Abstract
Both stress and dysfunction of prefrontal cortex are linked to psychological disorders, and structure and function of medial prefrontal cortex (mPFC) are altered by stress. Chronic restraint stress causes dendritic retraction in the prelimbic region (PL) of mPFC in rats. Dopamine release in mPFC increases during stress, and chronic administration of dopaminergic agonists results in dendritic remodeling. Thus, stress-induced alterations in dopaminergic transmission in PL may contribute to dendritic remodeling. We examined the effects of dopamine D1 receptor (D1R) blockade in PL during daily restraint stress on dendritic morphology in PL. Rats either underwent daily restraint stress (3 h/day, 10 days) or remained unstressed. In each group, rats received daily infusions of either the D1R antagonist SCH23390 or vehicle into PL prior to restraint; unstressed and stressed rats that had not undergone surgery were also examined. On the final day of restraint, rats were euthanized and brains were processed for Golgi histology. Pyramidal neurons in PL were reconstructed and dendritic morphology was quantified. Vehicle-infused stressed rats demonstrated dendritic retraction compared to unstressed rats, and D1R blockade in PL prevented this effect. Moreover, in unstressed rats, D1R blockade produced dendritic retraction. These effects were not due to attenuation of the HPA axis response to acute stress: plasma corticosterone levels in a separate group of rats that underwent acute restraint stress with or without D1R blockade were not significantly different. These findings indicate that dopaminergic transmission in mPFC during stress contributes directly to the stress-induced retraction of apical dendrites, while dopamine transmission in the absence of stress is important in maintaining normal dendritic morphology.
Keywords: prefrontal cortex, dopamine, D1 receptor, corticosterone, stress, dendrites
1. Introduction
Stress is linked to many psychological disorders, including post-traumatic stress disorder, depression, and schizophrenia (e.g., Leff et al., 1973; Harder et al., 1980; Brown and Harris, 1989). These stress-sensitive psychological disorders are often characterized by prefrontal cortex dysfunction (e.g., Weinberger et al., 1986; Berman et al., 1987; Berman et al., 1988; Breier et al., 1992; Drevets et al., 1992; Glantz and Lewis, 2000; Liotti et al., 2002; Milad et al., 2009; Cisler et al., 2013). Thus, understanding how stress alters prefrontal cortex may elucidate how stress contributes to psychological disorders.
Chronic (Cook and Wellman, 2004), mild (Brown et al., 2005), and acute stressors (Izquierdo et al., 2006) cause retraction of apical dendrites of pyramidal neurons in rat medial prefrontal cortex (mPFC). Chronic stress in rats impairs prefrontally mediated behaviors such as retrieval of extinction of conditioned fear (Miracle et al., 2006; Baran et al., 2009; Farrell et al., 2010), working memory (Hains et al., 2009; Mika et al., 2012), and attentional set-shifting (Liston et al., 2006). Further, prior stress dampens mPFC single-unit firing during extinction (Wilber et al., 2011) and impairs induction of long-term potentiation (LTP) in mPFC (Maroun and Richter-Levin, 2003; Rocher et al., 2004; Richter-Levin and Maroun, 2010).
Both NMDA and glucocorticoid receptors have been implicated in the stress-induced dendritic retraction in mPFC (Liu and Aghajanian, 2008; Martin and Wellman, 2011). However, during an acute stressor, dopamine levels in mPFC are markedly increased (Abercrombie et al., 1989; Gresch et al., 1994). Given that chronic administration of dopaminergic agonists such as amphetamine and cocaine produces dendritic proliferation in mPFC (e.g., Robinson and Kolb, 2004; Ball et al., 2010), stress-induced alterations in mPFC dopamine release could also contribute to dendritic remodeling.
The D1 receptor (D1R) is the most plentiful dopamine receptor in mPFC, at levels twenty times greater than D2-family receptors (Goldman-Rakic et al., 2000), and has been linked to stress and stress-sensitive cognitive processes. For instance, blockade of D1 but not D2 receptors in mPFC inhibits stress-induced increases in dopamine release in nucleus accumbens (Doherty and Gratton, 1996), and chronic stress impairs dopamine receptor-dependent long-term potentiation (LTP) in ventral mPFC (Goldwater et al., 2009) and working memory (Hains et al., 2009). Intra-mPFC D1R blockade impairs consolidation of extinction of conditioned fear (Hikind and Maroun, 2008) and working memory (Dent and Neill, 2012). Infusion of a D1R agonist in mPFC at low doses improves working memory while higher doses impair working memory performance (e.g., Zahrt et al., 1997; Lidow et al., 2003; Cools and D’Esposito, 2011; Dent and Neill, 2012). D1 but not D2 receptor antagonism blocks LTP maintenance (Huang et al., 2004). Given the prominent role of D1R activation in stress and prefrontally mediated behaviors, D1R activation during stress may contribute to dendritic remodeling in mPFC. To test this hypothesis, we examined dendritic morphology of pyramidal neurons in the prelimbic region (PL) of mPFC in rats that underwent daily restraint stress with intra-mPFC infusion of either the D1R-family antagonist SCH23390 or vehicle.
2. Materials and Methods
2.1. Effects of D1 Blockade on Dendritic Morphology
2.1.1. Subjects and Surgery
Male Sprague-Dawley rats (57±1.2 days old at surgery; Harlan Indianapolis IN; total N = 45) were housed individually in standard laboratory cages (48 cm × 20 cm × 26 cm), with food and water available ad libitum, ambient temperature 23-25 °C, and a 12:12 h light/dark cycle (lights on at 0700 h). All experimental procedures occurred between 0800 h and 1900 h, were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Bloomington Institutional Animal Care and Use Committee.
Rats (n = 29) underwent surgery for implantation of bilateral guide cannulae aimed at prelimbic cortex. Rats were anesthetized with IP ketamine (74 mg/kg), xylazine (3.7 mg/kg), and acepromazine (0.74 mg/kg) and given sc Rimadyl (5 mg/kg). Rats were placed in a stereotaxic apparatus, and 22-gauge guide cannulae (Plastics One, Roanoke VA) were implanted bilaterally just dorsal to PL (2.6 mm anterior to bregma; ±0.5 mm from midline, 2.7 mm ventral to bregma), allowing infusion cannulae, which project 1mm below the guide cannulae, to extend into PL. Guide cannulae were secured to skull screws with dental acrylic, and skin was sutured around the headstage.
