Abstract
Numerous crystal structures have been reported for the isolated extracellular region and tyrosine kinase domain of the epidermal growth factor receptor (EGFR) and its relatives, in different states of activation and bound to a variety of inhibitors used in cancer therapy. The next challenge is to put these structures together accurately in functional models of the intact receptor in its membrane environment. The intact EGFR has been studied using electron microscopy, chemical biology methods, biochemically, and computationally. The distinct approaches yield different impressions about the structural mode of communication between extracellular and intracellular regions. They highlight possible differences between ligands, and also underline the need to understand how the receptor interacts with the membrane itself.
Introduction
Growth factor receptor tyrosine kinases (RTKs) such as the epidermal growth factor receptor (EGFR) have been the subjects of intense study for many years [1,2]. There are 58 RTKs in the deduced human proteome, and all play key roles in regulating cellular processes such as proliferation, differentiation, cell survival and metabolism, cell migration, and cell cycle control [3]. Importantly, aberrant activation of RTK signaling by mutation, gene amplification, gene translocation or other mechanisms has been causally linked to cancers, diabetes, inflammation, and other diseases. These observations have prompted the development of many targeted therapies that inhibit RTKs such as EGFR [4•], Kit, VEGFR, or their ligands – typically employing therapeutic antibodies [5] or small molecule tyrosine kinase inhibitors [6].
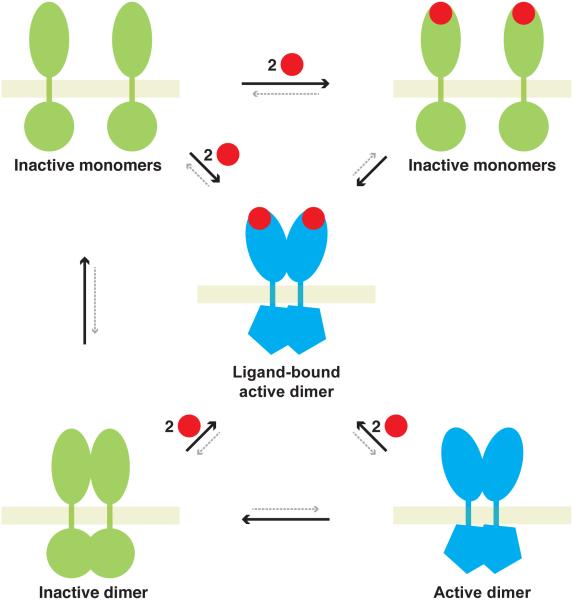
Following the initial discoveries for EGFR [7] and the platelet-derived growth factor receptor (PDGFR) [8] that ligand-stabilized dimers are essential for RTK signaling, structural studies over the past decade or so have guided development of quite sophisticated mechanistic views [1]. Each RTK has a ligand-binding extracellular region (ECR) that is linked by a single transmembrane α-helix to an intracellular tyrosine kinase domain (TKD). Structures of the isolated ECRs and TKDs from several RTKs point to surprising mechanistic diversity across the larger family [1]. Unliganded RTKs exist as an equilibrium mixture of inactive monomers, inactive dimers and active dimers (Figure 1), except for the extreme case of the insulin receptor (IR), which is covalently dimerized [9]. Extracellular ligand can bind to monomers, to inactive dimers, or to active dimers – in each case pushing the equilibria shown in Figure 1 towards the central ligand-bound active dimer. Thus, ligand binding can drive receptor dimerization (Figure 1, upper), or can promote inactive-to-active conformational transitions in dimers (Figure 1, lower). Regardless of pathway, the intracellular TKD of the ligand-stabilized dimer becomes activated either through trans-autophosphorylation or through induced allosteric changes [1,10]. Roles for other parts of the receptor in RTK activation, including the juxtamembrane (JM) and transmembrane (TM) segments, have also become clearer. The key current challenge for the field is to assemble data from many studies of isolated RTK parts into coherent views of how the intact receptors are regulated in their native membranes. We will focus here on recent efforts to do this for the EGFR (or ErbB receptor) family.
Figure 1.
Equilibria involved in activation of RTKs with the EGF receptor as an example. Inactive species are green, active species blue. Inactive monomers are in equilibrium with inactive dimers, the structural nature of which remains unclear. These in turn are in equilibrium with active dimers. Ligand binding activates the receptor by driving dimerization of inactive monomers, by inducing conformational changes in inactive dimers [24], or simply by stabilizing active dimers.
The missing links in intact RTKs: Flexible or rigid?
