Abstract
Cholesterol is a risk factor for breast cancer although the mechanisms by which this occurs are not well understood. One hypothesis is that dyslipidemia results in increased cholesterol content in cell membranes thus impacting membrane fluidity and subsequent signaling. Additionally, studies demonstrate that the metabolite, 27-hydroxycholesterol (27HC), can function as an estrogen, increasing the proliferation of estrogen receptor positive breast cancer cells. This was unexpected as 27HC and other oxysterols activate the liver X receptors resulting in the reduction of intracellular cholesterol. Resolution of this paradox will require a dissection of the molecular mechanisms by which ER and LXR converge in breast cancer cells. Regardless, the observation that 27HC influences breast cancer provides rationale for strategies that target cholesterol metabolism.
Breast Cancer Risk Factors
Breast cancer continues to be the most commonly diagnosed cancer in women, and is the second highest cause of cancer deaths [1]. Thus, notwithstanding the importance of developing new and effective therapeutics, there is significant interest in defining breast cancer risk factors and exploiting this information to develop chemopreventative and lifestyle modification strategies that can help reduce the burden of this disease. Among the best-studied risk factors for breast cancer are loss of function mutations in the gene coding for the tumor suppressor proteins BRCA1 and BRCA2 (breast cancer 1 and 2, early onset), and while they account for less than 10% of cases, their identification has had a significant impact on patient care [2]. Less well defined, although supported by a wealth of epidemiological data, are other risk factors, primarily associated with the development of estrogen receptor (ER) positive breast cancers, such as early thelarche, early menarche, and later age of first pregnancy, all of which may be related to increased exposure to estrogens [3]. More recently, however, obesity and the metabolic syndrome have emerged as particularly important risk factors for breast cancer, an association of significance given the current obesity epidemic [4, 5]. Although the mechanisms linking obesity and breast cancer are complex, it is likely that increases in circulating insulin and insulin-like growth factors, local production of estrogens in adipose tissue, and the influence of adipokines and inflammatory cytokines, are involved in disease pathogenesis [6]. Interestingly, there is a wealth of data linking these factors to the estrogen receptor (ER), and thus it is not surprising that obesity-related breast cancer is most prevalent in postmenopausal women, where ER-positive tumors are most common [4]. Finally, it has also become apparent that elevated cholesterol, LDL- and VLDL-cholesterol being a comorbidity of obesity [7–10], may be an independent risk factor for breast cancer, and it is only relatively recently that the mechanisms underlying this pathology have been defined.
Cholesterol as a Breast Cancer Risk Factor
One of the first observations linking cholesterol and cancer was made in 1909 in a study which noted the presence of crystals of a “fatty nature” in tumor sections prepared without alcohol fixation [11]. However, over a hundred years later the cause and effect relationships between cholesterol and increased cancer risk remain unclear. This issue has been addressed in a large number of retrospective clinical studies although these have yielded equivocal results, with many finding no relationship, some indicating a protective effect, while others implicating cholesterol as a significant risk factor [12]. Some of these discrepancies may relate to the differences in the impact of cholesterol on different subtypes of cancer; a possibility that needs to be explored further. Among the most interesting results are those from a recent cohort study in which it was demonstrated that patients with established breast cancer had higher LDL-cholesterol and VLDL-cholesterol, although no association with HDL or total cholesterol and breast cancer was evident [13]. It was also demonstrated, in another study, that when adjusted for obesity, dietary consumption of cholesterol was strongly associated with increased breast cancer risk in postmenopausal but not in premenopausal women [14]. These observations have been corroborated by other epidemiological studies and a large prospective study that suggest a link between dietary cholesterol consumption and breast cancer risk [15, 16]. Of note, elevated cholesterol has also been associated with other cancers, such as prostate cancer [17].
Statins (see glossary) are a class of drugs that inhibit HMG-CoA reductase (HMGCR), the rate-limiting enzyme in cholesterol biosynthesis, and thus lower the de novo synthesis of cholesterol. These drugs are widely used in the treatment of hypercholesterolemia and although their impact on breast cancer incidence has been investigated, no clear relationships have emerged [18]. Indeed, a recent meta-analyses suggested that statins can have either a positive or a negative effect on breast cancer incidence [19]. However, given the patient demographics of most of the studies where statins have been evaluated (patients at risk for cardiovascular disease, better access to healthcare, more likely to be screened) and considering that breast cancer was not a primary endpoint in any of these studies it is not clear if a significant impact on breast cancer risk would be observable. A placebo-controlled study evaluating the impact of statins on cancer incidence in women who are at elevated risk of ER-positive breast cancer is definitely warranted. Indeed, this type of study should model that of the Selective Estrogen Receptor Modulator (SERM) prevention trials for tamoxifen and raloxifene, in which the chemoprotective activity of these drugs were evaluated in women at high risk of breast cancer. There are compelling data, however, that statin use is associated with a decreased risk of recurrence in patients with breast cancer [20–22]. The reader is directed to a recently published perspective that addresses this issue and which concludes that much of the noted discrepancy can be attributed to differences in the pharmacological activities of the statins been studied. These authors build a very compelling argument for future studies in breast cancer patients using simvastatin (Zocor) over other statins; a position that is supported by a considerable amount of clinical and preclinical data [23].