2.1.2. Infusion and Chronic Restraint Stress
All rats were allowed approximately one week of recovery from surgery. To assess the effects of D1R blockade on chronic stress-induced dendritic remodeling in PL, rats were randomly assigned to vehicle (n = 8 unstressed and 7 stressed) or SCH23390 (n = 7 unstressed and 7 stressed) infusion groups. To control for potential effects of surgery and handling, a final group of unstressed (n = 8) and stressed (n = 8) rats that had not undergone surgery were also examined. See Figure 1A for schematic of experimental design.
Figure 1.
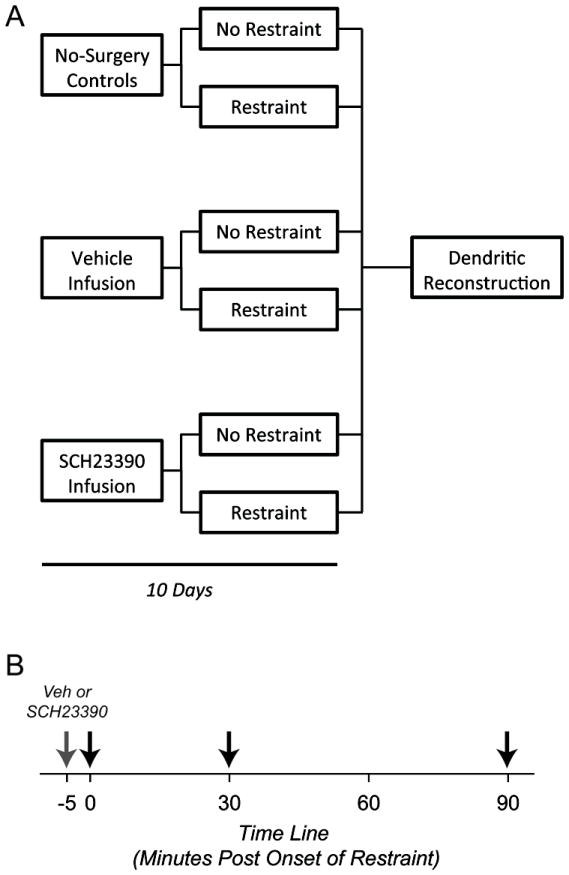
A. Schematic representation of the experimental design for assessing effects of D1 blockade during daily restraint stress on dendritic morphology in PL. B. Schematic representation of the time line for assessing effects of D1 receptor blockade on acute HPA axis response to stress. Gray arrow indicates time at which infusion of either vehicle or SCH23390 occurred; black arrows, times at which blood samples were taken.
All rats receiving infusions were handled for 3 days prior to stress and infusions. Infusions were performed daily for 10 days using two blunt-tipped 2 μl Hamilton syringes connected to infusion cannulae (Plastics One, Roanoke VA) with microdialysis tubing (Bioanalytical Systems, West Lafayette IN), through which the selective D1R antagonist SCH23390 (Sigma, St. Louis MO; 0.25μg/side in 0.5 μl sterile saline) or vehicle (0.5 μl sterile saline) was infused. Infusion of this volume and concentration of SCH23390 into mPFC is sufficient to impair fear extinction consolidation in rats undergoing auditory fear conditioning (Hikind and Maroun, 2008). Infusion cannulae were left in place 5 min following the infusion to ensure sufficient diffusion away from the cannula tip. All rats were weighed for verification of the stress manipulation. Immediately after weighing and infusions, stressed rats were placed in plastic semi-cylindrical restrainers (6.35 cm diameter × 15.24 cm length, modified so the tail piece locks into place; Braintree Scientific) in their home cages for 3 h, a manipulation that produces significant increases in plasma corticosterone levels (Cook and Wellman, 2004). Unstressed rats were returned to the vivarium. Daily restraint occurred for 10 days.
2.1.3 Histology and Dendritic Analysis
Within 12 hours of the final stress or infusion session, rats were deeply anesthetized with urethane and transcardially perfused with saline. Adrenal glands were removed and weighed for verification of the stress manipulation. Brains were removed and processed using Glaser and Van der Loos’ modified Golgi stain, as described previously (Glaser and Van der Loos, 1981; Martin and Wellman, 2011). Coronal sections were cut at 200 μm on a sliding microtome (American Optical AO860, Buffalo NY).
Pyramidal neurons in layer II-III of PL (Zilles and Wree’s Cg3; Zilles and Wree, 1985) were drawn from 4 sections evenly spaced through the anterior-posterior extent of PL (Figure 3A). Pyramidal neurons were defined by the presence of at least two basilar dendritic trees with at least third-order branches, a distinct, single apical dendritic tree with an identifiable apical tuft, and dendritic spines. Within each section, all pyramidal neurons in layer II-III that did not have truncated branches and were unobscured by neighboring neurons were identified, and using a random number generator, one neuron per hemisphere was randomly selected for reconstruction, for a total of 8 neurons per animal (see Martin and Wellman, 2011). All neurons were drawn at a final magnification of 600× and morphology of apical and basilar arbors was quantified in three dimensions using a computer-based neuron tracing system (Neurolucida, MBF Biosciences, Williston, VT) with the experimenter blind to condition.
Figure 3.