A central goal in extrapolating to the intact RTKs from studies of isolated soluble domains is to understand how the individual parts of the receptor communicate with one another. The methods that have been used to produce and study the isolated domains inevitably yield the impression that inter-domain linkers are flexible and disordered. For example, extracellular juxtamembrane regions have typically only been observed as C-terminal extensions of the soluble ECR. Similarly, intracellular juxtamembrane regions have been encountered predominantly as N-terminal extensions of TKD constructs, or as short peptides. In each of these contexts, the JM regions are incomplete, and may appear disordered and flexible simply because key structural restraints have been removed. Nonetheless, this possible artifact has strongly influenced thinking about linkages between the extra- and intra-cellular regions [11], and in turn about mechanisms of RTK signaling. Highly flexible linkages between extra- and intra-cellular regions of RTKs are fully consistent with simpler ligand-induced dimerization models for transmembrane signaling by RTKs. However, it is more difficult to understand how subtle allosteric communication across the membrane could be achieved if the linkages are truly flexible. For example, since flexible linkage implies structural independence of the extra- and intra-cellular regions, it is difficult to envision how a transition from inactive to active dimer in Figure 1 could be controlled precisely by ligand without more rigid (or restricted) connections.
Recent experimental studies with intact – or nearly intact – EGFR differ in the impressions they provide about how flexibly or rigidly the extra- and intra- cellular regions are linked. Springer’s laboratory used cysteine crosslinking and mutagenesis approaches to investigate this issue for EGFR expressed in Ba/F3 cells [12]. They were unable to identify any specific JM or TM region interfaces that were required for EGFR signaling, leading them to argue that the linkage across the membrane is too flexible to transmit a specific orientation between the extracellular and intracellular regions. Consistent with this, negative-stain electron microscopy studies of (nearly) full-length EGFR in dodecylmaltoside micelles showed that a given extracellular dimer can be linked to several different arrangements of the intracellular kinase domain [13••,14]. Similarly, dimers driven by inhibitor binding to the intracellular TKD could couple to multiple different ECR conformations [13••]. Biochemical studies are also consistent with such structural independence of the extra- and intra-cellular regions [15,16••]. Contrasting with these observations, however, Schepartz and colleagues have reported that different precise conformations within the EGFR intracellular region can be induced by distinct activating ligands [17••]. They used a method called bipartite tetracysteine display that reports on formation of a chemically detectable tetracysteine motif when two cysteine pairs come together at the dimer interface. EGF activation of the receptor led to formation of a tetracysteine motif that requires the intracellular JM helix [18] shown in Figure 2A to form an antiparallel coiled-coil dimer (Figure 2B/C) as proposed by Kuriyan and colleagues [19,20••]. Surprisingly, transforming growth factor-α (TGFα), which also activates EGFR, did not bring these two cysteine pairs together in the same way – arguing that TGFα does not induce formation of the same intracellular antiparallel coiled coil. Instead, activation of EGFR with TGFα (but not EGF) stabilized an alternative tetracysteine motif, consistent with a different intracellular JM structure. Evidence for ‘inside-out’ signaling in EGFR has also been reported, where alterations in the intracellular JM region directly influence allosteric EGF binding to the ECR of the intact receptor analyzed in CHO cells [21-23]. The contradictory views of flexibility versus rigidity in linkages between the domains leave the path to understanding the intact receptor unclear, although it seems reasonable to doubt that the inactive dimers known to form in the absence of ligand [24-26] could be regulated by extracellular ligand if all linkages are always highly flexible.
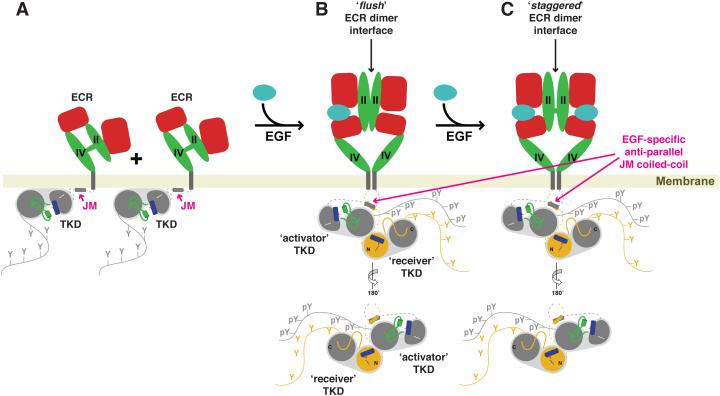
Figure 2.