Cholesterol is implicated as a breast cancer risk factor in animal models
It was first noted in the early 1950s that obesity and elevated total cholesterol increase tumor incidence in mouse models of breast cancer [24]. More recently, however, it was shown that a high fat, high cholesterol (HFHC, Western) diet decreases the latency and increases the growth and metastasis of tumors in the murine MMTV-PyMT model of mammary cancer [25]. MMTV-PyMT mice are transgenic for the viral polyoma middle T-antigen under the control of the mouse mammary tumor virus (MMTV) promoter. They develop spontaneous tumors of the mammary gland and are a model of luminal ER+ cancer making them an appropriate choice for the study of the impact of obesity on breast cancer [26, 27]. The role of dyslipidemia in breast cancer growth and metastasis has also been explored in severely hypercholesterolemic mice lacking apolipoprotein E (ApoE−/−), a protein essential for the transport of lipoprotein particles and therefore lipid homeostasis. It was shown that mammary tumors grew at a significantly enhanced rate and exhibited increased metastasis, when propagated in ApoE−/− mice fed a HFHC diet, compared to their wild type counterparts [28]. MMTV-PyMT mice deficient in adiponectin, an adipose derived insulin sensitizing hormone, on a HFHC diet, also exhibit increased tumor growth [29]. However, in these studies a diet high in both fat and cholesterol was used making it difficult to assess the specific contributions of cholesterol on tumor growth and metastasis. To address this issue, the impact of elevated cholesterol on breast tumor pathogenesis was recently evaluated in the MMTV-PyMT mouse model [30]. In this study, it was noted that a diet high in cholesterol but normal in fat content (HCD) significantly decreased tumor latency and increased tumor growth, supporting the idea that cholesterol itself can impact tumor pathophysiology. Furthermore, using a humanized transgenic mouse model, where the mouse ApoE gene was replaced with a human homologue, ApoE3 [31], thus recapitulating high-fat diet (HFD) induced hypercholesterolemia seen in humans, statin treatment blocked de novo cholesterol synthesis and attenuated tumor growth [30]. Further, it was also demonstrated that the effects of a HFHC diet on the growth of breast cancer xenografts, in mice, was completely negated after administration of the intestinal cholesterol uptake inhibitor, Ezetimibe [32]. Together these studies implicate cholesterol as a pathogenic agent in breast cancer and suggest that at least some of the impact of HFD on breast cancer risk can be attributed to cholesterol.
Mechanisms underlying the pathological actions of cholesterol in breast cancer
Free cholesterol in most cells is maintained at a very constant level by a series of homeostatic processes that regulate (a) partitioning into the plasma and endoplasmic membranes, (b) efflux, uptake, and de novo synthesis, and (c) esterification by acyl-CoA:cholesterol acyltransferase (ACAT) [33]. One of the key regulators of intracellular cholesterol levels is Sterol Regulatory Element Binding Protein-2 (SREBP2), a transcription factor that functions as a sensor for cholesterol and whose activity is negatively regulated by free cholesterol [34]. SREBP2 is maintained in an inactive state as part of a large multiprotein complex associated with the endoplasmic reticulum. When cholesterol levels fall, the integrity of this complex is disturbed, and SREBP2, facilitated by the chaperone SREBP cleavage activating protein (SCAP), migrates to the Golgi where it undergoes a series of proteolytic processing events that results in its activation. Upon entering the nucleus, SREBP2 upregulates the expression of genes responsible for cholesterol synthesis such as HMGCR and for cholesterol import (i.e. low density lipoprotein receptor, LDLR) [34–36]. In addition to SREBP2, the liver X receptors (LXRs) are also involved in maintaining intracellular cholesterol homeostasis. The LXRs are members of the nuclear receptor superfamily of ligand-regulated transcription factors whose activity is positively regulated by oxysterol ligands that are derived from cholesterol within cells [37]. LXR activation returns cells to normo-cholesterol conditions by increasing the expression of genes (A) regulating cholesterol uptake such as inducible degrader of the low-density lipoprotein receptor (IDOL) which is an E3 ubiquitin ligase that triggers the lysosomal degradation of LDLR, and (B) involved in cholesterol reverse transport; ATP-binding cassette subfamily A1 (ABCA1) and ATP-binding cassette subfamily G member 1 (ABCG1) [38, 39].
Given the complexity and redundancy of the mechanisms that regulate intracellular cholesterol homeostasis, it has been difficult to understand how increases in circulating cholesterol can impact cancer pathogenesis. However, it is clear that under conditions of high cholesterol demand, as occurs during rapid proliferation, cells must be able to disengage the processes that function to maintain cholesterol homeostasis. Insight into how this may occur has come from studies of cholesterol biology in activated T lymphocytes (T-cells), cells crucial in immunity. Specifically, it has been demonstrated that activation of the T-cell receptor (TCR), results in increased expression of SULT2B1, an enzyme that sulfates and inactivates the oxysterol ligands of LXR. The resultant loss of LXRs activity enables the cells to accumulate the cholesterol needed for new membrane synthesis [40]. It will be interesting to see whether breast cancer cells, or cancer cells in general, have adopted a similar mechanism to accumulate the cholesterol needed for cell proliferation. Along these lines, it is important to note that agonist-activated ER inhibits LXR mediated gene transcription with a particularly striking effect on ABCA1 expression in ER+ breast cancer cells [30]. It will be important to elucidate the mechanisms that enable this cross-talk between the ER and LXR and determine how cholesterol homeostasis is disrupted in rapidly proliferating breast cancer cells.
