Abstract
Antimicrobial preservatives (APs) are included in liquid multi-dose protein formulations to combat the growth of microbes and bacteria. These compounds have been shown to cause protein aggregation, which leads to serious immunogenic and toxic side-effects in patients. Our earlier work on a model protein cytochrome c (Cyt c) demonstrated that APs cause protein aggregation in a specific manner. The aim of this study is to validate the conclusions obtained from our model protein studies on a pharmaceutical protein. Interferon α-2a (IFNA2) is available as a therapeutic treatment for numerous immune-compromised disorders including leukemia and hepatitis c, and APs have been used in its multi-dose formulation. Similar to Cyt c, APs induced IFNA2 aggregation, demonstrated by the loss of soluble monomer and increase in solution turbidity. The extent of IFNA2 aggregation increased with the increase in AP concentration. IFNA2 aggregation also depended on the nature of AP, and followed the order m-cresol > phenol > benzyl alcohol > phenoxyethanol. This specific order exactly matched with that observed for the model protein Cyt c. These and previously published results on antibodies and other recombinant proteins suggest that the general mechanism by which APs induce protein aggregation may be independent of the protein.
Keywords: Interferon alpha-2a, preservatives, aggregation, formulation, benzyl alcohol, m-cresol
1. Introduction
Multi-dose protein formulations comprise approximately one third of protein-based pharmaceuticals available on the global market (Meyer et al. 2007). These formulations are beneficial in terms of economics and patient compliance, and require the inclusion of at least one antimicrobial preservative (AP) in order to inhibit the growth of microbes and bacteria during administration (Akers et al. 2002, Meyer et al. 2007).
It has become increasingly important to study APs in protein pharmaceuticals because these necessary compounds have been linked to protein aggregation in the liquid state. One of the earliest reports demonstrated that the addition of various aromatic compounds induced the aggregation of recombinant human growth hormone (Maa and Hsu 1996). Numerous studies have shown the ability of APs to cause destabilization and aggregation of many proteins (Gupta and Kaisheva 2003, Tobler et al. 2004, Zhang et al. 2004, Roy et al. 2005). Aggregates in these formulations cause a decrease in the effective concentration of the delivered drug as well as result in immunogenic and toxic responses in patients (Ratner et al. 1990, Bucciantini et al. 2002, Hermeling et al. 2006, Rosenberg 2006, Fradkin et al. 2009, Sauerborn et al. 2010, Vazquez-Rey and Lang 2011). In order to minimize AP-induced protein aggregation, an understanding of the interactions between APs and proteins is critical.
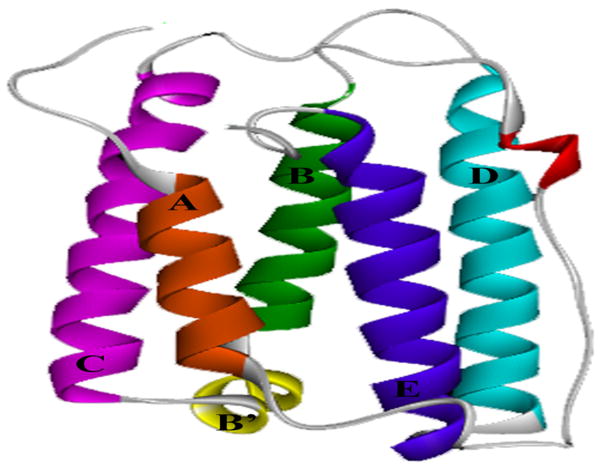
Previous work from our laboratory has demonstrated that a specific order exists in which individual APs induce the aggregation of a model protein cytochrome c (Cyt c) (Hutchings et al. 2013). The aim of the present study is to examine the effects of various APs on a pharmaceutically relevant protein and validate the results obtained with the model protein. We chose interferon α-2a (IFNA2) (Figure 1) for this purpose, which is available as a therapeutic treatment for numerous immune-compromised disorders including leukemia and hepatitis C. IFNA2 has been shown to aggregate in its formulation state (Braun and Alsenz 1997, Braun et al. 1997, Hochuli 1997, Ryff 1997). APs have been used in IFNA2 multi-dose as well as single-dose liquid formulations. However, it is unknown whether APs cause IFNA2 aggregation. Here, we demonstrate that IFNA2 aggregation is enhanced in the presence of APs and that the extent and order of these effects match exactly to what we observed earlier in the case of the model protein Cyt c (Hutchings et al. 2013).
Figure 1.
Molecular structure of interferon α-2a (IFNA2; 1ITF.pdb). The protein is α-helical in nature. In the structure, helices are colored according to their organization in the three-dimensional structure: helix A, residues 11–21 (orange); helix B, residues 52–68 (green); helix B′, residues 70–75 (yellow); helix C, residues 78–100 (purple); helix D, residues 110–132 (cyan); helix E, residues 137–157 (blue). Residues 22–51 comprise the AB-loop, with residues 40–43 usually found in a 310 helix (red).