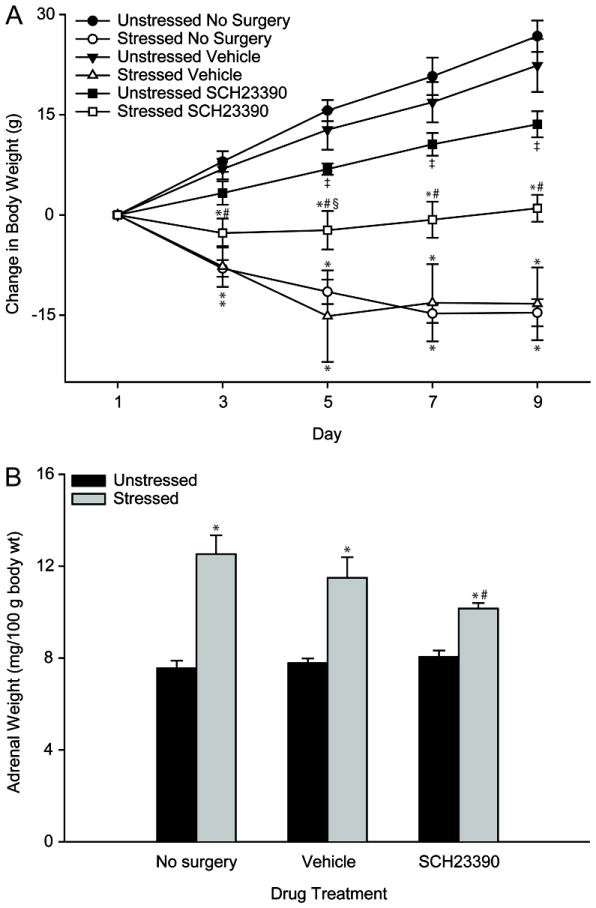
A. Mean weight change in unstressed versus stressed rats receiving no surgery, vehicle infusion, or SCH23390 infusion. Stress attenuated weight gain in each drug treatment group, but there was less attenuation in SCH23390-infused stressed rats. Vertical bars represent SEM values. B. Mean adrenal weight in unstressed versus stressed rats receiving no surgery, vehicle infusion, or SCH23390 infusion, expressed in mg/100 g body weight. Stress increased relative adrenal weight in each drug treatment group, but the increase was smaller in SCH23390-infused stressed rats. Vertical bars represent SEM values. For both A and B, *, significant differences between unstressed and stressed rats within drug treatment groups; ‡, significant difference relative to unstressed no-surgery group; #, significant difference relative to stressed no-surgery group; §, significant difference relative to stressed vehicle-infused group.
2.1.4. Statistical Analyses
Chronic stressors such as restraint or immobilization attenuate normal weight gain (e.g., Martí et al., 1994; Cook and Wellman, 2004) and increase relative adrenal weight in rats (e.g., Heiderstadt et al., 2000). Therefore, to verify the stress manipulation, weight gain was compared across groups using 3-way ANOVA (stress group × drug treatment × day), while adrenal weights were compared across groups using 2-way ANOVA (stress group × drug treatment). To assess changes in dendritic morphology, total length and number of basilar and apical dendrites were compared across groups using 2-way ANOVAs (stress group × drug treatment). Follow-up planned comparisons were performed when appropriate, and consisted of two-group F tests done within the context of the overall ANOVA (Hays, 1994).
2.2. Effects of mPFC D1 Blockade on Acute Stress-induced Corticosterone Release
Previous studies suggest a role for mPFC in modulating stress-induced HPA axis activation (Ulrich-Lai and Herman, 2009). To assess potential effects of D1R blockade in PL on HPA axis response to acute stress, we assessed plasma corticosterone levels in rats (N=41) that underwent acute restraint stress with or without D1R blockade. To minimize confounding effects of repeated blood sampling, a cross-sectional design was employed. Male Sprague-Dawley rats (68 ± 1.7 days old at surgery; Harlan, Indianapolis, IN) underwent implantation of guide cannulae as described above. After at least one week recovery from surgery, during which rats were handled daily, rats received infusions of either SCH23390 or vehicle, as described above. After the 5-min infusion period, rats were either anesthetized immediately (0 min group; see below) or placed in plastic restrainers for 30 min. This manipulation significantly increases plasma corticosterone concentrations (Viau and Sawchenko, 2002). Rats were deeply anesthetized with urethane either immediately after infusion (0 min post-onset of stress; n= 7 Vehicle and 4 SCH23390), immediately after the 30-min restraint stress (30 min; n= 8 Vehicle and 9 SCH23390), or 1 h after the cessation of stress (i.e., 90 min after the onset of stress; 90 min group; n= 8 Vehicle and 5 SCH23390). Blood samples were taken via cardiac puncture within a median of 2 min after urethane injection. All infusions and blood sampling occurred between 1230 h and 1730 h. See Figure 1B for a schematic of the experimental design.
Blood was transferred to heparinized vials and centrifuged at 2000 × g for 15 minutes to obtain plasma. Corticosterone titers were assessed using a competitive enzyme immunoassay kit (Enzo Life Sciences, Plymouth Meeting PA). This assay has low cross-reactivity with other major steroid hormones, sensitivity of <27 pg/mL, and coefficients of variation within and across assays of <8.0% and <13.1%, respectively. Corticosterone concentrations were compared across groups using 2-way ANOVA (time after onset of restraint × drug treatment). Significant effects were followed up with Fisher’s PLSD post hoc tests.
Immediately after blood sampling, rats were transcardially perfused with saline followed by 10% buffered formalin. Brains were removed, postfixed, and cryoprotected. Coronal sections through the rostral forebrain were cut at 40 μm on a sliding microtome (Leica Histoslide 2000, Buffalo Grove IL). Sections were stained with cresyl echt violet and examined under a light microscope to verify cannulae placement.
3. Results
3.1. Verification of Cannula Placement and Chronic Stress Manipulation
Guide cannula placement was verified based on standard cytoarchitectural criteria (Paxinos and Watson, 1998), and is shown in Figure 2.
Figure 2.
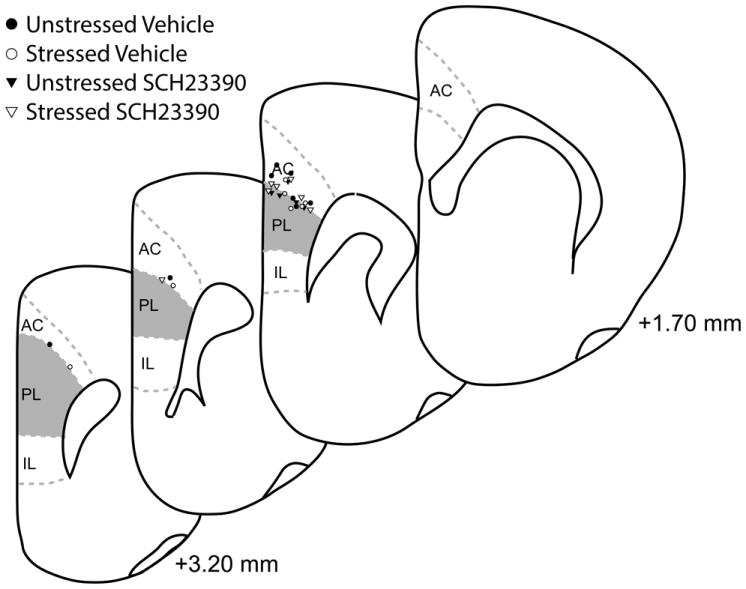
Guide cannula placement based on standard cytoarchitectural criteria (Paxinos and Watson, 1998). All guide cannulae were located just dorsal to prelimbic cortex. Infusion cannulae extended 1 mm beyond the tip of the guide cannulae. Although placements were bilateral, for simplicity only one hemisphere is shown.