Whereas it is clear that cholesterol is required for membrane synthesis in dividing cells and may be a limiting factor, it is unlikely that this activity per se is pathogenic (Figure 1). However, there is some evidence that in knockout mice lacking homeostatic control of cholesterol (LXR knockout mice), high cholesterol diets can lead to prostatic intra-epithelial neoplasia secondary to increases in the expression of the methyl transferase EZH2 and subsequent down–regulation of tumor suppressors [41]. A HFHC diet was used in these studies making it difficult to attribute the carcinogenic activities noted to cholesterol. On the other hand, there are data suggesting that increased cholesterol content alters the biophysical properties of membranes, facilitating the formation of lipid rafts and increasing the activity of signaling events that initiate at the membrane.
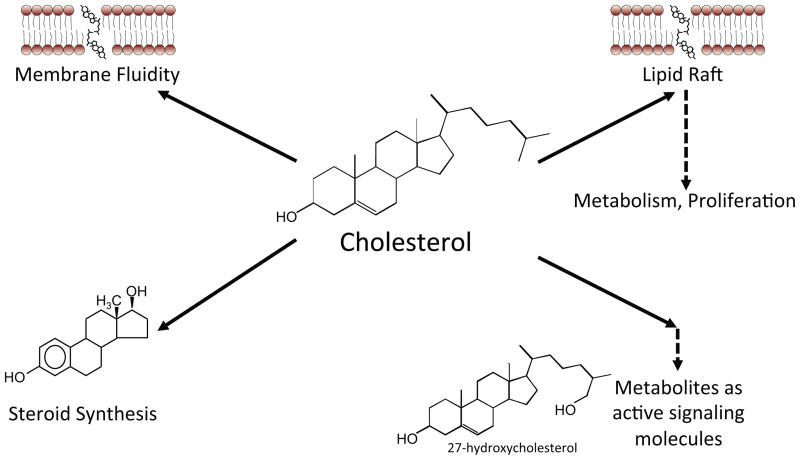
Figure 1. Potential mechanisms by which cholesterol impacts breast cancer physiology.
Cholesterol is central to multiple key cellular functions. It is a required component of membrane synthesis and may become a limiting agent in rapidly dividing cancer cells. Cholesterol is also required for lipid raft formation and subsequent signaling such as AKT signaling. Cholesterol is also the precursor for steroid synthesis. Recently, it has been shown that cholesterol metabolites such as 27-hydroxycholesterol have the capacity to signal through the estrogen receptor (ER) or liver x receptor (LXR), impacting breast cancer pathophysiology.
Indeed, an analysis of the molecular mechanisms involved in tumor growth of the above mentioned ApoE−/− mice fed a HFHC diet, revealed increased PI3K activation and AKT phosphorylation [28]. Furthermore, treatment of these mice with the PI3K inhibitor, BKM120, attenuated breast tumor growth, implicating the PI3K/AKT signaling pathway in the pathological actions of cholesterol in tumors. However, the relevance of this finding is unclear, given that the plasma cholesterol levels in this mouse model exceed 2000mg/dL, far greater than what would be considered “hypercholesterolemic” in humans (~240 mg/dL). It has also been demonstrated in vitro that the level of exogenous cholesterol needed to stimulate cell proliferation is far lower than that required for lipid raft formation and AKT phosphorylation [28]. These latter data would argue against the hypothesis that cholesterol impacts tumor pathology by increasing lipid raft formation and membrane signaling, and are more compatible with the idea that cholesterol, or a derivative, is functioning as a signaling molecule in cancer cells. Along these lines, the cholesterol metabolite 27-hydroxycholesterol (27HC) becomes relevant, as it was found to act as an endogenous ER modulator (SERM) as well as an LXR agonist [42, 43]. Specifically, while 27HC functions as an antagonist blocking ER activation in the cardiovascular system, it acts as an agonist, and activates ER in osteoblasts and ER-positive breast cancer [42–46]. Therefore, it is reasonable to expect that some of the pathogenic actions of cholesterol in ER-positive tumors may require its conversion to 27HC.