More detailed view of EGF-induced activation of EGFR, as described in text. In the absence of ligand (A), the ECR adopts a tethered conformation, with an autoinhibitory tether interaction between domains II and IV. The TKD and JM regions lie against the membrane, making what are believed to be additional autoinhibitory interactions. Domains I and III of the ECR are colored red, and domains II and IV are green. The JM helix is shown as a short cylinder and labeled in magenta. The N-and C-lobes of the kinase are also labeled, and both helix αC (blue) and the short helix in the activation loop (green) that interacts with αC to inhibit the TKD [50] are shown. The C-tail is also depicted as a curve bearing 5 tyrosines. As described in the text, binding of a single ligand (B) induces formation of a singly-liganded dimer with a ‘flush’ (presumed asymmetric) ECR dimer interface. The JM region forms an anti-parallel helix, as labeled in magenta, and the TKDs form an asymmetric dimer in which the activator (grey) allosterically activates the receiver (shown with an amber N-lobe). It is not clear how the extra- and intracellular asymmetry is structurally related, if at all. Finally, a second ligand binds to yield a more symmetric dimer with the ‘staggered’ ECR interface (C) described in the text.
Does the membrane hold the key?
All of the studies that support direct conformational communication between the extra- and intra-cellular regions of EGFR were performed in cells [17••,21,22]. By contrast, most of those that explicitly suggest otherwise were performed in detergent micelles [13••-15] – where the potentially important influences of specific membrane lipids (or membrane geometry) are absent. Studies of intact EGFR in liposomes with defined lipid compositions [27] have shown that the ganglioside GM3 inhibits ligand-independent activation (and dimerization) of the receptor, apparently through interactions with a site in its extracellular JM region. McLaughlin and colleagues [28,29] also proposed a model in which interaction of the intracellular JM region (and TKD) with anionic phospholipids in the inner leaflet of the plasma membrane (notably PtdIns(4,5)P2) exerts an inhibitory effect that must be overcome in order for EGFR to signal. Association of the JM and TM regions with specific membrane lipids is likely to define specific structures in the linkages between extra- and intra-cellular regions of RTK that are more well-defined structurally (and potentially rigid) than is typically appreciated.
Recent studies have begun to shed some structural light on how membrane interactions with the intracellular JM region of EGFR might influence the signaling mechanism. Endres et al. [20••] found that simply tethering the complete intracellular region of EGFR to the inner leaflet of the plasma membrane maintains the TKD in a largely monomeric state and inhibits its kinase activity. Parallel computational studies [30•] suggest that this results from the previously proposed [29] inhibitory interaction of the JM and TKD regions of EGFR with the negatively-charged membrane surface. The data of Endres et al. [20••] further indicated that TM-mediated dimerization reverses this inhibitory effect. Moreover, NMR studies of a 60-residue peptide containing the TM and part of the JM region solubilized in lipid bicelles led them to conclude that specific TM dimerization through an N-terminal GxxxG motif stabilizes formation of an antiparallel coiled-coil between the two JM fragments in the dimer – the same JM coiled coil shown in Figure 2B/C that was investigated in the bipartite tetracysteine display studies of intact EGF-bound EGFR described above [17••,19]. Independent solid-state NMR studies of a similar TM-JM peptide from the EGFR relative ErbB2 in vesicles containing acidic phospholipids [31•] further suggested that an activating mutation in the TM domain leads to release of the JM region from the anionic membrane surface. Collectively, these data suggest that ligand-induced dimerization of the receptor (or reorientation of receptors within a dimer) may engage the TM domain in a specific dimer that promotes both the formation of activating interactions in the JM region and disruption of inhibitory interactions between the JM region (and possibly TKD) and the membrane surface.
Less is known about the specifics of how lipids in the extracellular leaflet of the membrane interact with and influence the ECR. Several studies have applied Förster resonance energy transfer (FRET) approaches to investigate the conformation of the ECR of intact EGFR in membranes – and how it changes upon ligand binding – as well as the relationship between the plane of the membrane surface and the long axis of the ECR [32-34]. Modeling studies have also suggested that the ECR of the receptor may associate with the membrane surface [30•,32], providing opportunities for specific lipid interactions that may influence the properties of the receptor. There has been some advance in understanding the effects of the ganglioside GM3 on EGFR activity [27,35], but structural details remain sparse and other direct effects of membrane lipids on the ECR and extracellular JM region remain to be explored.
