Abstract
The urinary angiotensinogen (AGT) excretion rate could be a novel biomarker for the intrarenal activity of the reninangiotensin system. Little is known about the circadian rhythm of AGT levels in plasma or urine. In this short article, making use of data in plasma and urine of healthy volunteers and patients with chronic kidney diseases, we first report that we were unable to find evidence for a circadian rhythm of AGT under any condition. Next we critically discuss to what degree elevated urinary AGT levels might be considered an independent biomarker that is not simply the non-specific consequence of proteinuria.
Keywords: Angiotensinogen, circadian rhythm, healthy volunteers, CKD, plasma, urine
Introduction
The intrarenal renin-angiotensin system (RAS) is regulated by multiple independent mechanisms.1 Recent basic and clinical studies have demonstrated that the intrarenal RAS plays an important role in the progression of chronic kidney disease (CKD).1 Experimental studies suggest that the intrarenal angiotensin (Ang) II levels in several animal models of renal injury, including Dahl salt-sensitive rats on a high salt diet,2,3 spontaneously hypertensive rats,4 and diabetic nephropathy rats,5 are increased. The urinary angiotensinogen (AGT) excretion rate could be a novel biomarker for the activity of the RAS in the kidney.6 A novel sandwich enzyme-linked immunosorbent assay (ELISA) for human AGT has made it possible to measure a large quantity of specimens over time.7 Several components of systemic RAS are known to display a circadian rhythm. For example, plasma renin activity, Ang II, and aldosterone rise in the morning and decrease at night.8,9 However, little is known about the circadian rhythm of AGT in plasma or urine.10 In this short article, we first report our findings on the lack of a circadian rhythm of AGT levels in plasma and urine, both in healthy volunteers and in patients with CKD, and secondly we discuss to what degree urinary AGT might truly be considered an independent biomarker.
Circadian rhythm of plasma and urinary AGT concentrations
The experimental protocols of these studies were approved by the institutional review board and by the Clinical and Translational Research Center at Kagawa University (#22-057). Written informed consent was obtained after patients had received an oral and written explanation of the trial from the attending physicians. Clinical characteristics of the subjects in each study are summarized in Table 1. Plasma and urine were frozen at −80°C after centrifugation. Plasma and urinary AGT concentrations were measured by human AGT ELISA kit (IBL, Gunma, Japan).7 Urinary concentrations of creatinine (Cr) were measured by auto-analyzer (Hitachi, Tokyo, Japan). Urinary concentrations of AGT were normalized by urinary concentrations of Cr.
Table 1.
Clinical characteristics of participants in each study.
| Study I | Healthy volunteers (n = 43) |
| Men/women (n) | 17/26 |
| Age (years) | 34 ± 7 |
| Systolic blood pressure (mmHg) | 116 ± 12 |
| Diastolic blood pressure (mmHg) | 75 ± 9 |
| Study II | Healthy volunteers (n = 24) |
| Men/women (n) | 13/11 |
| Age (years) | 32 ± 6 |
| Systolic blood pressure (mmHg) | 123 ± 3 |
| Diastolic blood pressure (mmHg) | 74 ± 1 |
| Study III | Patients with CKD (n = 8) |
| Men/women (n) | 3/5 |
| Age (years) | 38 ± 22 |
| Systolic blood pressure (mmHg) | 117 ± 23 |
| Diastolic blood pressure (mmHg) | 62 ± 13 |
| eGFR(ml/min/ 1.73 m2) | 65.4 ± 18.0 |
| Urinary protein excretion (g/day) | 2.7 ± 3.5 |
CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate.
Plasma AGT in healthy volunteers
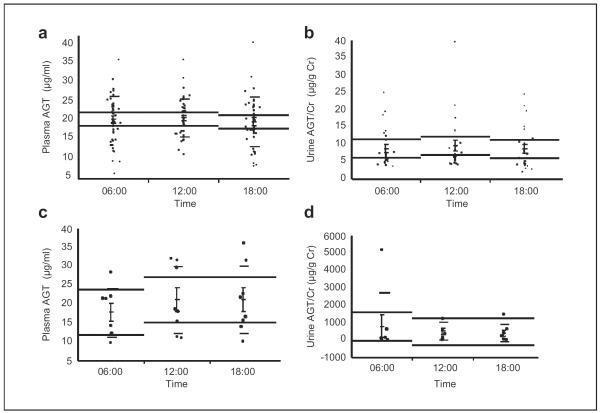
Plasma samples were collected from 43 healthy Japanese volunteers recruited from Kagawa University. Plasma samples were collected at 06:00, 12:00, and 18:00. Plasma samples were taken in a sitting position. Volunteers having a history of diabetes mellitus, hypertension, urine disorder, or metabolic syndrome were excluded. Those who were taking any medication or had previously had any cardiovascular events were also excluded. Plasma AGT in healthy volunteers did not have a circadian rhythm (Figure 1(a), 20.4 ± 0.9 μg/ml at 06:00, 20.7 ± 0.8 μg/ml at 12:00, and 19.8 ± 1.0 μg/ml at 18:00, p = 0.76). The relative ratio was calculated for each volunteer on the basis of measured values of plasma AGT at 06:00. The plasma AGT in healthy volunteers (relative ratio) did not have a circadian rhythm (1.0 ± 0.0 at 06:00, 1.1 ± 0.0, at 12:00, and 1.0 ± 0.0 at 18:00, p = 0.39) (Study I).11
Figure 1.