2. Materials and Methods
2.1 Materials
Synthetic cDNA corresponding to IFNA2 was obtained from Operon (Huntsville, Alabama), and was cloned into the pET-SUMO expression vector (a generous gift from Christopher Lima, Sloan-Kettering Institute). Protein was expressed in Escherichia coli BL21(DE3) cells, and the soluble protein was purified using a Nickel Sepharose 6 Fast Flow column (GE Healthcare Life Sciences, Pittsburgh, Pennsylvania). The SUMO tag was cleaved using the Ulp1 protease, leaving no additional amino acids. The final protein sequence is identical to that of the pharmaceutical protein. Detailed expression and purification protocols, and biophysical characterization of the protein were described in our earlier publication (Bis et al. 2014).
Preservatives were obtained in their highest available purity (Table 1). All experiments were performed in the buffer conditions used for IFNA2 formulations (0.01 M ammonium acetate, 0.12 M sodium chloride, pH 5.0), unless otherwise noted.
Table 1.
Antimicrobial preservatives (AP) used in this study.
| AP and Typical Use Concentration | Molecular Structure | Molecular weight (Da) | Source | Purity |
|---|---|---|---|---|
| Benzyl alcohol (BA) 1% vol/vol |
|
108.1 | Merck | 97% |
| m-Cresol (CR) 0.3% vol/vol |

|
108.1 | Sigma | 99% |
| Phenol (PH) 0.5% vol/vol |
|
94.1 | Sigma | 99.5% |
| 2-phenoxyethanol (PE) 1% vol/vol |
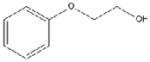
|
138.2 | Fluka | 99.5% |
2.2 Preservative Efficacy Test
To confirm the antimicrobial activity of APs, a simplified preservative efficacy test was performed (Sutton and Porter 2002, Hutchings et al. 2013). A primary culture of Escherichia coli BL21(DE3) cells was incubated overnight at 37°C in a shaker. Aliquots of 0.5 ml were transferred into five 50 ml culture flasks containing either no preservative (control), or one of the four APs (Table 1). Cultures were incubated at 37°C with shaking for six hours, and optical density at 600 nm was used to measure the cell count.
2.3 Size Exclusion Chromatography
To monitor the effects of various APs on protein aggregation, IFNA2 (10 μM in 0.01 M ammonium acetate, 0.12 M sodium chloride, pH 5) was incubated at 50°C in borosilicate vials (Kimble Chase Life Science, #60910 L-12, Vineland, New Jersey) and samples were taken at desired intervals. Samples were centrifuged to remove insoluble aggregates prior to HPLC injection. Concentration of monomer was estimated by injecting 70 μL onto a TSKgel 5 μM G3000SWxl column (Tosoh Bioscience LLC, San Francisco, California) on an Agilent 1100 HPLC (Santa Clara, California) at room temperature. The mobile phase used was 0.01 M ammonium acetate, 0.12 M sodium chloride, pH 5, at a flow rate of 1 mL min−1. Absorbance at 280 nm was used to determine the monomer content.
2.4 Isothermal Incubation Experiments
IFNA2 (10 μM) was incubated at the desired temperature with various APs, and the changes in optical density at 350 nm were measured as a function of the incubation time (Eberlein et al. 1994, Eckhardt et al. 1994). Buffer and protein do not absorb at this wavelength. The aggregation kinetics were monitored until the signal reached a plateau. At longer incubation times, the aggregates started to settle down to the bottom of the cuvette, resulting in decreased optical density. At that point, the experiment was stopped.
2.5 Thermal Scanning Method
The aggregation temperature (TmAgg) of the protein was measured on an UV-Visible spectrophotometer (Agilent Technologies, Santa Clara, California). The temperature was increased at a rate of 1°C/min followed by 90 sec equilibration, and changes in the optical density at 450 nm were recorded (Charman et al. 1993, Kurganov 2002). TmAgg was determined as the temperature at half the maximum optical density. For these experiments, 10 μm IFNA2 in formulation buffer was used with varying AP concentration.