27-hydroxycholesterol and breast cancer
The synthesis of 27HC from cholesterol is catalyzed by the enzyme CYP27A1, in what is called the ‘alternative’ or ‘acidic’ pathway for bile acid synthesis. It has been observed, both in humans and in animals, that the circulating levels of 27HC closely mirror those of cholesterol, and that hypercholesterolemia results in commensurately high levels of 27HC [47]. It has also been demonstrated that 27HC is an LXR agonist and as such serves to limit cholesterol accumulation in cells [45, 48, 49]. Further, there are data suggesting that this oxysterol can inhibit HMGCR activity in an indirect fashion, although the mechanism behind this activity is unclear [50]. Based on this established biology it was expected that 27HC would inhibit the growth of cancer cells, as has been observed for other LXR agonists [51–53]. However, recent studies have shown that 27HC actually promotes the proliferation of ER-positive breast cancer cell lines, in vitro [42, 54]. This paradox was somewhat resolved by the finding that 27HC can function as an ER-agonist in the cellular models of breast cancer examined. It was also demonstrated that activated ER could, by an as yet to be determined mechanism, inhibit LXR signaling, thus negating the protective effects of LXR [30].
The pro-tumor effects of 27HC have now been confirmed in several preclinical in vivo models of breast cancer, including MCF7 cell derived xenografts, and MMTV-PyMT transgenic mice [30, 55]. The ability to reverse the growth promoting effects of 27HC by co-administration of an ER-antagonist confirmed that ER is the primary target of this oxysterol, in tumors. Notable also is the observation that in the MMTV-PyMT mouse model, deletion of the enzyme responsible for the catabolism of 27HC (CYP7B1−/−) increased circulating levels of 27HC, and this was associated with increased tumor growth in the CYP7B1−/− mice [30]. The data suggest that cholesterol needs to be converted to its metabolite 27HC, in order to have an effect on tumor growth. This hypothesis was confirmed in the MMTV-PyMT mouse model by showing that although a HCD increased mammary tumor growth, it was without effect in this mouse model when CYP27A1, the enzyme responsible for the conversion of cholesterol to 27HC was ablated [30].
Oxysterols have also been shown to activate the hedgehog pathway by binding to the oncoprotein smoothened [56]. This may have direct effects on cancer cells, although the impact of oxysterols on breast cancer via this pathway has not yet been formally tested. While activation of the LXRs by oxysterols would reduce cholesterol content of cells and thus reduces cellular proliferation of cancer cells, it has been found that in dendritic cells, LXR activation inhibits the expression of chemokine receptor 7 (CCR7) thereby dampening their antitumor activity [57]. Thus, 27HC is likely to influence tumor biology by allowing cancer cells to escape host immune surveillance and through its ability to activate ER.
27HC as an autocrine/paracrine modulator of tumor growth
It is clear that the circulating levels of 27HC mirror those of cholesterol, suggesting that strategies to lower cholesterol will have a commensurate effect on 27HC [47]. However, it has also become apparent that the development of approaches to mitigate the impact of 27HC on tumor pathogenesis will also have to address how best to reduce local production of this oxysterol. It is likely that the majority of circulating 27HC is derived from the liver, a tissue where CYP27A1 is highly expressed. However, this enzyme is also highly expressed in macrophages [58], and most importantly, it is abundantly expressed in tumor associated macrophages in breast cancer [30]. It is well established that breast tumor infiltration by macrophages is associated with a poor prognosis [59]. Therefore, while macrophages contribute to the pathophysiology of breast cancer in several ways, it is possible that they also provide a local source of 27HC which might activate ER in these cells, and bypass the need for the synthesis of 17β-estradiol. In addition to macrophages, cancer cells themselves, can also express CYP27A1 [30]. Importantly, the expression level of the CYP27A1 enzyme is associated with the grade of the tumor; the higher the expression, the more likely that the biopsied tumor will be classified as a higher grade [30]. Conversely, expression of the 27HC catabolic enzyme Cyp7b1, is decreased in breast cancer, compared to normal tissue [55]. Furthermore, Cyp7b1 expression is associated with an increased time to relapse in patients with the luminal A subtype of breast cancer [30, 55]. This indicates that 27HC metabolism (both anabolism and catabolism), and resulting local concentrations of 27HC play important roles in breast tumor pathophysiology. It is significant, therefore, that human breast cancer tumors have been shown to have significantly higher 27HC concentrations than normal breast tissue [55]. These data highlight the likely importance of both circulating and intra-tumoral 27HC in ER-positive breast cancers.
Linking obesity, high fat diet, 27HC and cancer biology
As mentioned, obesity is a risk factor for several types of cancer, and this association is particularly apparent when looking at the incidence of ER-positive breast cancers in post-menopausal women [4, 5]. While several biochemical mechanisms have been identified that link obesity and breast cancer [6], the specific contribution of cholesterol, a comorbidity of obesity, in cancer pathogenesis, has been underappreciated. We believe that this may be due in part to the fact that mice do not exhibit elevated cholesterol in response to a HFD. To address this, ApoE3 humanized mice were fed a HFD and circulating cholesterol levels were measured [30]. Unlike previous studies that compared mice on a low fat low cholesterol diet to mice on a HFHC diet, this study controlled for cholesterol content thereby isolating the effects of fat on tumor pathophysiology. Thus, the observed increase in circulating cholesterol in HFD fed mice result from increased de novo synthesis. Importantly, the effect of a HFD on tumor growth was attenuated by statin treatment or by inhibition of CYP27A1 [30]. These observations confirm the importance of cholesterol and dyslipidemia in breast cancer, and highlight the importance of 27HC, and ER, as a mediator of these effects (Figure 2).