The circadian rhythm of plasma AGT and urinary AGT in healthy volunteers (a, b) and patients with CKD (c, d). AGT: angiotensinogen; CKD: chronic kidney disease; Cr: creatinine.
Urinary AGT in healthy volunteers
Urine samples were collected from 24 healthy Japanese volunteers recruited from Kagawa University. Urine samples were collected at 06:00, 12:00, and 18:00. The urinary AGT/Cr ratio in healthy volunteers did not have a circadian rhythm (Figure 1(b), 8.7 ± 1.2 μg/g Cr at 06:00, 9.5 ± 1.6 μg/g Cr at 12:00, and 8.6 ± 1.3 μg/g Cr at 18:00, p = 0.48). The relative ratio was calculated for each volunteer on the basis of measured values of urinary AGT/Cr ratio at 06:00. Urinary AGT/Cr ratio in healthy volunteers (relative ratio) did not have a circadian rhythm (1.0 ± 0.0 at 06:00, 1.1 ± 0.1 at 12:00, and 1.0 ± 0.1 at 18:00, p = 0.40) (Study II).10
Plasma and urinary AGT in CKD patients
Participants were eight Japanese CKD patients with continuous proteinuria. They were admitted to the Kagawa University Hospital from June 2011 to October 2011 for the purpose of diagnostic renal biopsy. Plasma and urine samples were collected at 06:00, 12:00, and 18:00. Plasma samples were taken in a sitting position. The final diagnosis of renal biopsy of eight patients was as follows: immunoglobulin A (IgA) nephropathy: three, minimal change nephrotic syndrome: two, membranous nephropathy: one, secondary focal segmental glomerulosclerosis: one, and minor glomerular abnormalities: one. The plasma AGT in CKD patients did not have a circadian rhythm (Figure 1(c), 17.6 ± 2.3 μg/ml at 06:00, 20.9 ± 3.1 μg/ml at 12:00, and 21.0 ± 3.2 μg/ml at 18:00, p = 0.66). The plasma AGT in CKD patients (relative ratio) did not have a circadian rhythm (1.0 ± 0.0 at 06:00, 1.2 ± 0.1 at 12:00, and 1.2 ± 0.1 at 18:00, p = 0.16). The urinary AGT/Cr ratio in CKD patients did not have a circadian rhythm (Figure 1(d), 762 ± 633 μg/g Cr at 06:00, 462 ± 179 μg/g Cr at 12:00, and 359 ± 174 μg/g Cr at 18:00, p = 0.75). Urinary AGT/Cr ratio in CKD patients (relative ratio) did not have a circadian rhythm (1.0 ± 0.0 at 06:00, 6.6 ± 3.5 at 12:00, and 2.9 ± 1.1 at 18:00, p = 0.19) (Study III).12
AGT ELISA recognizes both intact AGT and des-Ang I AGT
It is well known that renin is excreted into urine13 and that inactive renin is cryoactivated.14 Therefore, the multiple freeze-and-thaw cycles of urine samples may activate renin in urine samples and may cleave intact AGT into Ang I and des-Ang I AGT. However, the antibodies used in the AGT ELISA in this study recognize both intact AGT and des-Ang I AGT.7 Therefore, even though the cryoactivated renin in urine samples might have fragmented intact AGT into Ang I and des-Ang I AGT, the measured values of urinary AGT concentrations would not change in this AGT ELISA. In order to further address this possibility, a new ELISA for intact AGT is required.
Urinary AGT: A potential biomarker of an early stage of renal diseases?
Although most of the circulating AGT is produced in and secreted by the liver, the kidneys also produce AGT.1 Intrarenal AGT messenger RNA (mRNA) and protein have been localized to proximal tubular cells,15 indicating that the intratubular Ang II could be derived from locally formed and secreted AGT. Renal injury increases the excretion of biomarkers that are conventionally used. For example, glomerular injury increases urine protein. Ideally, one measures causative markers at a very early stage, rather than markers that increase as a consequence of the disease. In this regard, it is of interest to note that the levels of urinary AGT were increased in patients with type 1 diabetes even before the onset of proteinuria or microalbuminuria.16 Urinary AGT might therefore be a biomarker of an early stage of renal diseases.
