 2007;30(7):357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cerebral cortex. 2010;20(5):1175–1186. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- Herz DM, Eickhoff SB, Lokkegaard A, Siebner HR. Functional neuroimaging of motor control in parkinson's disease: A meta-analysis. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschmann J, Ozkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Differential modulation of STN-cortical and cortico-muscular coherence by movement and levodopa in Parkinson's disease. NeuroImage. 2013;68:203–213. doi: 10.1016/j.neuroimage.2012.11.036. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. 1992. Neurology. 2001;57(10 Suppl 3):S34–S38. [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. NeuroImage. 2010;52(2):571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider GH, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Experimental neurology. 2009;215(2):380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Muller ML, Dayalu P, Seidler RD. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Frontiers in systems neuroscience 4, 143. 2010 doi: 10.3389/fnsys.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain : a journal of neurology. 2002;125(6):1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Luo C, Song W, Chen Q, Zheng Z, Chen K, Cao B, Yang J, Li J, Huang X, Gong Q, Shang HF. Reduced functional connectivity in early-stage drug-naive Parkinson's disease: a resting-state fMRI study. Neurobiol Aging. 2014;35(2):431–441. doi: 10.1016/j.neurobiolaging.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Moran A, Bergman H, Israel Z, Bar-Gad I. Subthalamic nucleus functional organization revealed by parkinsonian neuronal oscillations and synchrony. Brain : a journal of neurology. 2008;131(12):3395–3409. doi: 10.1093/brain/awn270. [DOI] [PubMed] [Google Scholar]
- Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, Gaura V, Rascol O, Samson Y, Agid Y. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Archives of neurology. 2004;61(8):1307–1313. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- Pollok B, Krause V, Martsch W, Wach C, Schnitzler A, Südmeyer M. Motor-cortical oscillations in early stages of Parkinson's disease. J Physiology. 2012;590(13):3203–3212. doi: 10.1113/jphysiol.2012.231316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE. Region of interest template for the human basal ganglia: comparing EPI and standardized space approaches. NeuroImage. 2008;39(3):956–965. doi: 10.1016/j.neuroimage.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 97-9. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain connectivity. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Basal ganglia hypoactivity during grip force in drug naive Parkinson's disease. Human brain mapping. 2010;31(12):1928–1941. doi: 10.1002/hbm.20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, Chan P. Regional homogeneity changes in patients with Parkinson's disease. Human brain mapping. 2009;30:1502–1510. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Long X, Wang L, Hallet M, Zang Y, Li K, Chan P. Functional connectivity of cortical motor areas in the resting state in Parkinson's disease. Human brain mapping. 2011 Aug;32(9):1443–1457. doi: 10.1002/hbm.21118. Epub 2010 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material – Figure 1. Functional connectivity maps of the right STN in controls/less affected STN in PD patients and the regions of the Human Motor Area Template (HMAT). The HMAT mask used in the analysis includes the following structures: primary motor cortex (M1), sensory cortex (S1), dorsal premotor cortex (PMd), ventral premotor cortex (PMv), supplementary motor area (SMA), and pre-supplementary motor area (Pre-SMA). Results are given in MNI space, thresholded at P < 0.05 (corrected using 3dClustSim in AFNI) and presented on sagittal, coronal and axial slices of the Collin 27 average brain. The color scale provides information about the strength and sign of the correlation. L = left, R = right.
Supplementary Material – Figure 2. Between-group differences in right/less affected STN – sensorimotor cortex functional connectivity. The sensorimotor cortex is represented by the regions of the Human Motor Area Template (HMAT): primary motor cortex (M1), sensory cortex (S1), dorsal premotor cortex (PMd), ventral premotor cortex (PMv), supplementary motor area (SMA), and pre-supplementary motor area (Pre-SMA). Results are in MNI space, thresholded at P < 0.05 (corrected using 3dClustSim in AFNI) and presented on axial slices of the Collin 27 average brain. The color scale provides information about the strength of between-group differences (expressed as t-value). L = left, R = right.
Supplementary Material – Figure 3. Default mode network (DMN) in controls, de novo PD and moderate PD as found using a control seed placed within the posterior cingulate cortex. Results are shown in MNI space, thresholded at P < 0.05 (corrected using 3dClustSim in AFNI), and overlaid on the Collin 27 average brain. The color scale provides information about the strength and sign of the correlation. L = left, R = right.