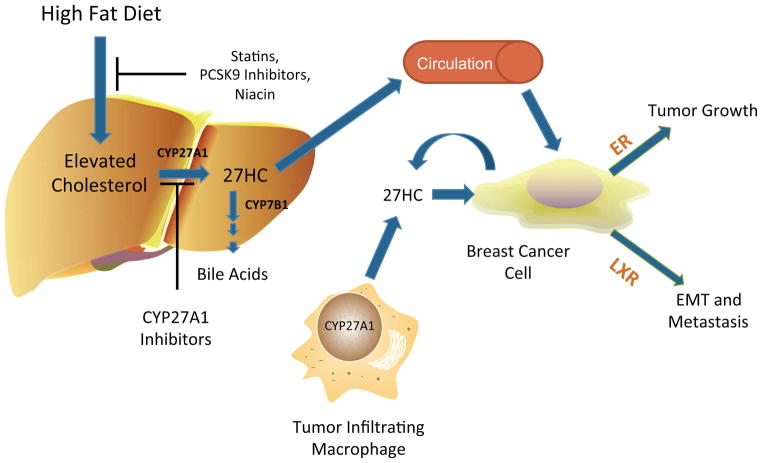
Figure 2. 27-hydroxycholesterol biochemically links obesity and elevated cholesterol to breast cancer.
Obesity leads to elevated cholesterol synthesis, primarily in the liver. This increase can be inhibited by HMGCoAR inhibitors (statins), PCSK9 inhibitors, or niacin. Cholesterol can be catabolized by CYP27A1 into 27-hydroxycholesterol (27HC). High CYP27A1 expression is found in the liver, tumor infiltrating macrophages, or the breast cancer cells themselves. Thus 27HC can originate from an endocrine, paracrine or autocrine source. The enzyme which catabolizes 27HC, CYP7B1, is also expressed in breast cancer cells, and its expression is associated with an increased relapse free survival of breast cancer patients. 27HC acts on the estrogen receptors (ERs) to drive breast cancer tumor growth and the liver X receptors (LXRs) to influence metastasis.
27-hydroxycholesterol and breast cancer metastasis
Several epidemiological studies have identified a positive association between either obesity or cholesterol, and breast cancer metastases (relapse-free survival) [60, 61]. Based on the ability of 27HC to activate the ER and promote tumor growth an obvious hypothesis is that the effects of cholesterol on metastasis were mediated in a similar manner. However, when investigating the effects of 27HC on metastasis, it was determined that the ER ligand, 17β-estradiol did not increase the metastatic tumor burden in the MMTV-PyMT mouse model [30]. While several mechanisms may be at play, such as increased lipid raft formation and subsequent downstream signaling, it also appears that LXR activation may be important. In support of this idea are data indicating that treatment of MMTV-PyMT mice with the synthetic LXR agonist, GW3965, also increased metastatic burden [30]. However, GW3965 was far less robust in promoting metastasis, compared to 27HC. This unexpected finding suggests that 27HC and synthetic LXR agonists may be selective LXR modulators, eliciting unique gene transcriptional profiles. This would be similar to the well-established actions of SERMs and the selective androgen receptor modulators. Alternatively, 27HC may signal through an as yet unidentified receptor. In support of the latter hypothesis is the observation that various oxysterols can facilitate the migration of cells within the myeloid lineage via a CXCR2 (chemokine receptor 2) mediated mechanism [62]. Studies determining whether CXCR2 is a bona fide target of 27HC, and whether 27HC interacts with any other receptors are warranted. Regardless, LXR activation itself does have the capacity to induce genes and morphological changes associated with the epithelial to mesenchymal transition (EMT), a typical requirement for cancer cells to metastasize [30]. As LXR activation has been reported to have antimetastatic activities in models of melanoma [51], it is especially important that the downstream mechanisms by which LXR induces EMT and promotes metastases in breast cancer are further investigated. Finally, as cholesterol and 27HC are produced locally, the impact of cholesterol and/or 27HC on the tumor microenvironment requires evaluation.
Concluding remarks and future perspectives
In addition to increased insulin, IGFs, adipokines and local production of estrogens, there is clear evidence to support an independent role for cholesterol as a mediator of the effects of dyslipidemia and/or obesity on breast cancer pathogenesis. One major mechanism by which cholesterol imparts its effects on breast cancer is via its metabolite, 27HC, a molecule with SERM activity. Of the oxysterols, 27HC circulates at the highest concentrations. However, it has been shown that other oxysterols such as 25-hydroxychoelsterol can also activate ER and stimulate the proliferation of ER+ breast cancer cells [63]. Although the circulating concentrations of oxysterols other than 27HC are low, their production in the local tumor microenvironment has not yet been studied and their roles on breast cancer pathogenesis remain to be determined.