3. Results
3.1 APs Cause IFNA2 Aggregation
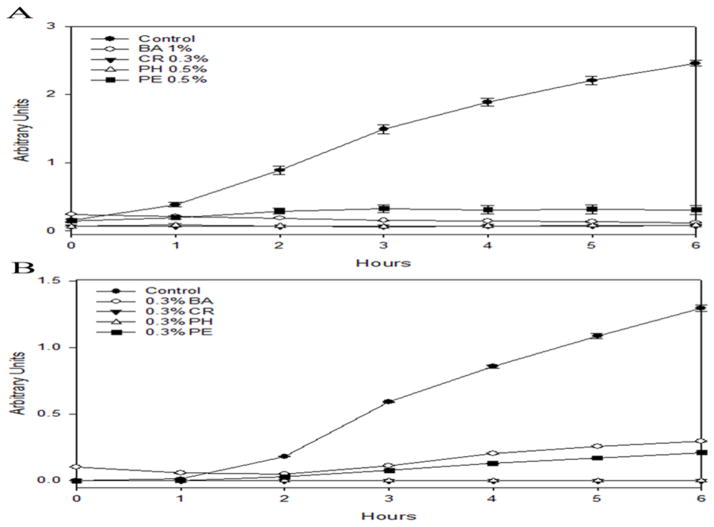
Four commonly employed APs in liquid protein formulations were used in this study. Table 1 lists the concentrations of these APs used in formulations. Figure 2A shows the antimicrobial efficacy of these APs, measured using a simplified test (Sutton and Porter 2002, Hutchings et al. 2013). We tested their effect on the growth of Escherichia coli bacteria. For this purpose, we used BL21(DE3) cells in LB media and monitored the cell count by measuring changes in the optical density at 600 nm as a function of the growth time. Without APs, the growth curve showed an exponential increase (Figure 2A). However, the optical density increase was minimal with the addition of any of the four APs when compared with the growth curve with no AP, indicating that these molecules inhibited bacterial growth. Table 2 lists the minimum inhibitory concentration (MIC) values for these APs against various organisms (Lucchini et al. 1990, Simpson and Wuthiekanun 2000, Meyer et al. 2007, Rowe et al. 2009, Abd-Elsalam et al. 2011).
Figure 2.
Preservative efficacy test of APs on BL21(DE3) E. coli cells (Sutton and Porter 2002, Hutchings et al. 2013). The cell count was monitored by measuring the relative optical density at 600 nm as a function of the growth time. Addition of APs inhibited bacterial growth. (A) Preservative efficacy at the AP concentrations used in protein formulations. Closed circles represent the data untreated with APs, while the other symbols represent the data treated with various APs: 1% v/v benzyl alcohol (BA, open circles), 0.3% v/v m-cresol (CR, closed triangles), 0.5% v/v phenol (PH, open triangles), and 0.5% v/v phenoxyethanol (PE, closed squares), respectively. (B) Preservative efficacy at 0.3% v/v AP concentrations. Closed circles, untreated control; open circles, BA; closed triangles, CR; open triangles, PH; closed squares, PE.
Table 2.
Minimum inhibitory concentration (MIC) values of APs measured using bacterial and microbial organisms (Lucchini et al. 1990, Simpson and Wuthiekanun 2000, Meyer et al. 2007, Rowe et al. 2009, Abd-Elsalam et al. 2011).
| Preservative and Typical Use Concentration | Typical Use Concentration [μg/ml] | Organism Tested | MIC [μg/ml] |
|---|---|---|---|
| Benzyl Alcohol, 1% vol/vol | 10400 | Aspergillus niger Candida albicans Escherichia coli Pseudomonas aeruginosa Staphylococcus aureus |
5000 2500 2000 2000 25 |
| m-Cresol, 0.3% vol/vol | 3090 | Aspergillus niger Bacillus subtilis Burkholderia pseudomallei Candida albicans Pseudomonas aeruginosa Staphylococcus aureus |
2000 1000 125 2500 1000 1000 |
| Phenol, 0.5% vol/vol | 5350 | Aspergillus niger Bacillus aerogenes Enterococcus faecium Escherichia coli Pseudomonas aeruginosa Staphylococcus aureus |
311 697 3600 2500 1800 1800 |
| Phenoxyethanol, 1% vol/vol | 11000 | Aspergillus niger Candida albicans Escherichia coli Pseudomonas aeruginosa Staphylococcus aureus |
3300 5400 3600 3200 8500 |
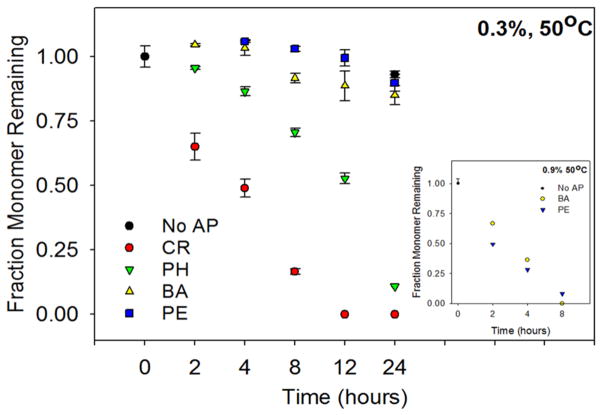
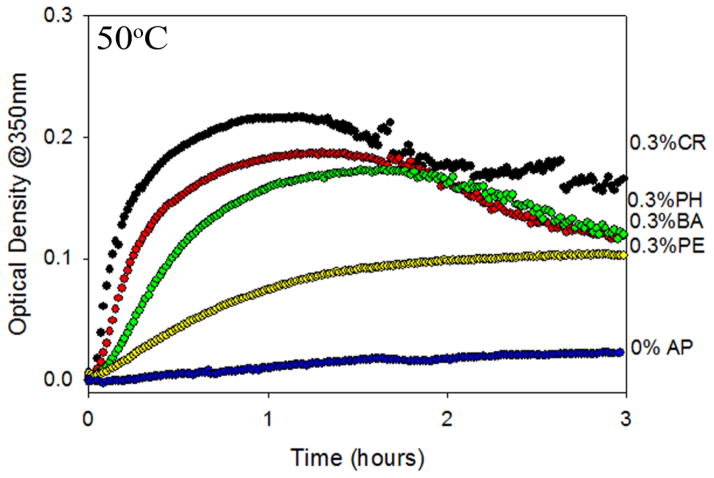
In previous studies, APs have been shown to induce protein aggregation over a period of days or months (Maa and Hsu 1996, Katakam and Banga 1997, Remmele Jr. et al. 1998, Gupta and Kaisheva 2003, Tobler et al. 2004, Roy et al. 2006, Thirumangalathu et al. 2006). In order to test the effects of different APs on protein aggregation on a convenient laboratory timescale, we accelerated aggregation kinetics by conducting isothermal incubation studies at an elevated temperature. Using elevated temperatures to accelerate protein aggregation is becoming a commonly used method for scanning the effect of a large number of solution conditions on protein stability and aggregation (Brummitt et al. 2011, Nashine et al. 2013, Chaudhuri et al. 2014). Initially, we demonstrated that APs induce IFNA2 aggregation. For comparing the effect of the four APs on IFNA2 aggregation, we chose a concentration of 0.3% v/v AP due to the low