Abstract
Studies of hemodialysis patients have shown a U-shaped association between systolic blood pressure (SBP) and mortality. These studies have largely relied on dialysis-unit SBP measures and have not evaluated whether this U-shape also exists in advanced chronic kidney disease (CKD), prior to starting hemodialysis. We determined the association between SBP and mortality at advanced CKD and again after initiation of hemodialysis. This was a prospective study of Chronic Renal Insufficiency Cohort (CRIC) participants with advanced CKD followed through initiation of hemodialysis. We studied the association between SBP and mortality when participants: 1) had an estimated glomerular filtration rate <30 ml/min/1.73m2 (N=1,705); 2) initiated hemodialysis and had dialysis-unit SBP measures (N=403) and; 3) initiated hemodialysis and had out-of-dialysis-unit SBP measured at a CRIC study visit (N=326). Cox models adjusted for demographics, cardiovascular risk factors and dialysis parameters. A quadratic term for SBP was included to test for a U-shaped association. At advanced CKD, there was no association between SBP and mortality (HR 1.02 [95% CI: 0.98–1.07] per every 10 mm Hg increase). Among participants who started hemodialysis, a U-shaped association between dialysis-unit SBP and mortality was observed. In contrast, there was a linear association between out-of-dialysis-unit SBP and mortality (HR 1.26 [95% CI: 1.14–1.40] per every 10 mm Hg increase). In conclusion, more efforts should be made to obtain out-of-dialysis-unit SBP which may merit more consideration as a target for clinical management and in interventional trials.
Keywords: CKD, hypertension, dialysis, mortality, ESRD
INTRODUCTION
Hypertension is very common in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) on hemodialysis.1, 2 While there is considerable information on the association of higher systolic blood pressure (SBP) and progression of CKD,3–6 less is known about the association of blood pressure with mortality. Previous studies have suggested a U-shaped association between SBP and risk of mortality in moderate stages of CKD,7, 8 however less is known about these relationships among patients with advanced stages of CKD, when estimated glomerular filtration rate (eGFR) is <30 ml/min/1.73 m2, which is a particularly high-risk subgroup of the overall CKD population.
Furthermore, numerous studies have also reported a U-shaped association between SBP and risk of mortality in hemodialysis patients, with higher mortality associated with both lower (even within the “normal” range) and very high blood pressures.9–13 A limitation of these investigations has been reliance on SBP measured in the dialysis-unit. Prior single-center studies have suggested that the setting of SBP measurement (dialysis-unit vs. out-of-dialysis-unit) may impact the measurement and associated outcomes of SBP. Consequently, national and international guidelines have concluded that “there is uncertainty about how to measure blood pressure in hemodialysis patients and a poor understanding of the association between blood pressure and risk of adverse outcomes”2 and “it is unclear which blood pressure reading should be used as the guide for therapy” for dialysis patients.14
Thus, there remains uncertainty on SBP targets in patient with advanced CKD and those on hemodialysis, particularly in relation to important outcomes such as mortality. A long-term, multi-center, observational study of men and women with chronic kidney disease, the Chronic Renal Insufficiency Cohort (CRIC) Study, provided the opportunity to study the association of SBP and all-cause mortality among participants with advanced stages of CKD followed longitudinally through the initiation of hemodialysis. The goal of this study was to: (1) better delineate the shapes and strengths of the association of SBP with mortality at eGFR<30 ml/min/1.73 m2 (but not on dialysis) and; (2) to compare the association between dialysis-unit SBP vs. out-of-dialysis-unit SBP with mortality among the participants who progressed to ESRD and initiated hemodialysis.
METHODS
Study Population
We studied participants of the Chronic Renal Insufficiency Cohort (CRIC) Study. The CRIC Study is a multi-center prospective observational cohort15–17 study which enrolled participants age 21 to 74 years with estimated glomerular filtration rate (eGFR) 20–70 ml/min/1.73m2 by the MDRD equation.18 Exclusion criteria included New York Heart Association Class III or IV heart failure and severe liver disease. Study participants were followed annually in-person and with six-month interim telephone calls.