To date, studies of 27HC and breast cancer have largely been limited to murine models. However, as discussed above, it has been shown that CYP27A1 expression is associated with tumor grade, and CYP7B1 expression is associated with increased relapse free survival. Further, 27HC levels are elevated in breast tumor biopsies compared to normal breast tissue. Therefore, it is highly likely that 27HC plays a significant role in human disease. Moving forward, it will be very important to determine if associations exist between 27HC and breast cancer risk and prognosis; a study which is currently underway. Regardless, recent work on this axis highlights the immediate therapeutic potential of modulating cholesterol, either by diet or by chemical interventions such as statins, PCSK9 inhibitors, or niacin. In this regard, it has been shown that the statin pravastatin significantly reduces circulating 27HC [64]. The effect of statins on circulating concentrations of 27HC in breast cancer patients as well as intra-tumoral concentrations are important studies to consider. Finally, controlled studies of the efficacy of statins in preventing breast cancer targeted to at risk women are warranted, as is an examination of the potential role of CYP27A1 inhibitors as breast cancer therapeutics.
BOX 1. OUTSTANDING QUESTIONS.
Do cholesterol lowering therapy (for example statins) result in decreased risk of breast cancer in at risk populations?
What impact do other oxysterols have on breast cancer?
How does 27HC circumvent the cholesterol homeostatic and thus anti-proliferative properties of LXR activation in breast cancer cells?
Would CYP27A1 inhibitors be effective anti-breast cancer therapeutics?
Highlights.
Elevated cholesterol is associated with breast cancer risk
Cholesterol promotes breast tumor growth and metastasis in murine models
Cholesterol metabolite acts as an estrogen receptor agonist to promote tumor growth
Novel strategies to target cholesterol/oxysterol metabolism
Acknowledgments
This work was supported by: Department of Defense Idea Expansion Award W81XWH-13-1-0366 (to D.P.M.), National Cancer Institute of the National Institutes of Health K99CA172357 (to E.R.N.) and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health R37DK048807 (to D.P.M.).
GLOSSARY
- 27-hydroxycholesterol (27HC)
a metabolite of cholesterol in the alternate or acidic bile acid synthesis pathway. 27HC is a ligand for the ERs and LXRs
- Apolipoprotein E
a protein involved in the transport of lipoproteins and cholesterol in the blood and lymphatic systems. In mice there is only one allele (ApoE). In humans, the most common allele is ApoE3. Unlike wildtype mice, mice lacking APOE or transgenic for the human APOE3 develop hypercholesterolemia when fed a high fat normal cholesterol diet
- ATP-binding cassette subfamily A1 (ABCA1) and ATP-binding cassette subfamily G member 1 (ABCG1)
proteins involved in the reverse transport of cholesterol
- CYP27A1
cytochrome P450, family 27, subfamily A, polypeptide 1, also known as 27-hydroxylase, is the first enzyme in the acidic bile acid synthesis pathway and converts cholesterol into 27-hydroxycholesterol
- CYP7B1
cytochrome P450, family 7, subfamily B, polypeptide 1 is an enzyme responsible for the catabolism of 27HC
- Estrogen receptor (ER)
a member of the nuclear receptor family of ligand inducible transcription factors. Activation of ERs stimulate the proliferation of ER-positive breast cancer
- ER-positive breast cancer
estrogen receptor positive breast cancer comprises the 65–80% of all breast cancer cases. The majority of postmenopausal women with breast cancer present with ER-positive disease
- HMG-CoA reductase
is the rate limiting enzyme in the synthesis of cholesterol, converting 3-hydroxy-3-methylglutaryl-CoA to mevalonic acid. Statins inhibit this enzyme and effectively reduce circulating cholesterol
- Liver X Receptor (LXR)
a member of the nuclear receptor family of ligand inducible transcription factors, are responsible for homeostatic regulation of cholesterol levels
- Low-Density Lipoprotein Receptor (LDLR)
responsible for the endocytosis of cholesterol associated with LDL
- MMTV-PyMT mouse model
these transgenic mice express the transforming oncogene polyoma middle T (PyMT) antigen under the control of the murine mammary tumor virus long terminal repeat promoter, which specifically directs expression to the mammary epithelium. Mice spontaneously develop mammary gland tumors, that closely recapitulate human breast cancer progression
- Oxysterol
a hydroxylated cholesterol. These chemicals play important roles in cholesterol homeostasis by activating the LXRs. Some oxysterols such as 27HC also activate the ER
- Selective Estrogen Receptor Modulator (SERM)
Chemicals such as tamoxifen or 27-hydroxycholesterol that have the ability to exert differential actions on the ER in a tissue and context specific manner. Tamoxifen has been extensively used to antagonize the ER and thereby treat breast cancer
- Statins
orally administered inhibitors of HMG-CoA reductase, effectively reducing circulating cholesterol
- Sterol Regulatory Element Binding Protein-2 (SREBP2)
under low cholesterol levels this transcription factor initiates the expression of several genes involved in cholesterol synthesis and import
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2011–2012. Atlanta: American Cancer Society, Inc; [Google Scholar]