solubility of m-cresol above this concentration. At this concentration of 0.3% v/v, all the APs retained their antimicrobial efficacy (Figure 2B). Isothermal aggregation kinetics were performed at 50°C and the concentration of IFNA2 monomer was monitored as a function of the incubation time (Figure 3). With all the APs, only soluble monomer was detected using SE-HPLC. After a period of one day in the absence of AP, approximately 90% IFNA2 monomer remained in solution. No monomer was detected after eight hours in the presence of 0.3% v/v m-cresol (CR) and after 12 hours in the presence of 0.3% v/v phenol (PH). Both benzyl alcohol (BA) and phenoxyethanol (PE) exerted a marginal effect on monomer loss, losing approximately 20% monomer content over the course of 24 hours. To differentiate the impact of BA and PE on IFNA2 monomer loss, an isothermal incubation study was performed using 0.9% v/v AP concentration (Figure 3 inset). In 0.9% BA, all IFNA2 monomer disappeared within eight hours at 50°C, while monomer was detected up to eight hours in PE samples. These results followed the order CR > PH > BA > PE in terms of their effects on IFNA2 aggregation. We also monitored the change in optical density as a function of the incubation time, in order to correlate monomer loss with protein aggregation (Figure 4). In these cases, the same pattern of AP-induced destabilization was observed: CR > PH > BA > PE. No IFNA2 aggregation was observed in the absence of APs. This order in which APs induce IFNA2 aggregation is identical to what we observed earlier in the case of the model protein Cyt c: CR > PH > BA > PE (Hutchings et al. 2013).
Figure 3.
Effects of APs on IFNA2 monomer concentration under isothermal conditions. Soluble monomer remaining in solution during 24 hours of incubation at 50°C in the presence and absence of 0.3% (v/v) AP as measured by size exclusion chromatography. Black – native (no AP); red – m-cresol (CR); green – phenol (PH); yellow – benzyl alcohol (BA); blue – phenoxyethanol (PE). Error bars indicate triplicate data. Individual time points are normalized with respect to the sample with no AP at hour zero.
Figure 4.
Isothermal kinetics of IFNA2 aggregation at 50°C as measured by the changes in optical density at 350 nm in the presence of 0.3% v/v APs.
3.2 IFNA2 Aggregation Increases with AP Concentration
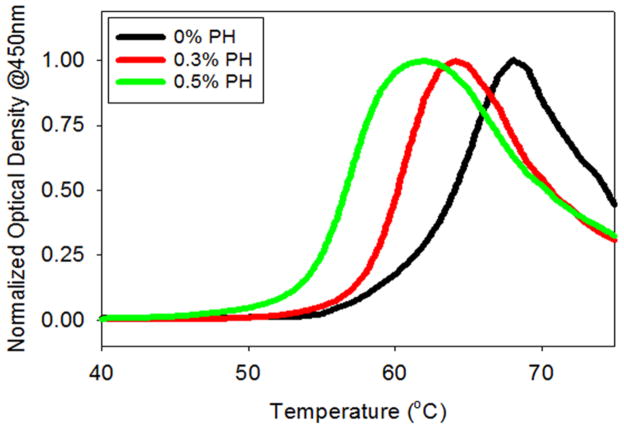
Another method used in the literature to monitor the effect of solution conditions on protein aggregation is thermal scanning (Brummitt et al. 2011, Nashine et al. 2013, Chaudhuri et al. 2014). This is very similar to using denaturant melts to determine the protein stability, although denaturant is not present in protein formulations. Here, we used temperature as the perturbant, rather than the denaturant. We utilized the thermal scanning technique to determine the effects of preservative concentration on IFNA2 aggregation. We measured the changes in the optical density at 450 nm as a function of increasing solution temperature. This wavelength was selected because neither the protein nor any solution components absorb in this range, and the changes in optical density can be attributed solely to protein aggregation. In buffer alone without APs, the optical density initially increased with temperature in a sigmoidal variation (Figure 5). At higher temperatures, aggregated protein particles began to settle to the bottom of the cuvette, causing an observable decrease in the optical density. We performed these thermal scanning experiments in the presence of increasing concentrations of PH (Figure 5). With the inclusion of PH, the midpoint aggregation temperature (TmAgg) decreased. In the absence of PH, the TmAgg of IFNA2 was 63.9 ± 0.9°C. The addition of 0.5% PH decreased the TmAgg to 56.7 ± 0.7°C, indicating that the presence of PH accelerated the aggregation of IFNA2. A similar phenomenon was observed with Cyt c whose aggregation increased with an increase in AP concentration (Hutchings et al. 2013).