Negative cooperativity
A key characteristic of ligand binding at the cell surface to EGFR [36], IR [37], and other receptors [38] is negative cooperativity – which is lost when soluble forms of the ECR from human EGFR [39] or IR [40] are studied in isolation. Several studies have shown that intracellular and/or transmembrane regions are required for this negative cooperativity to be manifest [21,22,40,41], implying that these parts of the receptor contribute to breaking the symmetry of the dimer – as required for the two sites to have distinct binding properties [42]. Such propagation of dimer asymmetry across the membrane would surely require defined structures in the regions that connect extra- and intracellular regions, and is difficult to reconcile with highly flexible JM linkers. It is important to note, however, that the Drosophila EGFR does retain negative cooperativity in binding to its ligands even when the ECR is studied in isolation [43] – and this has allowed the structural basis of negative cooperativity to be defined (Figure 2). In brief, binding of one ligand stabilizes a singly-liganded asymmetric dimer in which the unoccupied ligand-binding site is compromised [43]. The binding affinity of the second ligand is thus reduced, constituting a half-of-the-sites mode of negative cooperativity [44]. Leahy’s group has provided important evidence consistent with a similar mechanism in the cases of human EGFR and ErbB4 [16••]. They generated receptor variants with a debilitating mutation either in the ligand-binding site or the TKD. Neither variant could signal when introduced into cells on its own, but coexpression of the two restored signaling ability, arguing that a singly-liganded dimer with only one active TKD is capable of transmembrane signaling. By comparing human ErbB receptor ECR dimer crystal structures with different bound ligands, Leahy and colleagues went on to identify two types of dimer interface [16••], a ‘flush’ interface that resembles the asymmetric (singly-liganded) dimer seen for the Drosophila EGFR [43] and a ‘staggered’ interface seen in the ECRs from EGFR (with bound EGF [12]) and ErbB4 (with bound neuregulin1β [16••]). Consistent with results from the Drosophila receptor [43], with models based on detailed fitting of cell-surface binding data [36], and with molecular modeling studies [30•], these observations suggest that the ‘flush’ interface drives the most stable dimers, which are singly liganded (Figure 2B). Binding of the second ligand is weaker, and also forces the dimer interface into the less stable ‘staggered’ conformation (Figure 2C). Taken together, these findings suggest both a structural basis for negative cooperativity and a possible structural distinction between singly- and doubly- liganded ErbB receptor dimers, which may signal differently to allow the nature of the signal to vary with ligand concentration [45] and possibly to provide ligand specificity in ErbB signaling [46].
It should be noted that conclusions differ as to whether the asymmetry in the intracellular regions (where the TKD is allosterically activated) must match that in the extracellular region or ErbB receptor dimers [16••,47•]. This will be an important issue to resolve – as it lies at the heart of understanding the nature of the linkage been extra- and intracellular regions. Recent computational studies have also suggested that direct association of membrane lipids with the ECR [32] and/or the receptor-bound ligands [48] may contribute to imposing the asymmetry that manifests itself as negative cooperativity. These calculations underline further the need to understand interactions between the receptor and the membrane.
A model for EGFR activation
The model shown in Figure 2 summarizes key proposed steps in activation of human EGFR. In the absence of ligand, the ECR exists in a tethered conformation with the domain II ‘dimerization arm’ engaged in an intramolecular interaction with domain IV that occludes the dimer interface [49]. The TKDs and the N-terminal portions of each intracellular JM region are thought to be engaged in autoinhibitory interactions with the membrane surface [20••,28-30•]. Following ligand binding, it appears likely that a singly-liganded dimer of the ECR can form [16••,36], using the ‘flush’ dimer interface observed in a crystal structure of the asymmetric singly-liganded ECR from Drosophila EGFR [43]. As a result of ligand-induced ECR dimerization, the TKDs also dimerize, forming the activating asymmetric dimer identified by Kuriyan and colleagues [50]. The intracellular JM region dissociates from the membrane surface [20••,31•], and contributes directly to receptor dimerization both by ‘cradling’ the C-lobe of its neighbor [18,19] and through formation of the antiparallel coiled coil seen in NMR studies [20••] and inferred using bipartite tetracysteine display [17••]. As discussed in this article, it remains unclear whether the asymmetry in the extracellular region of this singly-liganded dimer has a fixed relationship to that in the intracellular region [16••,47•]. This is a central issue for understanding how EGFR functions. Most studies suggest a flexible linkage [12-15], but key questions still remain. The second ligand binding event, leading to the doubly-liganded dimer, is thought to induce a transition from a ‘flush’ to a ‘staggered’ dimerization interface that is more symmetric [16••,30•], and may actually be weaker – possibly allowing subunit exchange. We do not include the inactive dimer species from Figure 1 in this more detailed model, since its nature is unclear. Modeling studies have argued that a domain II-mediated dimer can exist in the absence of ligand [30•,32], but there is no experimental evidence that this type of unliganded ECR dimer is independently stable for the human receptor. It has only been observed for the Drosophila receptor [43]. Models of specific unliganded dimeric structures have led to the suggestion that the ECR serves to inhibit dimerization driven by the JM and TKD regions in the absence of ligand [20••,30•] – and that ligand binding serves to relieve this inhibition. This hypothesis seems unlikely to be correct, since the ECR appears to form no specific dimer in the absence of ligand [12,13••], yet dimerizes quite strongly once ligand is bound [39]. It therefore seems more likely that the ECR provides a substantial proportion of the driving force for ligand-induced receptor dimerization (rather than playing a passive role). Indeed, if ligand binding activates EGFR simply by removing an ECR-mediated impedance to TKD dimerization, it would be difficult to understand why single amino acid substitutions in the dimerization interface block EGF-induced signaling [51,52], and conversely why mutations that destabilize the tethered configuration are not activating [53,54].