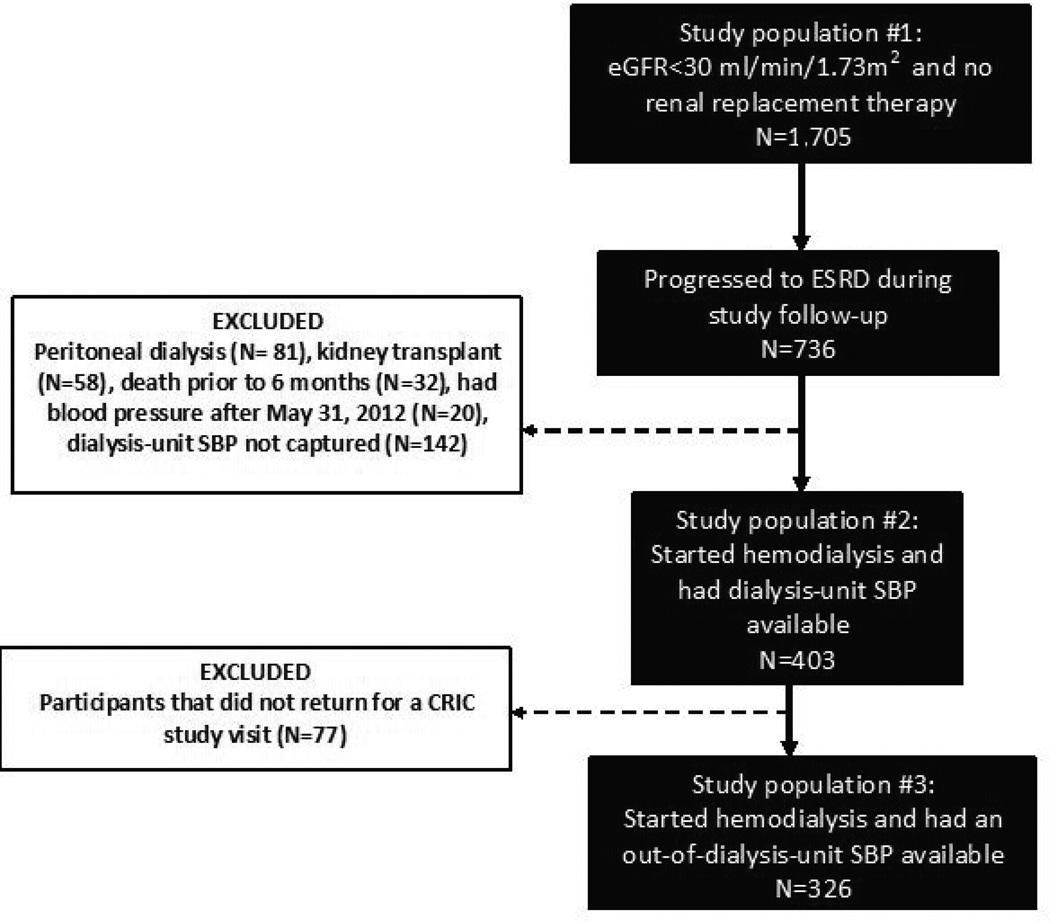
We studied three nested subgroups of CRIC participants (Figure 1). We first examined those who had stage 4 CKD, eGFR<30 ml/min/1.73 m2, at or after enrollment into CRIC (but before May 31, 2012) and available study visit SBP (N=1,705) (Figure 1 study population #1). Second, we examined the subgroup of the N=1,705 who subsequently progressed to require hemodialysis by May 31, 2012 and had dialysis-unit SBP measurements available (N=403) (Figure 1 study population #2). Third, we examined the subgroup of the N=403 who additionally had out-of-dialysis-unit SBP measurements from a CRIC Study clinical visit after initiation of hemodialysis (N=326) (Figure 1 study population #3).
Figure 1.
Derivation of study population
Predictors
We selected a priori systolic blood pressure (SBP) as the predictor variable.
Systolic blood pressure at eGFR<30 ml/min/1.73 m2
We first used the systolic blood pressure (SBP) obtained from the first CRIC study visit with eGFR observed to be <30 ml/min/1.73 m2, the threshold for “advanced CKD” (stage 4 and 5) in recent international guidelines19 (Figure 1; study population #1). SBP was measured by centrally trained staff using a standardized method20. The mean of three seated resting SBP readings was measured and used to define the advanced CKD SBP for our study.