Chronic stress significantly attenuated weight gain (Figure 3A; main effect of stress, F1,39 = 105.90, p ≤ .05; stress × day interaction, F4,156 = 68.99, p ≤ .05). There was no significant effect of drug treatment per se (F2,39 = 0.44, ns), but there were significant interactions of stress and drug treatment (F2,39 = 8.30, p ≤ .05), and stress, drug treatment, and day (F8,156 = 6.06, p ≤ .05). Planned comparisons revealed that within unstressed and stressed groups, weight was comparable between no-surgery and vehicle rats on all days (for unstressed rats, all Fs1,14 ≤ 0.90, ns; for stressed rats, all Fs1,13 ≤0.90, ns). In no-surgery, vehicle-, and SCH23390-infused rats, stress significantly reduced weight gain on days 3 through 9 compared to unstressed rats (for no-surgery rats, all Fs1,14 ≥ 64.58, p ≤ .05; for vehicle-infused rats, all Fs1,13 ≥ 15.30, p ≤ .05; for SCH23390-infused rats, all Fs1,12 ≥ 4.63, p ≤ .05). Within unstressed rats, SCH23390 infusion significantly attenuated weight gain on days 5 through 9 compared to no-surgery rats (Fs1,13 ≥ 8.99, p ≤ .05), though this effect was not significant compared to vehicle-infused rats on any day (all Fs1,13 ≤ 3.61, ns). However, within stressed groups, SCH23390-infused rats showed significantly increased weight gain on days 3 through 9 compared to stressed no-surgery rats (all F1,14 ≥ 4.75, p ≤ .05) and on day 9 compared to stressed vehicle-infused rats (F1,12 = 6.06, p ≤ .05). Thus, SCH23390 infusion had opposite effects on weight gain in unstressed versus stressed rats.
Relative adrenal weight was significantly increased in stressed compared to unstressed rats (Figure 3B; main effect of stress, F1,39 = 78.79, p ≤ .05). Although drug treatment per se did not significantly influence adrenal weight (F2,39 = 1.79, ns), there was a significant interaction between stress and drug treatment (F2,39 = 4.17, p ≤ .05). Planned comparisons demonstrated that within stress groups, vehicle-treated and no-surgery groups did not differ from each other (unstressed, F1,14 = 0.37, ns; stressed, F1,13 = 0.88, ns). In no-surgery, vehicle-, and SCH23390-infused rats, stress significantly increased relative adrenal weight compared to unstressed rats (no-surgery rats, F1,14 = 31.64, p ≤ .05; vehicle-infused rats, F1,13 = 28.99, p ≤ .05; SCH23390-infused rats, F1,12 =38.03, p ≤ .05). Although unstressed SCH23390-treated rats did not differ significantly from unstressed no-surgery or vehicle-infused rats (both Fs1,13 = 1.35, ns), within stressed rats, relative adrenal weight was significantly lower in SCH23390-infused rats compared to no-surgery (F1,13 = 6.85, p ≤ .05) but not vehicle-infused rats (F1,13 = 3.22, ns) .
3.2. Prefrontal D1R Blockade Alters Normal Morphology and Prevents Stress-induced Dendritic Retraction
To assess changes in apical dendrites, total branch number and length were compared across groups (Figure 4B,C). Although there was no significant overall effect of drug treatment (F2,39 = 1.28, ns) or stress (F1,39 = 0.33, ns) on apical branch number, the interaction between drug treatment and stress was significant (F2,39 = 5.79, p ≤ 0.05). Planned comparisons revealed that within the unstressed groups, treatment with SCH23390 significantly reduced apical branch number relative to either no-surgery or vehicle-infused controls (F1,13 = 5.93 and F1,13 = 9.80, respectively, both p ≤ 0.05). Unstressed no-surgery and vehicle-infused rats did not differ from each other (F1,14 = 0.56, ns). Stress did not significantly alter apical branch number in either no-surgery rats or vehicle-infused rats (F1,14 =3.47 and F1,13 = 3.59, respectively, both ns). However, in SCH23390-infused rats, stress significantly increased apical branch number (F1,12 = 4.75, p ≤ 0.05), restoring branch numbers to values comparable to those in each of the other treatment groups (all Fs ≤ 2.73, ns).
Figure 4.