Figure 5.
Variation in the optical density at 450 nm as a function of increasing solution temperature at different concentrations of phenol (PH).
3.3 Aggregation Temperature Decreases Linearly with AP Concentration
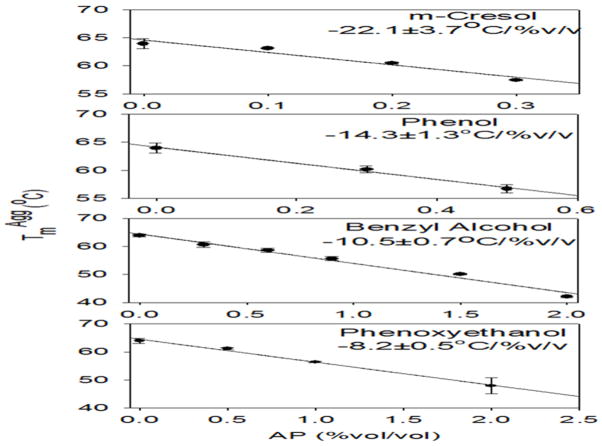
As seen with PH, the other APs also caused a decrease in TmAgg of IFNA2 with the increase in AP concentration (Figure 6). Interestingly, a linear correlation was observed between the TmAgg and the concentration of AP. This relationship is significant in that it is a qualitative measurement of how efficiently each AP causes IFN aggregation, which is represented in their individual slopes. This slope is analogous to the m-value commonly used in analyzing protein denaturant melts (Santoro and Bolen 1988), which represents the efficiency of a denaturant to denature a protein. Comparison of the slopes of TmAgg variation with the AP concentration demonstrates an effective order of CR > PH > BA > PE, with CR being the most effective AP in causing protein aggregation. These experiments indicate that for every percent concentration change of CR, the TmAgg of IFNA2 decreases by 22°C. In contrast, PE has a slope of 8.2°C/%v/v, nearly three-fold less in its effectiveness to aggregate IFNA2 than CR. This trend matches to that observed in the isothermal incubation studies (Figures 3 & 4), and is identical to our earlier observations on the model protein Cyt c (Hutchings et al. 2013).
Figure 6.
Dependence of aggregation temperature TmAgg on AP concentration. Individual panels indicate the slope of this variation.
4. Discussion
Protein therapeutics are marketed in a variety of dosage forms, including multi-dose formulations, which require the inclusion of at least one antimicrobial preservative in order to combat the growth of microbes and bacteria during repeated contact between the solution and a syringe needle (Cleland et al. 1993, Akers et al. 2002, Meyer et al. 2007). However, it has been shown that these preservatives cause protein aggregation (Maa and Hsu 1996, Katakam and Banga 1997, Remmele Jr. et al. 1998, Gupta and Kaisheva 2003, Tobler et al. 2004, Roy et al. 2006, Thirumangalathu et al. 2006). We have demonstrated previously that APs used in liquid protein formulations lead to protein destabilization and aggregation using the model protein Cyt c (Singh et al. 2010, Singh et al. 2011, Hutchings et al. 2013). The extent of this effect was dependent upon the nature of the AP, and the pattern of aggregation observed was CR > PH > BA > PE.
In this study, we tested the conclusions drawn from our model protein studies on a pharmaceutically relevant protein interferon α-2a. IFNA2 belongs to a family of cytokines that play crucial roles in the innate immune response, and is one of the numerous interferon-α subtypes found in humans. A number of interferon-α products exist in the pharmaceutical market and are used to treat various debilitating diseases including hairy cell leukemia and hepatitis c (Hiscott et al. 1984, Diaz et al. 1993, Kirkwood 2002).