AGT levels in patients with CKD are not a non-specific consequence of proteinuria
As AGT is a protein, one may think that the increased urinary excretion of AGT in CKD patients is a non-specific consequence of the increased urinary excretion of plasma protein. However, this possibility seems unlikely based on the following evidence. 1) It has previously been reported that urinary AGT and protein are enhanced in Ang II-infused rats (a model of RAS-dependent hypertension) and that urinary AGT is not augmented although urinary protein is elevated in deoxycorticosterone acetate rats (a model of RAS-independent hypertension).17 2) The urinary AGT/Cr ratios in patients with diabetic nephropathy (796 ± 296 μg/g Cr) or membranous nephropathy (504 ± 298 μg/g Cr) were much higher than the ratio in patients with CKD (273 ± 62 μg/g Cr), and were paralleled by an activated intrarenal RAS in both conditions.18 3) In contrast, the urinary AGT/Cr ratio in patients with minimal change nephrotic syndrome (8.3 ± 3.7 μg/g Cr) was similar to that in control participants (10.8 ± 3.4 μg/g Cr), even though these patients showed severe proteinuria.18 4) It was recently demonstrated that the vast majority of urinary AGT originates from the tubules rather than glomerular filtration by real-time in vivo imaging using a multiphoton microscopy.19 5) More recently, it was reported that AGT mRNA levels in tubules were significantly and negatively correlated with estimated glomerular filtration rate (eGFR) in patients with diabetes.20 These findings, taken together, argue against the possibility that the enhanced urinary AGT levels in patients with CKD are a non-specific consequence of proteinuria, and thus urinary AGT may be a promising biomarker of CKD.21
Acknowledgements
The authors would like to acknowledge A. H. Jan Danser, MSc, PhD (Erasmus Medical Center), for his constructive suggestions in order to polish the manuscript. The authors also thank Ms Yoshiko Fujita and Ms Aya Masuda (Kagawa University) for their excellent technical assistance.
Funding These studies were supported by grants from the Mitsui Life Social Welfare Foundation (Grant-in-Aid Medical Research) and Kagawa University (Strategic Research Fund, Special Encouragement for Research, and Grant-in-Aid from Alumni Association).
Footnotes
Conflict of interest None declared.
References
- 1.Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Kobori H, Nishiyama A, Abe Y, et al. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem Biophys Res Commun. 2004;315:746–750. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobori H, Ozawa Y, Suzaki Y, et al. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai Y, Yao L, Kobori H, et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobori H, Alper AB, Jr, Shenava R, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsurada A, Hagiwara Y, Miyashita K, et al. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–F960. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kala R, Fyhrquist F, Eisalo A. Diurnal variation of plasma angiotensin II in man. Scand J Clin Lab Invest. 1973;31:363–365. doi: 10.3109/00365517309084318. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki T, Ueno M, Uezono K, et al. Differences and similarities among circadian characteristics of plasma renin activity in healthy young women in Japan and the United States. Am J Med. 1980;68:91–96. doi: 10.1016/0002-9343(80)90177-1. [DOI] [PubMed] [Google Scholar]
- 10.Nishijima Y, Kobori H, Sofue T, et al. Important aspects of urine sampling for angiotensinogen measurement: Time and preservation conditions in healthy individuals. Tohoku J Exp Med. 2012;228:333–339. doi: 10.1620/tjem.228.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaifu K, Kobori H, Nishijima Y, et al. The circadian rhythm of plasma angiotensinogen in healthy volunteers. Hypertension. 2012;60:A399. doi: 10.1177/1470320314557584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishijima Y, Kobori H, Mizushige T, et al. Circadian rhythm of plasma and urinary angiotensinogen in patients with chronic kidney disease. Hypertension. 2013;62:A195. doi: 10.1177/1470320314557584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navar LG, Prieto MC, Satou R, et al. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol. 2011;11:180–186. doi: 10.1016/j.coph.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castrop H, Höcherl K, Kurtz A, et al. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 15.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito T, Urushihara M, Kotani Y, et al. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci. 2009;338:478–480. doi: 10.1097/MAJ.0b013e3181b90c25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobori H, Nishiyama A, Harrison-Bernard LM, et al. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobori H, Ohashi N, Katsurada A, et al. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–354. doi: 10.1016/j.jash.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano D, Kobori H, Burford JL, et al. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–1856. doi: 10.1681/ASN.2012010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamiyama M, Urushihara M, Morikawa T, et al. Oxidative stress/angiotensinogen/renin-angiotensin system axis in patients with diabetic nephropathy. Int J Mol Sci. 2013;14:23045–23062. doi: 10.3390/ijms141123045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns KD, Hiremath S. Urinary angiotensinogen as a biomarker of chronic kidney disease: Ready for prime time? Nephrol Dial Transplant. 2012;27:3010–3013. doi: 10.1093/ndt/gfs166. [DOI] [PubMed] [Google Scholar]