Conclusions
Our mechanistic understanding of EGFR and its relatives has advanced dramatically in recent years, and the past year or two has seen substantial progress in putting the results of studies with isolated domains together into initial views of how the intact receptor works. New insights into the origin of allosteric regulation of EGFR have been gained through a combination of innovative structural, biochemical, cellular, and computational studies. A self-consistent picture is beginning to emerge. Two key issues remain unclear, however, and represent the current frontiers in studies of EGFR. The first – for which we describe progress in this review – centers on the influence of specific interactions of the receptor with membrane lipids, which seem likely to define the structural ‘connections’ between extra- and intra-cellular regions of the receptor. The second centers on the role of the carboxy-terminal ~230 amino acids, which is believed to play a regulatory role for which little detail has so far been defined [55•].
Highlights.
Several studies suggest flexible linkage between extra- and intracellular regions
Others imply more rigid connections, required for allosteric regulation of dimers
Interactions with membrane lipids play important roles in EGFR regulation
Cellular studies suggest half-of-the-sites negative cooperativity for human EGFR
Acknowledgements
Work in the Lemmon laboratory on EGFR has been supported by grant number R01-CA079992 from the NIH. NJB was supported by an NIH Training Grant in Structural Biology (T32-GM008275) and a Predoctoral Fellowship from the Great Rivers Affiliate of the American Heart Association (10PRE4140108). DMF is supported by a Postdoctoral Fellowship from the NIH (F32-GM109688).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 3.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 4•.Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25:282–303. doi: 10.1016/j.ccr.2014.02.025. An excellent recent perspective on the ErbB receptors, their involvement in cancers, current therapeutic approaches for their inhibition in cancer patients, and clinical challenges. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–1198. doi: 10.1126/science.1241145. [DOI] [PubMed] [Google Scholar]
- 6.Jänne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8:709–723. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- 7.Schlessinger J. Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci. 1988;13:443–447. doi: 10.1016/0968-0004(88)90219-8. [DOI] [PubMed] [Google Scholar]
- 8.Williams LT. Signal transduction by the platelet-derived growth factor receptor. Science. 1989;243:1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- 9.Hubbard SR. The insulin receptor: both a prototypical and atypical receptor tyrosine kinase. Cold Spring Harb Perspect Biol. 2013;5:a008946. doi: 10.1101/cshperspect.a008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jura N, Zhang X, Endres NF, Seeliger MA, Schindler T, Kuriyan J. Catalytic control in the EGF receptor and its connection to general kinase regulatory mechanisms. Mol Cell Biol. 2011;42:9–22. doi: 10.1016/j.molcel.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bessman NJ, Lemmon MA. Finding the missing links in EGFR. Nat Struct Mol Biol. 2012;19:1–3. doi: 10.1038/nsmb.2221. [DOI] [PubMed] [Google Scholar]
- 12.Lu C, Mi LZ, Grey MJ, Zhu J, Graef E, Yokoyama S, Springer TA. Structural evidence for loose linkage between ligand binding and kinase activation in the epidermal growth factor receptor. Mol Cell Biol. 2010;30:5432–5443. doi: 10.1128/MCB.00742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Lu C, Mi LZ, Schurpf T, Walz T, Springer TA. Mechanisms for kinase-mediated dimerization of the epidermal growth factor receptor. J Biol Chem. 2012;287:38244–38253. doi: 10.1074/jbc.M112.414391. An important study showing how certain EGFR-targeted tyrosine kinase inhibitors (TKIs) stabilize dimers of the receptor in cells and detergent micelles, but fail to promote formation of a specific extracellular dimer. Dimerization of the transmembrane and juxtamembrane regions was revealed using cross-linking methods. Electron microscopy showed that the nature of the intracellular dimer was similar whether induced by kinase inhibitor or EGF, whereas the extracellular dimer was quite different - being undefined when driven by TKI. These studies argue that the EGFR extra- and intracellular regions are loosely coupled in the intact receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mi LZ, Lu C, Li Z, Nishida N, Walz T, Springer TA. Simultaneous visualization of the extracellular and cytoplasmic domains of the epidermal growth factor receptor. Nat Struct Mol Biol. 2011;18:984–989. doi: 10.1038/nsmb.