IFNA2 multi-dose formulations have been shown to aggregate in the liquid state (Braun and Alsenz 1997, Braun et al. 1997, Hochuli 1997, Ryff 1997) and contain APs. In order to validate the conclusions drawn from our studies on a model protein Cyt c, we studied the effect of multiple APs on IFNA2 aggregation using various biophysical techniques. Isothermal incubation studies showed that APs cause the loss of IFNA2 monomer (Figure 3), and the increase in optical density due to protein aggregation (Figure 4). The order in which APs induce IFNA2 aggregation was CR > PH > BA > PE, which is the same order we observed earlier in the case of the model protein Cyt c (Hutchings et al. 2013). Further, the addition of AP caused a decrease in TmAgg. The slopes indicate that CR was the most efficient in aggregating IFNA2, whereas PE was the least (Figure 6). Again, this order exactly matched the pattern we observed in our earlier studies on the model protein Cyt c (Hutchings et al. 2013). Based on these results, PE appears to be the best preservative choice for pharmaceutical formulations in causing less protein aggregation when compared with the commonly used BA. However, the perfect AP and its concentration in a multi-dose formulation should be such that it causes less protein aggregation and has sufficient antimicrobial activity (Hutchings et al. 2013).
Similar comparisons, although not as extensive as demonstrated in the case of IFNA2 or Cyt c, were made earlier on other proteins. Recombinant human growth hormone (rhGH) aggregation was monitored during freezing, high-temperature incubation, and agitation using changes in optical density and the percentage monomer loss by SEC. In this case, the three APs followed a similar order in causing rhGH aggregation: CR >PH >BA (Maa and Hsu 1996). In the case of a monoclonal antibody (IgG) (Gupta and Kaisheva 2003), aggregation was monitored during isothermal incubation using visual inspection of the samples, percent monomer loss using SEC, and light scattering. The three APs examined induced protein aggregation in the order: CR> PH> BA. Changes in interleukin-1 receptor (IL-1R) melting temperature assessed by DSC, and monomer content measured using SEC in the presence of preservatives also showed a similar relationship (Remmele Jr. et al. 1998). Taken together, the data on these five proteins (Cyt c, IFNA2, rhGH, IgG, IL-1R), suggest that the order in which APs induce protein aggregation may be independent of the nature of protein. Cyt c, IFNA2, and rhGH are α-helical proteins, whereas the IgG is primarily β-sheet and IL-1R contains both α-helix and β-sheet. The proteins also differ in their function: Cyt c plays a role in electron transport; IFNA2, IgG, and IL-1R have critical functions in the immune system; and rhGH is a multi-functional protein with emphasis on growth and regeneration.
The observation that the extent of AP effects on protein aggregation is independent of the nature of protein implies that APs may interact with common structural groups present in proteins. APs do not have strong binding sites on proteins (Maa and Hsu 1996, Roy et al. 2006, Singh et al. 2010, Singh et al. 2011). APs may hydrogen bond with the peptide backbone, suggested by earlier studies on BA and polyproline monitoring changes in amide I, amide II, and hydroxyl bands using infrared spectroscopy (Strassmair et al. 1969). The extent of aggregation seems to qualitatively correlate with the hydrophobicity of the AP (Singh et al. 2011, Hutchings et al. 2013). APs also have been suggested to interact with hydrophilic regions of proteins (Alford et al. 2011). Perhaps the mechanism by which APs affect protein aggregation is a combination of hydrogen bonding, hydrophobic interactions, and electrostatics, and further examination of the exact mechanism is necessary in order to develop stable and effective pharmaceuticals.
Acknowledgments
The authors would like to acknowledge John Carpenter, Theodore Randolph, David Bain, and LaToya Jones Braun for many helpful discussions and critical comments. This work was funded by the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences. Regina Bis was partially supported by a NIH Leadership training grant in Pharmaceutical Biotechnology (T32GM008732) and a predoctoral fellowship from the PhRMA Foundation (AWD-120487).
Abbreviations
- BA
benzyl alcohol
- Cyt c
cytochrome c
- CR
m-cresol
- IFNA2
interferon alpha-2a
- PE
phenoxyethanol
- PH
phenol
- SE-HPLC
size exclusion high pressure liquid chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd-Elsalam MA, Abdoon N, Al-Ahaidib MS. What is the optimum concentration of m-cresol in antivenoms? Journal of Venomous Animals and Toxins. 2011;17:12–22. [Google Scholar]
- Akers M, Vasudevan V, Stickelmeyer M. In: Formulation development of protein dosage forms. Development and manufacture of protein pharmaceuticals. AM, Nail SL, editors. New York City, New York: Kluwer Academic/Plenum Publishers; 2002. pp. 47–127. [DOI] [PubMed] [Google Scholar]