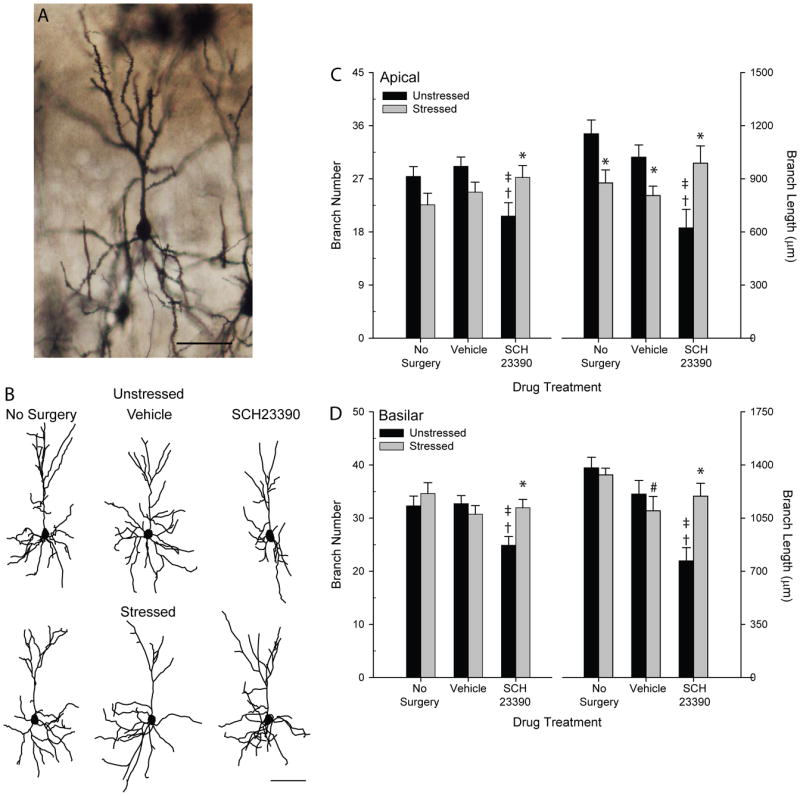
A. Digital micrograph of Golgi-stained neuron in layer II-III of PL. Scale bar = 75 μm For a color version of this micrograph, the reader is referred to the web version of the article. A. Computer-assisted reconstructions of Golgi-stained neurons in layers II-III of prelimbic cortex in unstressed and stressed, no-surgery, vehicle-infused, and SCH23390-infused rats. Neurons are representative of apical dendritic lengths near their respective group means. Scale bar = 75 μm. B. Mean apical branch number (left) and length (right) in unstressed versus stressed rats receiving no surgery, vehicle infusion, or SCH23390 infusion. Apical branch number and length were significantly reduced in unstressed SCH23390-infused rats relative to unstressed no-surgery and vehicle-infused rats. Stress significantly reduced apical branch length in no-surgery and vehicle-infused rats relative to unstressed no-surgery and vehicle-infused rats. SCH23390 infusion prevented stress-induced dendritic retraction. Vertical bars represent SEMs. C. Mean basilar branch number (left) and length (right) in unstressed versus stressed rats receiving no surgery, vehicle infusion, or SCH23390 infusion. Basilar branch number and length were significantly reduced in unstressed SCH23390-infused rats relative to unstressed no-surgery and vehicle-infused rats. Stress did not significantly alter basilar morphology in no-surgery and vehicle-infused rats relative to unstressed no-surgery and vehicle-infused rats, but SCH23390 infusion during stress normalized basilar morphology. For both C and D, *, significant differences between stressed and unstressed rats within a drug treatment group; ‡, significant difference relative to unstressed no-surgery group; †, significant difference relative to unstressed vehicle-infused group; #, significant difference relative to stressed no-surgery group.
For apical dendritic length (Figure 4B,C), the main effect of stress was not significant (F1,39 = 0.42, ns), but there was a significant effect of drug treatment (F2,39 = 3.40, p ≤ .05) and a significant interaction of drug treatment and stress (F2,39 = 9.44, p ≤ .05). Planned comparisons revealed that within the unstressed groups, SCH23390 infusion significantly reduced apical dendritic length relative to no-surgery (F1,13 = 16.95, p ≤ .05) and vehicle-infused rats (F1,13 = 10.54, p ≤ .05). Unstressed no-surgery and vehicle rats did not differ from each other (F1,14 = 1.60, ns). The effect of stress varied across drug treatments: in no-surgery and vehicle-infused rats, stress significantly decreased apical branch length relative to unstressed rats (F1,14 = 6.69 and F1,13 = 5.90, respectively, both p ≤ .05). However, in SCH23390-infused rats, stress significantly increased apical length compared to unstressed SCH23390-infused rats (F1,12 = 6.53, p ≤ .05), restoring branch lengths to values comparable to those in unstressed no-surgery and vehicle rats (all Fs ≤ 2.75, ns).
To assess changes in basilar dendrites, total branch number and length were compared across groups (Figure 4D). Although there was no main effect of stress on basilar branch number or length (F1,39 = 3.05 and 1.95, respectively, both ns), there was a significant effect of drug treatment (F2,39 = 4.33 and 11.34, respectively, both p ≤ .05), and an interaction between drug treatment and stress (F2,39 = 3.30 and 6.58, respectively, both p ≤ .05). Planned comparisons showed that within the unstressed groups, SCH23390 infusion reduced basilar branch number and length relative to either no-surgery controls or vehicle-infused controls (for branch number, F1,13 =5.93 and F1,13 = 9.80, respectively; for branch length, F1,13 =30.56 and F1,13 = 12.14, respectively; all p ≤ .05). Unstressed no-surgery and vehicle-infused rats did not differ from each other (F1,14 = 0.03 and 2.29, both ns). Stress did not significantly alter basilar branch number or length in either no-surgery or vehicle-infused rats (for no-surgery rats, F1,14 = 0.73 and 0.31; for vehicle-infused rats, F1,13 = 0.81 and 0.71; all ns). However, stress significantly increased basilar branch number and length in SCH23390-infused rats (F1,12 = 9.60 and 12.29, respectively; both p ≤ .05).
3.3. Prefrontal D1R Blockade Does Not Alter Acute Stress-Induced Increases in Corticosterone
Infusion cannulae placements were verified based on standard cytoarchitectural criteria (Figure 5A; Paxinos and Watson, 1998). Corticosterone levels in both vehicle-infused and SCH23390-infused animals exhibited typical HPA activity patterns, with low levels in the 0h group, peak corticosterone concentration occurring immediately after restraint stress, and levels returning to baseline by one hour after the end of restraint stress (Figure 5B). Accordingly, the effect of time point was significant (F2,35 = 90.52, p ≤ 0.05). Post-hoc comparisons collapsed across drug treatment groups demonstrated that plasma corticosterone concentrations were significantly elevated in the 30-minute groups relative to the 0-minute and 90-minute groups (p ≤ 0.05). Plasma corticosterone concentrations were significantly lower in the 90-minute groups relative to the 0-minute groups (p ≤ 0.05). No significant effect of drug infusion was observed on plasma corticosterone concentration (main effect of drug treatment, F1,35 = 0.001, ns), and this did not vary across time points (drug treatment × time point interaction, F2,35 = 0.71, ns).
Figure 5.