Systolic blood pressure after initiation of hemodialysis
Dialysis-unit SBP
For CRIC participants who started maintenance hemodialysis, study personnel obtained records from each patient’s dialysis-unit approximately 6 months after hemodialysis initiation (Figure 1; study population #2). SBP measurements were recorded at the start of each hemodialysis session. The median number of dialysis-unit SBP records was 4 (IQR 3–4), observed over a median time period of 7 days (IQR 5–9 days). The mean of the SBP measurements obtained from these dialysis-unit records was used to define “dialysis-unit SBP” in our study.
Out-of-dialysis-unit SBP
We used mean SBP obtained at the first CRIC research study visit after initiation of maintenance hemodialysis, which was measured using the same CRIC protocol used in advanced CKD patients. Among the 403 participants with advanced CKD who started hemodialysis, we analyzed SBP in 326 (81%) who returned for their first CRIC study visit after ESRD (“out-of-dialysis-unit” SBP) (Figure 1; study population #3).
Outcome
All-cause mortality was the primary outcome. Deaths were identified through report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and linkage with the Social Security Mortality Master File through May 31, 2012. For participants with eGFR<30 ml/min/1.73 m2, all-cause mortality included both deaths prior to and after ESRD. Participants were censored on May 31, 2012 for this report.
Covariates
History of cardiovascular disease and medication use were self-reported annually. All covariates were obtained from the same study visit as the SBP measurement of interest. For the analyses with dialysis-unit SBP, covariates were obtained from the closest study visit prior to the SBP measurement.
For the participants who subsequently started hemodialysis, selected measurements taken during routine clinical care including dose of dialysis (Kt/V), serum albumin and hemoglobin level were also abstracted from dialysis-unit records. CRIC protocol also calls for performance of research echocardiograms after incident ESRD, which was done in 218 of the 403 (54%) participants who had a dialysis-unit SBP.21–23 The echocardiograms were performed at the same study visit as the out of dialysis-unit SBP measurement (e.g. the first study visit after starting hemodialysis) in 164 out of the 218 (75%) participants.
Statistical methods
We examined the association of SBP and mortality in the three nested groups (Figure 1 study populations #1–#3): participants with SBP measured at eGFR<30 ml/min/1.73 m2, participants who initiated hemodialysis with dialysis-unit SBP measures and those additionally with out-of-dialysis-unit SBP measures. We began by performing multivariable Cox proportional hazard models with SBP modeled as a continuous variable. A quadratic term for SBP was included in these Cox models to test for linearity of the association. If the quadratic term was not statistically significant, we quantified and reported the association between SBP and mortality in a linear manner. If the quadratic term was significant, we explored the association between SBP and mortality using adjusted penalized smoothing splines with N0.2 evenly spaced knots among the inner 99% distribution of SBP in Cox models. This allowed us to display the relationship of SBP and mortality without making assumptions about the shape of the relationship. Models were adjusted for age, sex, race/ethnicity, tobacco use, body mass index (BMI), diabetes and history of cardiovascular disease. For those on hemodialysis, we also adjusted for Kt/V, serum albumin and hemoglobin level. In supplementary analysis, SBP values were grouped into quartiles.
We performed several sensitivity analyses. First, we adjusted for use of anti-hypertensive medications (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, β-blockers and diuretics) recorded at the study visit corresponding the SBP measure (or the closest study visit prior to the SBP measure for the dialysis-unit SBP analysis). Second, to explore whether these associations were altered because of attrition of the study population, we repeated the analyses examining associations between dialysis-unit SBP and out-of-dialysis-unit SBP in the subset of participants who had both measurements (N=326). For this analysis, “baseline” for the time-to-event analysis was set to the latter of the two SBP measures. Third, to explore whether the U-shaped association between SBP and mortality may be related to systolic heart failure,24, 25 we repeated the dialysis-unit SBP analysis including only persons with an ejection fraction ≥45% (N=157) after initiation of hemodialysis.