- Alford JR, Fowler AC, Wuttke DS, Kerwin BA, Latypov RF, Carpenter JF, Randolph TW. Effect of benzyl alcohol on recombinant human interleukin-1 receptor antagonist structure and hydrogen–deuterium exchange. Journal of pharmaceutical sciences. 2011;100(10):4215–4224. doi: 10.1002/jps.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bis RL, Stauffer TM, Singh SM, Lavoie TB, Mallela KMG. High yield soluble bacterial expression and streamlined purification of recombinant human interferon α-2a. Protein Expr Purif. 2014;99:138–146. doi: 10.1016/j.pep.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Alsenz J. Development and use of enzyme-linked immunosorbent assays (ELISA) for the detection of protein aggregations in interferon-alpha (IFN-α) formulations. Pharm Res. 1997;14:1394–1400. doi: 10.1023/a:1012168621337. [DOI] [PubMed] [Google Scholar]
- Braun A, Kwee L, Labow M, Alsenz J. Protein aggregations seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-α) in normal and transgenic mice. Pharm Res. 1997;14:1472–1478. doi: 10.1023/a:1012193326789. [DOI] [PubMed] [Google Scholar]
- Brummitt RK, Nesta DP, Roberts CJ. Predicting accelerated aggregation rates for monoclonal antibody formulations, and challenges for low-temperature predictions. J Pharm Sci. 2011;100:4234–4243. doi: 10.1002/jps.22633. [DOI] [PubMed] [Google Scholar]
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formingli L, Zurdo J, Taddei N, Ramponi G, Dobson C, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- Charman S, Mason K, Charman W. Techniques for assessing the effects of pharmaceutical excipients on the aggregation of porcine growth hormone. Pharm Res. 1993;10:954–962. doi: 10.1023/a:1018994102218. [DOI] [PubMed] [Google Scholar]
- Chaudhuri R, Cheng Y, Middaugh CR, Volkin DB. High-throughput biophysical analysis of protein therapeutics to examine interrelationships between aggregate formation and conformational stability. AAPS J. 2014;16:48–64. doi: 10.1208/s12248-013-9539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland J, Powell M, Shire S. The development of stable protein formulations - A close look at protein aggregation, deamidation and oxidation. Crit Rev Ther Drug Carrier Syst. 1993;10:307–377. [PubMed] [Google Scholar]
- Diaz M, Bohlander S, Allen G. Nomenclature of human interferon genes. J interferon Res. 1993;13:243–244. doi: 10.1089/jir.1993.13.243. [DOI] [PubMed] [Google Scholar]
- Eberlein G, Stratton P, Wang Y. Stability of rhbFGF as determined by UV spectroscopic measurements of turbidity. PDA J Pharm Sci Tech. 1994;48:224–230. [PubMed] [Google Scholar]
- Eckhardt B, Oeswein J, Yeung D, Milby T, Bewley T. A turbidimetric method to determine visual appearance of protein solutions. J Pharm Sci Tech. 1994;48:64–70. [PubMed] [Google Scholar]
- Fradkin A, Carpenter J, Randolph T. Immunogenicity of aggregates of recombinant human growth hormone in mouse models. J Pharm Sci. 2009;98:3247–3264. doi: 10.1002/jps.21834. [DOI] [PubMed] [Google Scholar]
- Gupta S, Kaisheva E. Development of a multidose formulation for a humanized monoclonal antibody using experimental design techniques. AAPS PharmSci. 2003;5:1–9. doi: 10.1208/ps050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermeling S, Schellekens H, Maas C, Gebbink MF, Crommelin DJ, Jiskoot W. Antibody response to aggregated human interferon alpha2b in wild-type and transgenic immune tolerant mice depends on type and level of aggregation. Journal of pharmaceutical sciences. 2006;95(5):1084–1096. doi: 10.1002/jps.20599. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Cantell K, Weissmann C. Differential expression of human interferon genes. Nucleic Acids Research. 1984;12:3727–3746. doi: 10.1093/nar/12.9.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochuli E. Interferon immunogenicity: technical evaluation of interferon-α2a. J Interferon Cytokine Res. 1997;17:S15–S21. [PubMed] [Google Scholar]
- Hutchings R, Singh S, Cabello-Villegas J, Mallela K. Effect of antimicrobial preservatives on partial protein unfolding and aggregation. J Pharm Sci. 2013;102:365–376. doi: 10.1002/jps.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam M, Banga A. Use of poloxamer polymers to stabilize recombinant human growth hormone against various processing stresses. Pharm Dev Technol. 1997;2:143–149. doi: 10.3109/10837459709022619. [DOI] [PubMed] [Google Scholar]
- Kirkwood J. Cancer immunotherapy: the interferon-alpha experience. Seminars in Oncology. 2002;29:18–26. doi: 10.1053/sonc.2002.33078. [DOI] [PubMed] [Google Scholar]
- Kurganov B. Kinetics of protein aggregation. Quantitative estimation of the chaperone-like activity in test-systems based on suppression of protein aggregation. Biochemistry. 2002;67:409–422. doi: 10.1023/a:1015277805345. [DOI] [PubMed] [Google Scholar]