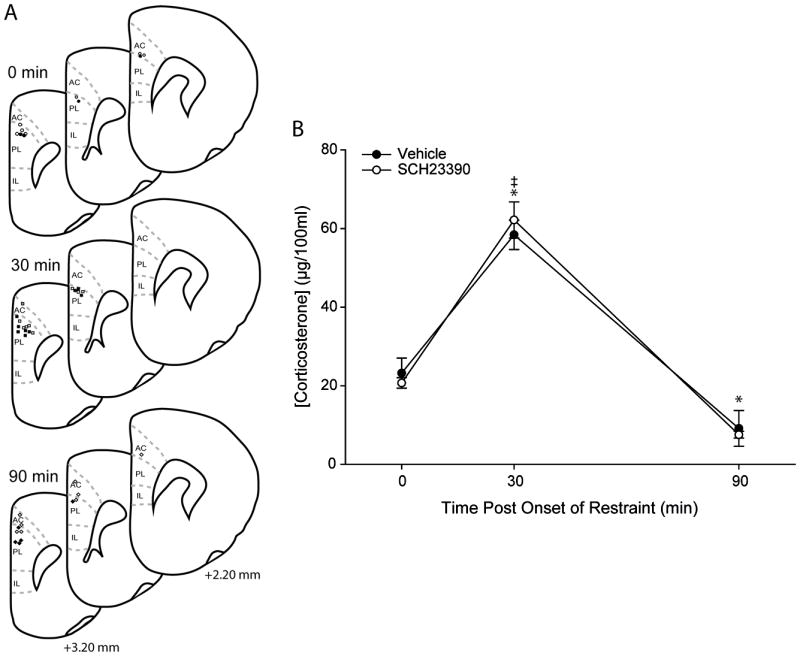
A. Guide cannula placement (based on standard cytoarchitectural criteria; Paxinos and Watson, 1998) in 0-min, 30-min, and 90-min rats receiving either vehicle (open markers) or SCH23390 (filled markers) infusions. All guide cannulae were located near the dorsal border of PL, and infusion cannulae extended 1 mm beyond the tip of the guide cannulae. Although placements were bilateral, for simplicity only one hemisphere is shown. B. Mean plasma corticosterone concentration for animals infused with either vehicle or SCH23390 and perfused either prior to restraint (0 minutes post onset of stress), immediately after cessation of restraint (30 minutes post onset of stress) or one hour after cessation of restraint (90 minutes post onset of stress). SCH23390 infusion into PL did not alter HPA response to acute stress. Vertical bars represent SEMs. *, significantly different from 0-min; ‡, significantly different from 90-min.
4. Discussion
We investigated the role of dopamine D1Rs in stress-induced dendritic remodeling in mPFC of male rats. Our major findings are that, in unstressed rats, daily infusion of the selective D1R antagonist SCH23390 into PL resulted in apical and basilar dendritic retraction in PL; in contrast, intra-PL D1R blockade during 10 days of daily restraint stress prevented stress-induced apical dendritic retraction in PL.
4.1. D1Rs in PL and HPA Axis Function
Consistent with previous findings (e.g., Heiderstadt et al., 2000; Cook and Wellman, 2004; Baran et al., 2009), chronic stress attenuated body weight gain and increased relative adrenal weight. Blockade of D1Rs in PL during repeated restraint stress reduced both of these effects, suggesting that D1 blockade in PL may have reduced the HPA axis response to stress, with the sparing of dendritic morphology being a secondary effect. However, this is unlikely for several reasons. First, even mild, brief stressors produce dendritic retraction (Brown et al., 2005; Izquierdo et al., 2006). Second, the effect of D1 blockade on relative adrenal weight was less pronounced that its effect on body weight, suggesting that the effect of D1 blockade on relative adrenal weight was driven by increased weight gain in stressed SCH23390-infused rats relative to stressed controls. This is consistent with evidence that stress-induced attenuation of weight gain is not mediated by corticosterone (Magariños and McEwen, 1995). Third, in the present study, infusion of SCH23390 into PL did not significantly alter plasma corticosterone concentrations during and after an acute 30-min restraint stressor. Previous studies suggest a role for PL in the inhibition of HPA axis function (e.g., Radley et al., 2006; Radley et al., 2008). Our findings suggest that D1Rs may not contribute to the role of PL in HPA axis negative feedback (though this may be due to the bilateral nature of our manipulation; see Sullivan, 2004). Instead, the prevention of dendritic retraction could be responsible for the attenuation of the stress effect on weight; i.e., the sparing of dendritic morphology may have preserved the inhibitory input of PL on structures responsible for the stress-induced weight loss. If this were the case, the physiological markers of decreased “stressfulness” of the restraint were more likely an effect of rather than a cause of the morphological changes.
4.2. D1Rs Maintain Normal Prefrontal Dendritic Morphology in Non-stress Conditions
Interestingly, in unstressed rats, 10 days of daily D1R blockade caused apical and basilar dendritic retraction. Thus, appropriate D1R activation in PL may contribute to maintenance of normal dendritic morphology. Previous studies showed that chronic administration of drugs that increase dopaminergic transmission causes dendritic proliferation in PFC (e.g., Robinson and Kolb, 2004; Ball et al., 2010), whereas constitutive D1R knockout resulted in basilar dendritic retraction (Wang et al., 2009). Our data are consistent with these studies, and suggest that prefrontal D1Rs may mediate the dendritic proliferation seen after chronic psychostimulant administration: basal levels of D1R activation in PL may be required to maintain normal dendritic morphology, and increases in D1R activation under non-stress conditions may contribute to dendritic proliferation (but see section 4.3).
4.3. D1Rs Contribute to Stress-Induced Dendritic Remodeling in PL
Chronic stress causes apical dendritic retraction in PL (e.g., Cook and Wellman, 2004; Radley et al., 2004; Hill et al., 2011; Martin and Wellman, 2011). Consistent with this, 10 days of restraint stress caused apical dendritic retraction in PL in both vehicle-infused and no-surgery control rats. Administration of the D1R antagonist SCH23390 during restraint stress prevented this retraction, suggesting that D1R activation during stress contributes to the stress-induced apical dendritic retraction. This finding is surprising, as chronic administration of psychostimulants results in dendritic proliferation (Robinson and Kolb, 2004). However, the contrasting results could be due to a number of factors, including systemic administration versus localized infusion—perhaps the psychostimulant-induced mPFC proliferation is due to changes in activity of afferents to mPFC rather than a direct effect on mPFC. Alternatively, given that stress-induced dendritic retraction can be prevented by blocking either glucocorticoid (Liu and Aghajanian, 2008), NMDA (Martin and Wellman, 2011), or D1Rs (present study), stress-induced dendritic remodeling in mPFC may depend on activation of these systems in concert. Similarly, dopamine-induced proliferation may depend on activation of several subtypes of dopamine receptors. We blocked only the D1-family receptors, so our study may not reflect the effect of global changes in dopaminergic transmission.