RESULTS
Characteristics of study population
A total of 1,705 CRIC study participants had eGFR of <30 ml/min/1.73m2 at a baseline or follow-up visit (Figure 1; study population #1). The mean (SD) SBP was 131 (±24) mm Hg and mean (SD) eGFR was 25 (±4) ml/min/1.73 m2. Participants with higher SBP were more likely to be Black, had higher levels of proteinuria, more likely to have diabetes and reported use of a greater number of anti-hypertensive medications (Table 1).
Table 1.
Characteristics of study population with advanced CKD (defined as eGFR < 30 ml/min/1.73 m2) by quartile of systolic blood pressure (mm Hg) (N=1705)
| Characteristic | All (N=1705) |
Q1 SBP (<114 mmHg) (N= 433) |
Q2 SBP (114–127 mmHg) (N= 423) |
Q3 SBP (128–145 mmHg) (N= 427) |
Q4 SBP (>145 mmHg) (N= 422) |
p value |
|---|---|---|---|---|---|---|
| Age (years) | 59.4 (11.4) | 58.7 (12.2) | 59.6 (11.6) | 59.2 (11.1) | 60.4 (10.4) | 0.1 |
| Female (%) | 46.7 | 47.6 | 42.3 | 48.2 | 48.8 | 0.2 |
| Race/ethnicity (%) | <0.001 | |||||
| Non-Hispanic White | 35.5 | 50.3 | 44.2 | 30.0 | 17.3 | |
| Non-Hispanic Black | 43.5 | 34.9 | 38.1 | 47.1 | 54.0 | |
| Hispanic | 17.4 | 11.6 | 13.0 | 19.4 | 25.4 | |
| Other | 3.7 | 3.2 | 4.7 | 3.5 | 3.3 | |
| Diastolic blood pressure (mmHg) | 69 (13) | 61 (10) | 67 (11) | 71(12) | 78 (14) | <0.001 |
| Pulse pressure | 68 (12) | 68(12) | 68 (11) | 68 (12) | 69 (12) | 0.6 |
| Body Mass Index (kg/m2) | 32.3 (8.1) | 31.5 (7.6) | 32.9 (8.7) | 33.0 (8.0) | 31.9 (8.0) | 0.01 |
| Estimated Glomerular Filtration Rate (ml/min/1.73 m2) | 25.0 (4.0) | 25.6 (3.7) | 25.9 (3.5) | 24.6 (4.0) | 23.6 (4.5) | <0.001 |
| Proteinuria (mg/24 hour) | 731 (166, 2640) | 238 (74, 960) | 461 (128, 1642) | 974 (282, 3232) | 2192 (630, 4851) | <0.001 |
| Hypertension (%) | 94.5 | 87.8 | 93.1 | 97.2 | 100.0 | <0.001 |
| Diabetes (%) | 60.1 | 45.5 | 55.8 | 67.2 | 72.3 | <0.001 |
| Cardiovascular Disease (%) | 43.8 | 41.8 | 42.3 | 43.3 | 47.6 | 0.3 |
| Peripheral Vascular Disease (%) | 11.1 | 8.1 | 11.4 | 13.1 | 11.9 | 0.1 |
| Congestive Heart Failure (%) | 16.1 | 16.9 | 12.8 | 17.8 | 16.8 | 0.2 |
| Stroke (%) | 12.7 | 12.0 | 12.3 | 11.9 | 14.7 | 0.6 |
| Current Smoker (%) | 14.1 | 14.8 | 12.5 | 13.8 | 15.4 | 0.6 |
| Alcohol Use (%) | 51.7 | 60.3 | 50.4 | 49.6 | 46.2 | <0.001 |
| Blood pressure medication use (%) | 96.1 | 94.7 | 96.9 | 96.0 | 96.7 | 0.3 |
| ACE inhibitor or ARB | 70.2 | 77.6 | 70.7 | 71.4 | 60.7 | <0.001 |