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Longo PA, Tarrant MK, Kim K, Head S, Leahy DJ, Cole PA. Mechanistic insights into the activation of oncogenic forms of EGF receptor. Nat Struct Mol Biol. 2011;18:1388–1893. doi: 10.1038/nsmb.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Liu P, Cleveland TE, 4th, Bouyain S, Byrne PO, Longo PA, Leahy DJ. A single ligand is sufficient to activate EGFR dimers. Proc Natl Acad Sci U S A. 2012;109:10861–10866. doi: 10.1073/pnas.1201114109. Comparison of existing crystal structures of the liganded EGFR extracellular region with the structure of the neuregulin-bound ErbB4 extracellular region (presented here) suggest two types of dimer interface - 'flush' and 'staggered', consistent with the model for negative cooperativity proposed in reference 43 for the Drosophila receptor. These observations led the authors to investigate (using mutated receptors) whether singly-liganded EGFR (or ErbB4) dimers could signal. The results suggest that the singly liganded receptors can signal, suggesting that the molecular basis for negative cooperativity is similar in human and invertebrate EGFRs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Scheck RA, Lowder MA, Appelbaum JS, Schepartz A. Bipartite tetracysteine display reveals allosteric control of ligand-specific EGFR activation. ACS Chem Biol. 2012;7:1367–1376. doi: 10.1021/cb300216f. An innovative chemical biology approach is used to probe specific structures in the intracellular juxtamembrane region of the ligand-activated EGFR in cells. Using a reagent (ReAsH) that gives a fluorescence signal only for cells in which a specific tetracysteine binding site is reconstituted, the authors provide evidence for EGF-induced formation of an antiparallel coiled-coil between juxtamembrane helices in the dimer, originally proposed in reference 19. Importantly, studies with TGFα suggested that this alternative EGFR-activating ligand induces a different intracellular juxtamembrane conformation in the activated receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Red Brewer M, Choi SH, Alvarado D, Moravcevic K, Pozzi A, Lemmon MA, Carpenter G. The juxtamembrane region of the EGF receptor functions as an activation domain. Mol Cell. 2009;34:641–651. doi: 10.1016/j.molcel.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, Wemmer DE, Zhang X, Kuriyan J. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009;137:1293–1307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Endres NF, Das R, Smith AW, Arkhipov A, Kovacs E, Huang Y, Pelton JG, Shan Y, Shaw DE, Wemmer DE, et al. Conformational coupling across the plasma membrane in activation of the EGF receptor. Cell. 2013;152:543–556. doi: 10.1016/j.cell.2012.12.032. Careful analyses of autophosphorylation of different EGFR fragments and chimera provide new insight into EGFR signaling. The results suggest that the intracellular module of the receptor is inhibited by binding of both the tyrosine kinase domain and intracellular juxtamembrane region to the membrane surface, and that these interactions are reversed by specific dimerization of the transmembrane domain. NMR structural analysis of a peptide containing the transmembrane and part of the juxtamembrane region support this hypothesis. The authors also suggest that the primary function of ligand binding to the extracellular region is to relieve an impediment to dimerization, which is critically discussed in the present review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald-Obermann JL, Pike LJ. The intracellular juxtamembrane domain of the EGF receptor is responsible for the allosteric regulation of EGF binding. J Biol Chem. 2009;284:13570–13576. doi: 10.1074/jbc.M109.001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adak S, Yang KS, Macdonald-Obermann J, Pike LJ. The membrane-proximal intracellular domain of the epidermal growth factor receptor underlies negative cooperativity in ligand binding. J Biol Chem. 2011;286:45146–45155. doi: 10.1074/jbc.M111.274175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoyab M, De Larco JE, Todaro GJ. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979;279:387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- 24.Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464:783–787. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- 25.Valley CC, Lidke KA, Lidke DS. The spatiotemporal organization of ErbB receptors: insights from microscopy. Cold Spring Harb Perspect Biol. 2014;6:a020735. doi: 10.1101/cshperspect.a020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arndt-Jovin DJ, Botelho MG, Jovin TM. Structure-function relationships of ErbB RTKs in the plasma membrane of living cells. Cold Spring Harb Perspect Biol. 2014;6:a008961. doi: 10.1101/cshperspect.a008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coskun Ü , Grzybek M, Drechsel D, Simons K. Regulation of human EGF receptor by lipids. Proc Natl Acad Sci U S A. 2011;108:9044–9048. doi: 10.1073/pnas.1105666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin S, Smith SO, Hayman MJ, Murray D. An electrostatic engine model for autoinhibition and activation of the epidermal growth factor receptor (EGFR/ErbB) family. J Gen Physiol. 2005;126:41–53. doi: 10.1085/jgp.200509274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sengupta P, Bosis E, Nachliel E, Gutman M, Smith SO, Mihályné G, Zaitseva I, McLaughlin S. EGFR juxtamembrane domain, membranes, and calmodulin: kinetics of their interaction. Biophys J. 2009;96:4887–4895. doi: 10.1016/j.bpj.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Arkhipov A, Shan Y, Das R, Endres NF, Eastwood MP, Wemmer DE, Kuriyan J, Shaw DE. Architecture and membrane interactions of the EGF receptor. Cell. 2013;152:557–569. doi: 10.1016/j.cell.2012.12.030. An impressive computational analysis, with very long timescale molcular dynamic simulations of EGFR, which accompanied reference 20••, with similar conclusions. One key outcome of the simulations is that specific stable unliganded extracellular dimers form that block constitutive dimerization of the receptor. As discussed in the present review, some experimental data contradict this conclusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Matsushita C, Tamagaki H, Miyazawa Y, Aimoto S, Smith SO, Sato T. Transmembrane helix orientation influences membrane binding of the intracellular juxtamembrane domain in Neu receptor peptides. Proc Natl Acad Sci U S A. 2013;29:1646–1651. doi: 10.1073/pnas.1215207110. Solid-state NMR and fluorescence studies, using a peptide containing the transmembrane and membrane-proximal juxtamembrane regions of ErbB2, support a model in which activating dimerization of the transmembrane region disrupts (autoinhibitory) interactions of the juxtamembrane portion with phosphoinositides in the membrane. Together with references 20•• and 27-30•, this study emphasizes the importance of understanding membrane interactions for a complete model of EGFR function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tynan CJ, Roberts SK, Rolfe DJ, Clarke DT, Loeffler HH, Kästner J, Winn MD, Parker PJ, Martin-Fernandez ML. Human epidermal growth factor receptor (EGFR) aligned on the plasma membrane adopts key features of Drosophila EGFR asymmetry. Mol Cell Biol. 2011;31:2241–2252. doi: 10.1128/MCB.01431-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb SE, Roberts SK, Needham SR, Tynan CJ, Rolfe DJ, Winn MD, Clarke DT, Barraclough R, Martin-Fernandez ML. Single-molecule imaging and fluorescence lifetime imaging microscopy show different structures for high- and low-affinity epidermal growth factor receptors in A431 cells. Biophys J. 2008;94:803–819. doi: 10.1529/biophysj.107.112623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziomkiewicz I, Loman A, Klement R, Fritsch C, Klymchenko AS, Bunt G, Jovin TM, Arndt-Jovin DJ. Dynamic conformational transitions of the EGF receptor in living mammalian cells determined by FRET and fluorescence lifetime imaging microscopy. Cytometry A. 2013;83:794–805. doi: 10.1002/cyto.a.22311. [DOI] [PubMed] [Google Scholar]
- 35.Yoon SJ, Nakayama K, Hikita T, Handa K, Hakomori SI. Epidermal growth factor receptor tyrosine kinase is modulated by GM3 interaction with N-linked GlcNAc termini of the receptor. Proc Natl Acad Sci U S A. 2006;103:18987–18891. doi: 10.1073/pnas.0609281103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacDonald JL, Pike LJ. Heterogeneity in EGF-binding affinities arises from negative cooperativity in an aggregating system. Proc Natl Acad Sci U S A. 2008;105:112–117. doi: 10.1073/pnas.0707080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Meyts P, Roth J, Neville DM, Jr., Gavin JR, 3rd, Lesniak MA. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973;55:154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]