On the other hand, chronic stress reduces baseline dopamine levels in mPFC (Gresch et al., 1994; Ramkumar et al., 2012). Blockade of D1Rs during stress may prevent stress-induced downregulation of dopamine release in mPFC. Our data from unstressed rats suggest that D1R activation is necessary for dendritic maintenance; thus, preventing downregulation of baseline dopamine release in mPFC could prevent stress-induced dendritic retraction. This is consistent with the recent finding that intracranial self-stimulation of the ventral tegmental area after stress restores baseline levels of mPFC dopamine and apical dendritic morphology (Ramkumar et al., 2012). Future studies measuring baseline levels of dopamine in mPFC could determine whether D1R blockade in PL during stress prevents stress-induced downregulation of dopamine release in mPFC.
A final alternative is that an inverted U-shaped relationship between D1R activation in mPFC and dendritic retraction, with either insufficient or excessive receptor activation causing dendritic retraction. This inverted U-shaped relationship is well documented for D1R activation and working memory tasks (e.g., Zahrt et al., 1997; Lidow et al., 2003; Cools and D’Esposito, 2011; Dent and Neill, 2012). Indeed, consistent with the notion of a U-shaped relationship, studies have shown both that application of a D1 receptor agonist potentiates NMDA receptor responses (Tseng and O’Donnell, 2004; Seong and Carter, 2012), and that feedforward cAMP-protein kinase A-calcium-protein kinase C signaling impairs working memory and weakens network connectivity in mPFC (e.g., Birnbaum et al., 2004; Arnsten, 2009; Arnsten et al., 2012). Indeed, inhibition of protein kinase C signaling prior to daily restraint stress prevents both dendritic spine loss and working memory impairment (Hains et al., 2009). Thus, D1R blockade in unstressed rats may cause insufficient D1R activation and result in dendritic retraction. Dopamine release in mPFC is increased during stress (Abercrombie et al., 1989), which may cause excessive D1R activation and result in dendritic retraction, as well as impairment of working memory (Mizoguchi et al., 2000; Hains et al., 2009; Mika et al., 2012). D1R blockade during stress may prevent stress-induced increases in dopaminergic transmission, normalizing D1R activation and preventing dendritic retraction.
Note, however, that SCH23390 blocks the D1 family of receptors; thus, it is possible that the present results are due at least in part to blockade of D5 receptors. Indeed, D5 receptors likely influence internal calcium stores and protein kinase C signaling (Paspalas and Goldman-Rakic, 2004). SCH23390 is also an agonist at 5HT1C and 5HT2C receptors, albeit at lower affinity than for the D1-family receptors (e.g., Wamsley et al., 1991; Millan et al., 2001). Given the role of serotonin in the stress response, we cannot rule out a role for altered in serotonergic transmission in our results.
Interestingly, a similar inverted U-shaped relationship exists between glucocorticoid activity and both working memory (Bardgett et al., 1994; Mizoguchi et al., 2004; Roozendaal et al., 2004) and mPFC dendritic remodeling (Wellman, 2001; Cerqueira et al., 2007). In fact, stress-induced changes in D1Rs in mPFC may contribute to stress-induced deficits in spatial working memory (Mizoguchi et al., 2000), and glucocorticoid effects on dopamine target neurons contribute to at least some of the behavioral effects of chronic psychosocial stress. For instance, selective inactivation of glucocorticoid receptors on dopaminoceptive neurons prevents the effects of chronic social defeat on social aversion (Barik et al., 2013). Further, we have previously demonstrated that NMDA receptor blockade during daily restraint stress also prevents prefrontal dendritic retraction (Martin and Wellman, 2011). Given the interactions between glucocorticoids and D1Rs (reviewed in Sinclair et al., 2014) and dopamine’s modulation of NMDA receptor-mediated currents via D1Rs (reviewed in Tritsch and Sabatini, 2012), it is interesting to speculate that D1Rs may be the final pathway by which stress-induced increases in glucocorticoids alter both dendritic morphology in mPFC and prefrontally mediated behaviors.
Highlights.
We blocked D1 receptors in prelimbic cortex in unstressed and stressed rats
In unstressed rats, 10 days of daily D1 receptor blockade caused dendritic retraction
D1 receptor blockade during daily restraint prevented stress-induced dendritic retraction
Prelimbic D1 receptor blockade did not alter corticosterone release in response to acute restraint
Prefrontal D1 receptors bidirectionally regulate dendritic morphology
Acknowledgments
This research was supported by National Institutes of Health grant MHR03087794 to C.L.W. and by Indiana University.
Role of the Funding Source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Contributors
G. Lin, C. Borders, and C. Wellman contributed to the conception and design of the study and analysis and interpretation of the data; G. Lin, C. Borders, L. Lundewall, and C. Wellman contributed substantially to data acquisition and drafting and revising the article. All authors have approved the final article.