| Calcium channel blocker | 51.5 | 37.4 | 49.9 | 58.6 | 60.7 | <0.001 |
| B-Blocker | 58.6 | 54.7 | 56.7 | 61.1 | 61.9 | 0.0994 |
| Diuretic | 69.9 | 65.8 | 67.4 | 72.4 | 74.2 | 0.0206 |
| # of BP drugs classes | 3.1 (1.5) | 2.9 (1.5) | 3.1 (1.5) | 3.3 (1.5) | 3.3 (1.5) | 0.0001 |
Association of SBP and mortality in participants with eGFR<30 ml/min/1.73 m2
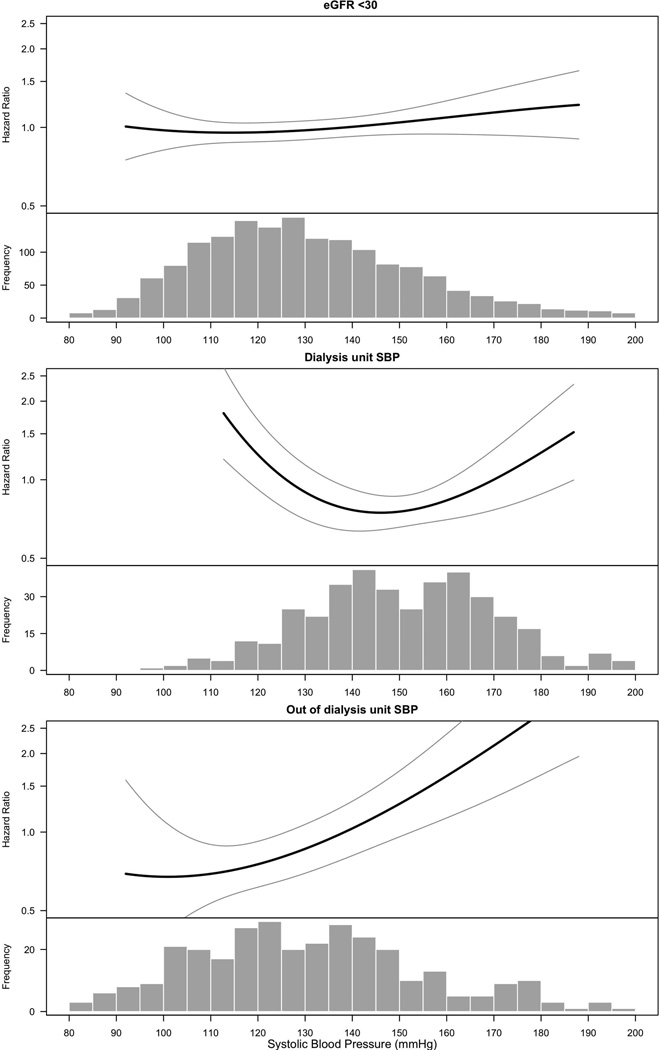
There were a total of 389 deaths during a mean follow-up of 4.74 (±2.31) years among these 1705 participants with eGFR<30 ml/min/1.73 m2 (Supplemental Table S1). There was no independent association between higher SBP and mortality (HR 1.02 [95% CI: 0.98–1.07] per every 10 mm Hg increase, p=0.3) (Figure 2A and Supplemental Table S1). The quadratic term for SBP was not statistically significant.
Figure 2.
Associations of systolic blood pressure (SBP) with mortality
The smooth spline estimates the hazard ratio of all-cause mortality, according to systolic blood pressure (mm Hg) among CRIC participants with SBP measured at (1) eGFR<30 ml/min/1.73 m2 (2) at maintenance hemodialysis by dialysis-unit measurements and (3) at maintenance hemodialysis by out-of-dialysis-unit measurements
All analyses are adjusted for age, gender, race/ethnicity, tobacco use, BMI, diabetes, history of cardiovascular disease. The analyses examining dialysis-unit and out of dialysis-unit systolic blood pressures are also adjusted for Kt/V, serum hemoglobin and serum albumin. Dotted lines represent 95% confidence intervals. Below each spline is the histogram of the distribution of systolic blood pressure to indicate the range of the majority of the data.
Association of dialysis-unit SBP and mortality
Among the 1,705 participants with advanced CKD, 403 subsequently started maintenance hemodialysis (Figure 1; study population #2). The median duration of time between hemodialysis initiation and the dialysis-unit SBP measurement was 188 (IQR 151, 276) days; 81.2% of participants had their dialysis-unit SBP measurement within 1 year of beginning hemodialysis. There were a total of 98 deaths over a mean follow-up of 2.72 (±1.67) years.
In multivariable Cox models, the quadratic term for SBP was statistically significant, suggesting a non-linear association (p<0.05). Spline analyses showed that both low and high ends of the SBP distribution were associated with higher rates of mortality (Figure 2B and Supplemental Table S1).