- 38.De Meyts P. The insulin receptor: a prototype for dimeric, allosteric membrane receptors? Trends Biochem Sci. 2008;33:376–384. doi: 10.1016/j.tibs.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Lemmon MA, Bu Z, Ladbury JE, Zhou M, Pinchasi D, Lax I, Engelman DM, Schlessinger J. Two EGF molecules contribute additively to stabilization of the EGFR dimer. EMBO J. 1997;16:281–294. doi: 10.1093/emboj/16.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Meyts P, Whittaker J. Structural biology of insulin and IGF1 receptors: Implications for drug design. Nat Rev Drug Discov. 2002;1:769–783. doi: 10.1038/nrd917. [DOI] [PubMed] [Google Scholar]
- 41.Ozcan F, Klein P, Lemmon MA, Lax I, Schlessinger J. On the nature of low- and high- affinity EGF receptors on living cells. Proc Natl Acad Sci U S A. 2006;103:5735–5740. doi: 10.1073/pnas.0601469103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koshland DE., Jr. The structural basis of negative cooperativity: receptors and enzymes. Curr Opin Struct Biol. 1996;6:757–761. doi: 10.1016/s0959-440x(96)80004-2. [DOI] [PubMed] [Google Scholar]
- 43.Alvarado D, Klein DE, Lemmon MA. Structural basis for negative cooperativity in growth factor binding to an EGF receptor. Cell. 2010;142:568–579. doi: 10.1016/j.cell.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemmon MA. Ligand-induced ErbB receptor dimerization. Exp Cell Res. 2009;315:638–648. doi: 10.1016/j.yexcr.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krall JA, Beyer EM, MacBeath G. High- and low-affinity epidermal growth factor receptor-ligand interactions activate distinct signaling pathways. PLoS One. 2011;6:e15945. doi: 10.1371/journal.pone.0015945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson KJ, Gilmore JL, Foley J, Lemmon MA, Riese DJ., 2nd Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol Ther. 2009;122:1–8. doi: 10.1016/j.pharmthera.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Macdonald-Obermann JL, Piwnica-Worms D, Pike LJ. Mechanics of EGF receptor/ErbB2 kinase activation revealed by luciferase fragment complementation imaging. Proc Natl Acad Sci U S A. 2012;109:137–142. doi: 10.1073/pnas.1111316109. Using an innovative approach in which luciferase is reconstituted from two fragments, the authors probe ligand-induced formation of an EGFR/ErbB2 heterodimer. Their results suggest that the symmetry of the extracellular region must match that in the intracellular region in this heterodimer - with EGFR both binding ligand extracellularly and functioning as the 'receiver' kinase (see Figure 2) intracellularly. This finding contrasts with results reported in reference 16•• for homodimers. Further studies will be required to determine whether heterodimers and homodimers differ in the structural relationships between extra- and intra-cellular regions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arkhipov A, Shan Y, Kim ET, Shaw DE. Membrane interaction of bound ligands contributes to the negative binding cooperativity of the EGF receptor. PLoS Comput Biol. 2014;10:e1003742. doi: 10.1371/journal.pcbi.1003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell. 2003;11:507–517. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Dawson JP, Berger MB, Lin D, Schlessinger J, Lemmon MA, Ferguson KM. EGF receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Mol Cell Biol. 2005;25:7734–7742. doi: 10.1128/MCB.25.17.7734-7742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 53.Mattoon D, Klein P, Lemmon MA, Lax I, Schlessinger J. The tethered configuration of the EGF receptor extracellular domain exerts only a limited control of receptor function. Proc Natl Acad Sci USA. 2004;101:923–928. doi: 10.1073/pnas.0307286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker F, Orchard SG, Jorissen RN, Hall NE, Zhang HH, Hoyne PA, Adams TE, Johns TG, Ward C, Garrett TPJ, et al. CR1/CR2 interactions modulate the functions of the cell surface epidermal growth factor receptor. J Biol Chem. 2004;279:22387–22398. doi: 10.1074/jbc.M401244200. [DOI] [PubMed] [Google Scholar]
- 55•.Lemmon MA, Schlessinger J, Ferguson KM. The EGFR family: not so prototypical receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2014;6:a020768. doi: 10.1101/cshperspect.a020768. A more detailed review on key current issues for understanding EGFR signaling mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]