Abstract
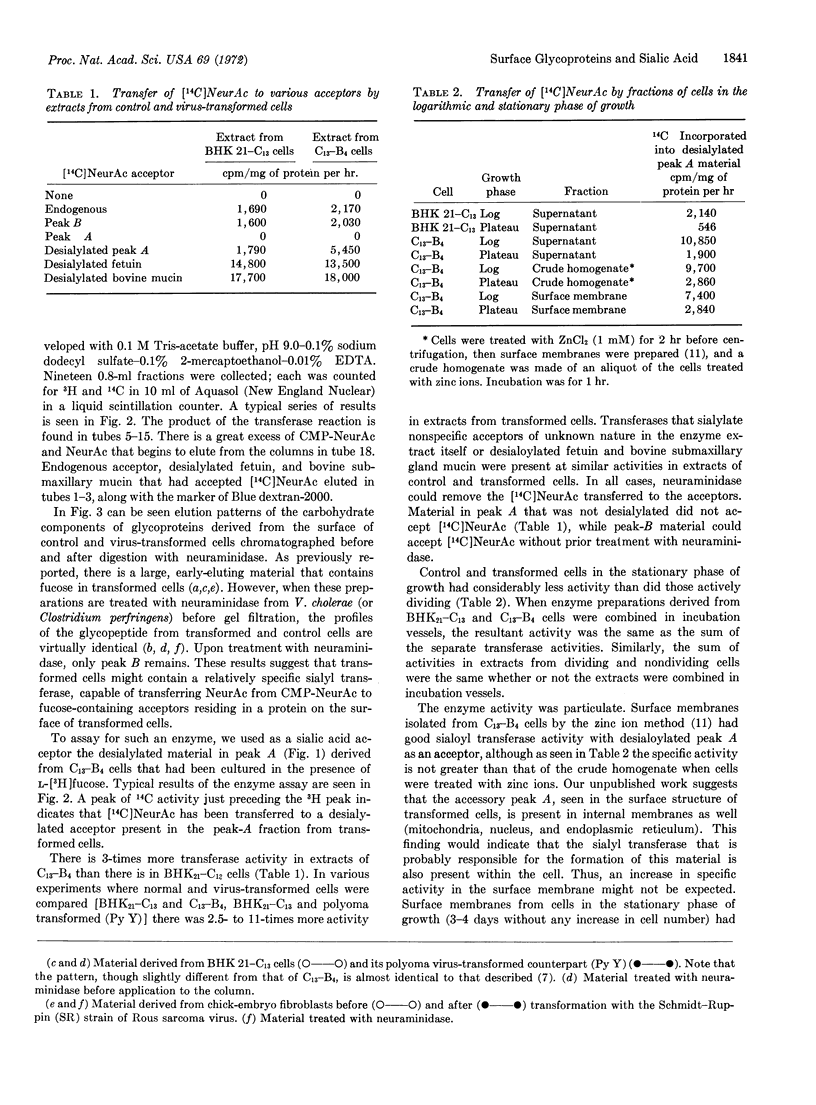
The pattern of elution from a column of Sephadex G-50 of a fucose-labeled carbohydrate component derived from the surface glycoproteins of transformed cells can be altered to resemble that from untransformed cells by enzymatic removal of the sialic acid. These results indicate that the consistent differences found between control and virus-transformed cells may depend upon a relatively specific sialyl transferase that is found in greater amounts (2.5- to 11-times) in transformed cells than in control cells, and in dividing cells as compared to nondividing cells.
Keywords: fucose-labeled surface components, malignant cells, temperature-sensitive oncogenic virus mutants, hamster, N-acetylneuraminic acid, chick
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosmann H. B., Hagopian A., Eylar E. H. Membrane glycoprotein biosynthesis: changes in levels of glycosyl transferases in fibroblasts transformed by oncogenic viruses. J Cell Physiol. 1968 Oct;72(2):81–88. doi: 10.1002/jcp.1040720202. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. Effect of growth on the glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1971 May 25;10(11):2176–2180. doi: 10.1021/bi00787a034. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. Glycopeptides from the surface of control and virus-transformed cells. Science. 1971 Apr 9;172(3979):169–171. doi: 10.1126/science.172.3979.169. [DOI] [PubMed] [Google Scholar]
- Cumar F. A., Brady R. O., Kolodny E. H., McFarland V. W., Mora P. T. Enzymatic block in the synthesis of gangliosides in DNA virus-transformed tumorigenic mouse cell lines. Proc Natl Acad Sci U S A. 1970 Oct;67(2):757–764. doi: 10.1073/pnas.67.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den H., Schultz A. M., Basu M., Roseman S. Glycosyltransferase activities in normal and polyoma-transformed BHK cells. J Biol Chem. 1971 Apr 25;246(8):2721–2723. [PubMed] [Google Scholar]
- Grimes W. J. Sialic acid transferases and sialic acid levels in normal and transformed cells. Biochemistry. 1970 Dec 22;9(26):5083–5092. doi: 10.1021/bi00828a007. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Meezan E., Wu H. C., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969 Jun;8(6):2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- Onodera K., Sheinin R. Macromolecular glucosamine-containing component of the surface of cultivated mouse cells. J Cell Sci. 1970 Sep;7(2):337–355. doi: 10.1242/jcs.7.2.337. [DOI] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971 Aug 10;246(15):4825–4833. [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J Cell Biol. 1971 Nov;51(21):536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin R., Onodera K. Studies on the biochemical properties of surface component of normal and SV-40 transformed 3T3 mouse cells. Can J Biochem. 1970 Aug;48(8):851–857. doi: 10.1139/o70-134. [DOI] [PubMed] [Google Scholar]
- Walborg E. F., Jr, Lantz R. S., Wray V. P. Isolation and chemical characterization of a cell-surface sialoglycopeptide fraction from novikoff ascites cells. Cancer Res. 1969 Nov;29(11):2034–2038. [PubMed] [Google Scholar]
- Warren L., Critchley D., Macpherson I. Surface glycoproteins and glycolipids of chicken embryo cells transformed by a temperature-sensitive mutant of Rous sarcoma virus. Nature. 1972 Feb 4;235(5336):275–278. doi: 10.1038/235275a0. [DOI] [PubMed] [Google Scholar]