Association of out-of-dialysis-unit SBP and mortality
Among 403 participants who started hemodialysis, 326 had an out-of-dialysis-unit SBP measurement (Figure 1; study population #3), 82.2% had the measurement taken within 1 year of initiating hemodialysis. Among these participants, 70 deaths were identified over a mean of 2.83 (±1.73) years of follow-up (Supplemental Table S1). The quadratic term for SBP was not statistically significant suggesting a linear association. The spline analyses confirmed this linear association (Figure 2C). In multivariable Cox models, the association between higher SBP and risk of mortality was statistically significant (HR 1.26 [95% CI 1.13–1.39], per every 10 mm Hg increase, p<0.001).
Sensitivity analyses
When we repeated all models also adjusting for anti-hypertensive medication use, similar results were seen (results not shown). When we repeated these analyses among the subset of participants who had both measures of dialysis-unit and out of dialysis-unit SBP measured (median time of 7 [IQR −106, 99] days apart), the results were similar to the main analysis (Supplemental Table S2). Finally, in the subgroup of hemodialysis patients with documented ejection fraction ≥45% (N=157), a U-shaped association between dialysis-unit SBP and risk of mortality remained (Supplemental Figure S1).
DISCUSSION
Our study provides new and important insights into the evolving association between SBP and all-cause mortality as patients progress from advanced CKD to start hemodialysis. We found that among patients with advanced CKD not on dialysis, SBP was not an independent risk factor for all-cause mortality. Among the subset of CRIC Study participants with advanced CKD who subsequently progressed to require hemodialysis, we observed a strong, linear association between out-of-dialysis-unit SBP and risk of all-cause mortality. This was in contrast to the U-shaped association with mortality that was observed when we analyzed dialysis-unit SBP. These findings suggest that out-of-dialysis-unit SBP may be an important measure to guide clinical management of hemodialysis patients.
Our finding of no association of SBP level with mortality in advanced CKD is consistent with a recent meta-analysis of 10 clinical trials which failed to find an association between risk of mortality (RR 0.94, 95% CI 0.84–1.05) among CKD patients treated to “intensive” goals (target approximately SBP 130 mmHg) vs. “standard” goals (target approximately SBP 140 mmHg).6 Our results may appear to contradict a recent large study of U.S. Veterans with moderate to advanced CKD which reported a U-shaped association between SBP (measured as part of routine clinical care) and all-cause mortality.7 However this association was substantially attenuated when baseline SBP rather than time-updated values were considered (the former is less likely to reflect physiology of terminal illness than the latter). Importantly, the increase in risk was primarily observed at SBP levels <110 mmHg or >170 mmHg, levels of blood pressure not often found in the CRIC Study participants we studied. Factors responsible for these more extreme SBP levels may include co-morbid conditions and/or lack of adherence to antihypertensive drug therapy. Within the range of SBP of approximately 110 to 170 mmHg, which encompasses the SBP target choices realistically facing contemporary physicians, there was also little correlation between SBP and risk of mortality in the U.S. Veterans study,7 which is similar to our results. Our findings differ from a recent study also based on U.S. Veterans which found that among patients with prevalent CKD and uncontrolled hypertension, those who had an intensification of their anti-hypertensive medications and a follow-up SBP <120 mm Hg had a higher risk of death compared to those with SBP 120–139 mm Hg.26 However, this study differed from ours in several important ways: participants had uncontrolled blood pressure at cohort entry, while participants in our study had relatively well controlled blood pressure; most patients in this study had moderate CKD with a mean eGFR of 48 ml/min/1.73 m2, while our study only included those with stage 4 and 5 CKD; and finally, this study examined almost exclusively male and predominantly white patients, which was not the case in ours.26 Our results may lend support to the newly revised Eighth Joint National Committee (JNC8) Guidelines for the Management of High Blood Pressure in Adults which did not recommend strict blood pressure control in CKD.27
A U-shaped association between dialysis-unit SBP and risk of mortality had been observed previously, where SBP within the “normal” range and within very high ranges are associated with higher risk of mortality.9–13, 28, 29 These paradoxical observations have led to uncertainty regarding the optimal blood pressure target for hemodialysis patients, since the non-linear association is observed in the range of SBP encompassing plausible treatment targets (for example, SBP 138–166 mm Hg in our study population; Supplemental Table S1). This has led some to question whether target blood pressure among hemodialysis patients should be higher than the conventional goal of 140 mmHg systolic.10, 12, 13, 30
The mechanisms to explain this U-shaped association between dialysis-unit SBP and mortality is unclear. Some have suggested that the U-shape association between SBP and mortality could be explained by the impact of including patients with severe systolic heart failure and low SBP due to low ejection fraction.25 However, this seems like an unlikely explanation since we found this same U-shaped association among participants with preserved ejection fraction. Other previous hypotheses to explain the U-shape have included: survival bias, competitive risk factors, or neurohormonal state unique to hemodialysis patients.13, 31 However, these other contributing factors are also unlikely since we observed a linear association between higher SBP and risk of mortality when out-of-dialysis-unit SBP in these same patients.