- 2.Shah R, Rosso K, Nathanson SD. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World journal of clinical oncology. 2014;5:283–298. doi: 10.5306/wjco.v5.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petracci E, Decarli A, Schairer C, Pfeiffer RM, Pee D, Masala G, Gail MH. Risk factor modification and projections of absolute breast cancer risk. J Natl Cancer Inst. 2011;103:1037–1048. doi: 10.1093/jnci/djr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 5.Capasso I, Esposito E, Pentimalli F, Crispo A, Montella M, Grimaldi M, Giordano A. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol Ther. 2011;10:1240–1243. doi: 10.4161/cbt.10.12.13473. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 7.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 8.Howard BV, Ruotolo G, Robbins DC. Obesity and dyslipidemia. Endocrinology and metabolism clinics of North America. 2003;32:855–867. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 9.Centers fo Disease Control and Prevention. Prevalence of Abnormal Lipid Levels Among Youths- United States, 1999–2006. MMWR. 2010;59:29–33. [PubMed] [Google Scholar]
- 10.Gostynski M, Gutzwiller F, Kuulasmaa K, Doring A, Ferrario M, Grafnetter D, Pajak A. Analysis of the relationship between total cholesterol, age, body mass index among males and females in the WHO MONICA Project. Int J Obes Relat Metab Disord. 2004;28:1082–1090. doi: 10.1038/sj.ijo.0802714. [DOI] [PubMed] [Google Scholar]
- 11.White CP. On the occurrence of crystals in tumors. Journal of Pathology and Bacteriology. 1909;13:3–10. [Google Scholar]
- 12.Danilo C, Frank PG. Cholesterol and breast cancer development. Current Opinion in Pharmacology. 2012;12:677–682. doi: 10.1016/j.coph.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Laisupasin P, Thompat W, Sukarayodhin S, Sornprom A, Sudjaroen Y. Comparison of Serum Lipid Profiles between Normal Controls and Breast Cancer Patients. Journal of laboratory physicians. 2013;5:38–41. doi: 10.4103/0974-2727.115934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L. Dietary cholesterol intake and cancer. Ann Oncol. 2012;23:491–500. doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- 15.Ronco AL, De Stefani E, Stoll M. Hormonal and metabolic modulation through nutrition: towards a primary prevention of breast cancer. Breast. 2010;19:322–332. doi: 10.1016/j.breast.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Boussac H, Pommier AJ, Dufour J, Trousson A, Caira F, Volle DH, Lobaccaro JM. LXR, prostate cancer and cholesterol: the Good, the Bad and the Ugly. American journal of cancer research. 2013;3:58–69. [PMC free article] [PubMed] [Google Scholar]
- 18.Gazzerro P, Proto MC, Gangemi G, Malfitano AM, Ciaglia E, Pisanti S, Bifulco M. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev. 2012;64:102–146. doi: 10.1124/pr.111.004994. [DOI] [PubMed] [Google Scholar]
- 19.Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat. 2012;135:261–269. doi: 10.1007/s10549-012-2154-x. [DOI] [PubMed] [Google Scholar]
- 20.Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Lash TL. Statin Prescriptions and Breast Cancer Recurrence Risk: A Danish Nationwide Prospective Cohort Study. J Natl Cancer Inst. 2011 doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109:573–579. doi: 10.1007/s10549-007-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 23.Ahern TP, Lash TL, Damkier P, Christiansen PM, Cronin-Fenton DP. Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncol. 2014;15:e461–468. doi: 10.1016/S1470-2045(14)70119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waxler SH, Tabar P, Melcher LR. Obesity and the time of appearance of spontaneous mammary carcinoma in C3H mice. Cancer Res. 1953;13:276–278. [PubMed] [Google Scholar]
- 25.Llaverias G, Danilo C, Mercier I, Daumer K, Capozza F, Williams TM, Frank PG. Role of cholesterol in the development and progression of breast cancer. Am J Pathol. 2011;178:402–412. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, MacLeod CL. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- 27.Pfefferle AD, Herschkowitz JI, Usary J, Harrell JC, Spike BT, Adams JR, Perou CM. Transcriptomic classification of genetically engineered mouse models of breast cancer identifies human subtype counterparts. Genome biology. 2013;14:R125. doi: 10.1186/gb-2013-14-11-r125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alikhani N, Ferguson RD, Novosyadlyy R, Gallagher EJ, Scheinman EJ, Yakar S, Leroith D. Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model. Oncogene. 2013;32:961–967. doi: 10.1038/onc.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Xu A, Lam KS, Wong NS, Chen J, Shepherd PR, Wang Y. Cholesterol-induced mammary tumorigenesis is enhanced by adiponectin deficiency: role of LDL receptor upregulation. Oncotarget. 2013;4:1804–1818. doi: 10.18632/oncotarget.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 32.Pelton K, Coticchia CM, Curatolo AS, Schaffner CP, Zurakowski D, Solomon KR, Moses MA. Hypercholesterolemia Induces Angiogenesis and Accelerates Growth of Breast Tumors in Vivo. The American journal of pathology. 2014;184:2099–2110. doi: 10.1016/j.ajpath.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das A, Brown MS, Anderson DD, Goldstein JL, Radhakrishnan A. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife. 2014:e02882. doi: 10.7554/eLife.02882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua X, Wu J, Goldstein JL, Brown MS, Hobbs HH. Structure of the human gene encoding sterol regulatory element binding protein-1 (SREBF1) and localization of SREBF1 and SREBF2 to chromosomes 17p11.2 and 22q13. Genomics. 1995;25:667–673. doi: 10.1016/0888-7543(95)80009-b. [DOI] [PubMed] [Google Scholar]