Conflict of Interest
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Miller BR, Rebec GV. Electrophysiological and structural alterations in striatum associated with behavioral sensitization to (±)3,4-methylenedioxymethamphetamine (Ectasy) in rats: role of drug context. Neuroscience. 2010;171:794–811. doi: 10.1016/j.neuroscience.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Taylor GT, Csernansky JG, Newcomer JW, Nock B. Chronic corticosterone treatment impairs spontaneous alternation behavior in rats. Behav Neural Biol. 1994;61:186–190. doi: 10.1016/s0163-1047(05)80074-3. [DOI] [PubMed] [Google Scholar]
- Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin J-P, Mombereau C, Faure P, Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- Berman KF, Illowsky BP, Weinberger DR. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. IV. Further evidence for regional and behavioral specificity. Arch Gen Psychiat. 1988;45:616–622. doi: 10.1001/archpsyc.1988.01800310020002. [DOI] [PubMed] [Google Scholar]
- Berman KF, Weinberger DR, Shelton RC, Zec RF. A relationship between anatomical and physiological brain pathology in schizophrenia: lateral cerebral ventricular size predicts cortical blood flow. Am J Psychiatr. 1987;144:1277–1282. doi: 10.1176/ajp.144.10.1277. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AFT. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science (New York, N Y ) 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiat. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Depression. In: Brown GW, Harris TO, editors. Life Events and Illness. Guilford; New York: 1989. pp. 49–93. [Google Scholar]
- Brown SM, Henning S, Wellman CL. Short-term, mild stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Cerqueira J, Taipa R, Uylings HBM, Almeida OFX, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17:1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, Hu XP, Mayberg HS, Nemeroff CB, Kilts CD. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological medicine. 2013;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatr. 2011;69:e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent MF, Neill DB. Dose-dependent effects of prefrontal dopamine on behavioral state in rats. Behav Neurosci. 2012;126:620–639. doi: 10.1037/a0029640. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- Drevets W, Videen T, Price J, Preskorn S, Carmichael S, Raichle M. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Sayed JA, Underwood AR, Wellman CL. Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiol Learn Mem. 2010;94:240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiat. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high-quality Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem. 1994;63:575–583. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MAT, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AFT. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. PNAS. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D, Strauss J, Kokes R, Ritzier B, Gift T. Life events and psychopathology severity among first psychiatric admissions. J Abnormal Psychol. 1980;89:165–180. doi: 10.1037//0021-843x.89.2.165. [DOI] [PubMed] [Google Scholar]
- Hays WL. Statistics. 5. Harcourt Brace; Fort Worth, TX: 1994. [Google Scholar]
- Heiderstadt KM, McLaughlin RM, Wright DC, Walker SE, Gomez-Sanchez CE. The effect of chronic food and water restriction on open-field behaviour and serum corticosterone in rats. Lab Anim. 2000;34:20–28. doi: 10.1258/002367700780578028. [DOI] [PubMed] [Google Scholar]
- Hikind M, Maroun M. Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol Learn Mem. 2008;90:217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, McEwen BS. Alterations in Corticolimbic Dendritic Morphology and Emotional Behavior in Cannabinoid CB1 Receptor–Deficient Mice Parallel the Effects of Chronic Stress. Cereb Cortex. 2011;21:2056–2064. doi: 10.1093/cercor/bhq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Simpson E, Kellendork C, Kandel ER. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. PNAS. 2004;101:3236–3241. doi: 10.1073/pnas.0308280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Rapid dendritic retraction in medial prefrontal neurons and impaired fear extinction following exposure to uncontrollable stress. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff JP, Hirsch SR, Gaind R, Rohde PD, Stevens BS. Life events and maintenance therapy in schizophrenic relapse. Br J Psychiatry. 1973;123:659–660. doi: 10.1192/bjp.123.6.659. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Koh P-O, Arnsten AFT. D1 dopamine receptors in the mouse prefrontal cortex: immunocytochemical and cognitive neuropharmacological analyses. Synapse. 2003;47:101–108. doi: 10.1002/syn.10143. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatr. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R-J, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí O, Gavalda A, Gomez F, Armario A. Direct evidence for chronic stress-induced facilitation of the adrenocorticotropin response to a novel acute stressor. Neuroendocrinology. 1994;60:1–7. doi: 10.1159/000126713. [DOI] [PubMed] [Google Scholar]
- Martin KP, Wellman CL. NMDA Receptor Blockade Alters Stress-Induced Dendritic Remodeling in Medial Prefrontal Cortex. Cereb Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika A, Mazur GJ, Hoffman AN, Talboom JS, Bimonte-Nelson HA, Sanabria F, Conrad CD. Chronic stress impairs prefrontal cortex-dependent response inhibition and spatial working memory. Behav Neurosci. 2012;126:605–619. doi: 10.1037/a0029642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatr. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M, Newman-Tancredi A, Quentric Y, Cussac D. The “selective” dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacol. 2001;156:58–62. doi: 10.1007/s002130100742. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci. 2004;24:5492–5499. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui D-H, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Microdomains for Dopamine Volume Neurotransmission in Primate Prefrontal Cortex. J Neurosci. 2004;24:5292–5300. doi: 10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar K, Srikumar BN, Venkatasubramanian D, Siva R, Shankaranarayana Rao BS, Raju TR. Reversal of stress-induced dendritic atrophy in the prefrontal cortex by intracranial self-stimulation. J Neural Transmission. 2012;119:533–543. doi: 10.1007/s00702-011-0740-4. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Maroun M. Stress and amygdala suppression of metaplasticity in the medial prefrontal cortex. Cereb Cortex. 2010;20:2433–2441. doi: 10.1093/cercor/bhp311. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong HJ, Carter AG. D1 Receptor Modulation of Action Potential Firing in a Subpopulation of Layer 5 Pyramidal Neurons in the Prefrontal Cortex. J Neurosci. 2012;32:10516–10521. doi: 10.1523/JNEUROSCI.1367-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D, Purves-Tyson T, Allen K, Weickert C. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacol. 2014;231:1581–1599. doi: 10.1007/s00213-013-3415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7:131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic Modulation of Synaptic Transmission in Cortex and Striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng K, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress. J Comp Neurol. 2002;445:293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, Hunt ME, McQuade RD, Alburges ME. [3H]SCH39166, a D1 dopamine receptor antagonist: binding characteristics and localization. Exp Neurol. 1991;111:145–151. doi: 10.1016/0014-4886(91)90001-s. [DOI] [PubMed] [Google Scholar]
- Wang H-D, Stanwood GD, Grandy DK, Deutch AY. Dystrophic dendrites in prefrontal cortical pyramidal cells of dopamine D1 and D2 but not D4 receptor knockout mice. Brain Res. 2009;1300:58–64. doi: 10.1016/j.brainres.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiat. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: Areal and laminar structure. In: Paxinos G, editor. The rat nervous system. Academic Press; Sydney: 1985. pp. 375–415. [Google Scholar]