We offer an alternative hypothesis to reconcile the U-shape paradox. We hypothesize that among patients on hemodialysis, the ability to mount an elevated blood pressure in response to fluid accumulated between hemodialysis sessions—reflected in the dialysis unit blood pressure documented at the start of each hemodialysis session--is a sign of relative health.
Our data confirm and extend prior single center studies of the importance of setting of SBP measurement among hemodialysis patients. In a study of 150 hemodialysis patients, one standard deviation increase in SBP measured by home ambulatory blood pressure monitoring was associated with a 35% increased risk of mortality, while there was no association between dialysis-unit SBP and mortality.32 Another report based on 326 hemodialysis patients found a very strong association with SBP measured at home and by ambulatory blood pressure monitoring with mortality but no association between dialysis-unit SBP and mortality.29 Our study expands on these results. We studied a large population of hemodialysis patients recruited from multiple centers around the country. We relied on blood pressures that are readily obtained outside the dialysis-unit in a regular office setting, rather than ambulatory blood pressure monitoring which is expensive and difficult to implement.
Our findings have important clinical implications. Despite acknowledgement that “it is unclear which blood pressure reading should be used as the guide for therapy” for dialysis patients, existing guidelines nevertheless suggest dialysis-unit SBP as the target of treatment and recommend a blood pressure goal of <140/90 mm Hg recorded at the start of each hemodialysis session.14 However, our data and others29, 32 strongly suggest that out-of-dialysis-unit SBP may be an important target for treatment in hemodialysis patients. In the absence of information from randomized clinical trials of hemodialysis patients,33 choice of blood pressure targets in this population can only rely on findings from observational studies. We believe that these data should also inform the design of clinical trials of blood pressure control in hemodialysis patients, which have traditionally targeted dialysis-unit SBP.33–35 Currently in clinical practice, many primary care providers and other specialists often defer treatment of blood pressure to the nephrologist; yet non-nephrologists actually observe out-of-dialysis-unit SBP readings whereas most nephrologists typically have access only to dialysis-unit SBP readings. It should be noted that in comparing the dialysis-unit SBP and out-of-dialysis-unit SBP in our study (although these were not taken contemporaneously), the majority of patients with dialysis-unit SBP ≥140 mmHg (144 out of 239 or 60%) actually had out-of-dialysis-unit SBP <140 mmHg.
Our study had several strengths, including a relatively large number of participants with advanced CKD recruited from multiple sites in the U.S. The participants were racially/ethnically diverse and over half had diabetes. SBP was measured in a standardized fashion by centrally trained study staff to minimize bias in ascertainment (vs. in routine clinical care, more ill patients tend to have more health care encounters and more frequently blood pressure readings which can bias associations). The longitudinal study design was unique and we were able to study the evolving associations of SBP with mortality with progression of CKD through ESRD in the same cohort of patients to yield unique insight into how the association between SBP and mortality differs by late stages of CKD. We were able to capture comorbid conditions uniformly using research grade data. We were able to take into account important physiological parameters such as cardiac function using research echocardiograms. Our study had several limitations. SBP was not captured for some study participants who progressed to hemodialysis (e.g. there was missing dialysis-unit SBP documentation in 142 out of 545 eligible patients). We did not have access to out-of-dialysis-unit SBP measures obtained as part of routine clinical care, which may differ from those measured at CRIC study visits. We could not determine cause of death. The number of patients who went on to receive peritoneal dialysis or kidney transplant was too small for analyses of these treatments for ESRD. We could not assess the extent to which our results could be explained by inaccuracies of dialysis-unit SBP readings.36 The majority of participants were taking anti-hypertensive medications; findings may differ in an untreated study population. SBP was relatively well controlled and we only studied those who volunteered to enroll in this prospective cohort study, which may limit generalizability.