- Lucchini JJ, Corre J, Cremieux A. Antibacterial activity of phenolic compounds and aromatic alcohols. Res Microbiol. 1990;141:499–510. doi: 10.1016/0923-2508(90)90075-2. [DOI] [PubMed] [Google Scholar]
- Maa Y, Hsu C. Aggregation of recombinant human growth hormone induced by phenolic compounds. Int J Pharm. 1996;140:155–168. [Google Scholar]
- Meyer BK, Ni A, Hu B, Shi L. Antimicrobial preservative use in parenteral products: past and present. Journal of Pharmaceutical Sciences. 2007;96(12):3155–3167. doi: 10.1002/jps.20976. [DOI] [PubMed] [Google Scholar]
- Meyer BK, Ni A, Hu B, Shi L. Antimicrobial preservative use in parenteral products: Past and present. J Pharm Sci. 2007;96:3155–3167. doi: 10.1002/jps.20976. [DOI] [PubMed] [Google Scholar]
- Nashine VC, Kroetsch AM, Sahin E, Zhou R, Adams ML. Orthogonal high-throughput thermal scanning method for rank ordering protein formulations. AAPS PharmSciTech. 2013;14:1360–1366. doi: 10.1208/s12249-013-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner R, Phillips T, Steiner M. Persistent cutaneous insulin allergy resulting from high molecular weight insulin aggregates. Diabetes. 1990;39:728–733. doi: 10.2337/diab.39.6.728. [DOI] [PubMed] [Google Scholar]
- Remmele R, Jr, Nightlinger N, Srinivasan S, Gombotz W. Interleukin-1 receptor (IL-1R) liquid formulation development using differential scanning calorimetry. Pharm Res. 1998;15:200–208. doi: 10.1023/a:1011902215383. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. Effects of protein aggregation: An immunologic perspective. AAPS J. 2006;8:E501–E507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RC, Sheskey PJ, Quinn ME, editors. Handbook of Pharmaceutical Excipients. Pharmaceutical Press; 2009. [Google Scholar]
- Roy S, Jung R, Kerwin BA, Randolph TW, Carpenter JF. Effects of benzyl alcohol on aggregation of recombinant human interleukin-1-receptor antagonist in reconstituted lyophilized formulations. J Pharm Sci. 2005;94(2):382–396. doi: 10.1002/jps.20258. [DOI] [PubMed] [Google Scholar]
- Roy S, Katayama D, Dong A, Kerwin B, Randolph T, Carpenter J. Temperature dependence of benzyl alcohol- and 8-anilinonaphthalene-1-sulfonate-induced aggregation of recombinant human interleukin-1 receptor antagonist. Biochemistry. 2006;45:3898–3911. doi: 10.1021/bi052132g. [DOI] [PubMed] [Google Scholar]
- Ryff J. Clinical investigation of the immunogenicity of interferon-α2a. Journal of Interferon and Cytokine Research. 1997;17:S29–S33. [PubMed] [Google Scholar]
- Santoro MM, Bolen DW. Unfolding free energy changes determined by the linear extrapolation method. 1. Unfolding of phenylmethanesulfonyl alpha-chymotrypsin using different denaturants. Biochemistry. 1988;27:8063–8068. doi: 10.1021/bi00421a014. [DOI] [PubMed] [Google Scholar]
- Sauerborn M, Brinks V, Jiskoot W, Schellekens H. Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci. 2010;31:53–59. doi: 10.1016/j.tips.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Simpson AJH, Wuthiekanun V. Interaction of insulin with Burkholderia pseudomallei may be caused by a preservative. J Clin Pathol. 2000;53:159–160. doi: 10.1136/jcp.53.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Cabello-Villegas J, Hutchings R, Mallela K. Role of partial protein unfolding in alcohol-induced protein aggregation. Proteins. 2010;78:2625–2637. doi: 10.1002/prot.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Hutchings R, Mallela K. Mechanisms of m-cresol-induced protein aggregation studied using a model protein Cytochrome c. J Pharm Sci. 2011;100:1679–1689. doi: 10.1002/jps.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmair H, Engel J, Zundel G. Binding of alcohols to the peptide CO-group of poly-L-proline in the I and II conformation. I. Demonstration of the binding by infrared spectroscopy and optical rotatory dispersion. Biopolymers. 1969;8(2):237–246. [Google Scholar]
- Sutton SVW, Porter D. Development of the antimicrobial effectiveness test as USP Chapter <51>. PDA J Pharm Sci Tech. 2002;56:300–311. [PubMed] [Google Scholar]
- Thirumangalathu R, Krishnan S, Brems DN, Randolph TW, Carpenter JF. Effects of pH, temperature, and sucrose on benzyl alcohol-induced aggregation of recombinant human granulocyte colony stimulating factor. J Pharm Sci. 2006;95(7):1480–1497. doi: 10.1002/jps.20619. [DOI] [PubMed] [Google Scholar]
- Tobler S, Holmes B, Cromwell M, Fernandez E. Benzyl alcohol-induced destabilization of interferon-γ. J Pharm Sci. 2004;93:1605–1617. doi: 10.1002/jps.10589. [DOI] [PubMed] [Google Scholar]
- Vazquez-Rey M, Lang D. Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng. 2011;108:1494–1508. doi: 10.1002/bit.23155. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Roy S, Jones LS, Krishnan S, Kerwin BA, Chang BS, Manning MC, Randolph TW, Carpenter JF. Mechanism for benzyl alcohol-induced aggregation of recombinant human interleukin-1 receptor antagonist in aqueous solution. J Pharm Sci. 2004;93(12):3076–3089. doi: 10.1002/jps.20219. [DOI] [PubMed] [Google Scholar]