- 35.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- 36.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci U S A. 2007;104:6511–6518. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szanto A, Benko S, Szatmari I, Balint BL, Furtos I, Ruhl R, Nagy L. Transcriptional regulation of human CYP27 integrates retinoid, peroxisome proliferator-activated receptor, and liver X receptor signaling in macrophages. Molecular and cellular biology. 2004;24:8154–8166. doi: 10.1128/MCB.24.18.8154-8166.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Reue K, Fong LG, Young SG, Tontonoz P. Feedback regulation of cholesterol uptake by the LXR-IDOL-LDLR axis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2541–2546. doi: 10.1161/ATVBAHA.112.250571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pommier AJ, Dufour J, Alves G, Viennois E, De Boussac H, Trousson A, Baron S. Liver x receptors protect from development of prostatic intra-epithelial neoplasia in mice. PLoS genetics. 2013;9:e1003483. doi: 10.1371/journal.pgen.1003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 44.DuSell CD, Nelson ER, Wang X, Abdo J, Modder UI, Umetani M, McDonnell DP. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology. 2010;151:3675–3685. doi: 10.1210/en.2010-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson ER, Dusell CD, Wang X, Howe MK, Evans G, Michalek RD, McDonnell DP. The oxysterol, 27-hydroxycholesterol, links cholesterol metabolism to bone homeostasis through its actions on the estrogen and liver x receptors. Endocrinology. 2011;152:4691–4705. doi: 10.1210/en.2011-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Umetani M, Ghosh P, Ishikawa T, Umetani J, Ahmed M, Mineo C, Shaul PW. The Cholesterol Metabolite 27-Hydroxycholesterol Promotes Atherosclerosis via Proinflammatory Processes Mediated by Estrogen Receptor Alpha. Cell Metab. 2014;20:172–182. doi: 10.1016/j.cmet.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karuna R, Holleboom AG, Motazacker MM, Kuivenhoven JA, Frikke-Schmidt R, Tybjaerg-Hansen A, Rentsch KM. Plasma levels of 27-hydroxycholesterol in humans and mice with monogenic disturbances of high density lipoprotein metabolism. Atherosclerosis. 2011;214:448–455. doi: 10.1016/j.atherosclerosis.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 48.Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 49.Song C, Liao S. Cholestenoic acid is a naturally occurring ligand for liver X receptor alpha. Endocrinology. 2000;141:4180–4184. doi: 10.1210/endo.141.11.7772. [DOI] [PubMed] [Google Scholar]
- 50.Corsini A, Maggi FM, Catapano AL. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacological research : the official journal of the Italian Pharmacological Society. 1995;31:9–27. doi: 10.1016/1043-6618(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 51.Pencheva N, Buss CG, Posada J, Merghoub T, Tavazoie SF. Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell. 2014;156:986–1001. doi: 10.1016/j.cell.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 52.Vedin LL, Lewandowski SA, Parini P, Gustafsson JA, Steffensen KR. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30:575–579. doi: 10.1093/carcin/bgp029. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Jiang H, Zhang J, Zhang Y, Liu A, Zhao Y, Yuan X. Liver X receptor activation induces apoptosis of melanoma cell through caspase pathway. Cancer cell international. 2014;14:16. doi: 10.1186/1475-2867-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cruz P, Torres C, Ramirez ME, Epunan MJ, Valladares LE, Sierralta WD. Proliferation of human mammary cancer cells exposed to 27-hydroxycholesterol. Experimental and therapeutic medicine. 2010;1:531–536. doi: 10.3892/etm_00000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Shaul PW. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell reports. 2013;5:637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nature chemical biology. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, Russo V. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 58.Hansson M, Ellis E, Hunt MC, Schmitz G, Babiker A. Marked induction of sterol 27-hydroxylase activity and mRNA levels during differentiation of human cultured monocytes into macrophages. Biochim Biophys Acta. 2003;1593:283–289. doi: 10.1016/s0167-4889(02)00398-1. [DOI] [PubMed] [Google Scholar]
- 59.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Bahl M, Ennis M, Tannock IF, Hux JE, Pritchard KI, Koo J, Goodwin PJ. Serum lipids and outcome of early-stage breast cancer: results of a prospective cohort study. Breast Cancer Res Treat. 2005;94:135–144. doi: 10.1007/s10549-005-6654-9. [DOI] [PubMed] [Google Scholar]
- 61.Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24:2506–2514. doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, Russo V. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210:1711–1728. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lappano R, Recchia AG, De Francesco EM, Angelone T, Cerra MC, Picard D, Maggiolini M. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor alpha-mediated signaling in cancer cells and in cardiomyocytes. PLoS One. 2011;6:e16631. doi: 10.1371/journal.pone.0016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thelen KM, Lutjohann D, Vesalainen R, Janatuinen T, Knuuti J, von Bergmann K, Laaksonen R. Effect of pravastatin on plasma sterols and oxysterols in men. European journal of clinical pharmacology. 2006;62:9–14. doi: 10.1007/s00228-005-0068-9. [DOI] [PubMed] [Google Scholar]