PERSPECTIVES
Among a large, diverse well characterized cohort of patients with advanced CKD, SBP was not associated with mortality. Among patients who subsequently initiated hemodialysis, a positive, linear association between out-of-dialysis-unit SBP and a U-shaped association between dialysis-unit SBP and mortality was observed. Greater effort to obtain out-of-dialysis unit SBP in hemodialysis patients should be made and may help guide clinical management.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new
This study suggests that hypertension at advanced kidney disease may not be an important contributor to risk of death.
Low and high blood pressure (e.g. U-shape) measured in the dialysis unit in hemodialysis patients is associated with higher risk of death.
When blood pressure is measured outside of the dialysis unit in these same hemodialysis patients, there is a linear association with higher blood pressure and higher risk of death.
What is relevant
Hypertension is extremely prevalent in kidney disease and there remains uncertainty on how best to manage these high-risk patients.
This study helps inform clinical management of kidney disease patients as well as may guide the design of future clinical trials of blood pressure reduction in kidney disease.
Summary
Greater effort to obtain out-of-dialysis unit blood pressure in hemodialysis patients should be made which may inform clinical practice and future studies.
ACKOWLEGEMENTS
We would like to thank Feng Lin and Cassianne Robinson-Cohen for their statistical support.
SOURCES OF FUNDING
This work was supported by the following grants: K23 DK088865 (Bansal), R01 DK70939 (Hsu), K24 DK92291 (Hsu). Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). This work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003 and K01 DK092353 (Anderson), Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Peralta CA, Hicks LS, Chertow GM, Ayanian JZ, Vittinghoff E, Lin F, Shlipak MG. Control of hypertension in adults with chronic kidney disease in the united states. Hypertension. 2005;45:1119–1124. doi: 10.1161/01.HYP.0000164577.81087.70. [DOI] [PubMed] [Google Scholar]
- 2.Levin NW, Kotanko P, Eckardt KU, Kasiske BL, Chazot C, Cheung AK, Redon J, Wheeler DC, Zoccali C, London GM. Blood pressure in chronic kidney disease stage 5d-report from a kidney disease: Improving global outcomes controversies conference. Kidney Int. 2010;77:273–284. doi: 10.1038/ki.2009.469. [DOI] [PubMed] [Google Scholar]
- 3.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of diet in renal disease study group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 4.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER, 3rd, Norris K, O'Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley-Brown D, Tisher CC, Toto RD, Wright JT, Jr, Xu S. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA. 2001;285:2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 5.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G. Renoprotective properties of ace-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 6.Lv J, Ehteshami P, Sarnak MJ, Tighiouart H, Jun M, Ninomiya T, Foote C, Rodgers A, Zhang H, Wang H, Strippoli GF, Perkovic V. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: A systematic review and meta-analysis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2013;185:949–957. doi: 10.1503/cmaj.121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Bleyer AJ, Molnar MZ, Ma JZ, Sim JJ, Cushman WC, Quarles LD, Kalantar-Zadeh K. Blood pressure and mortality in u.S. Veterans with chronic kidney disease: A cohort study. Ann Intern Med. 2013;159:233–242. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant. 2006;21:1257–1262. doi: 10.1093/ndt/gfk057. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Lacson E, Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48:606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P. "U" curve association of blood pressure and mortality in hemodialysis patients. Medical directors of dialysis clinic, inc. Kidney Int. 1998;54:561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 11.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33:507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 12.Duranti E, Imperiali P, Sasdelli M. Is hypertension a mortality risk factor in dialysis? Kidney Int Suppl. 1996;55:S173–S174. [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 14. http://www.kidney.org/professionals/KDOQI/guidelines_cvd/guide12.htm. [Google Scholar]
- 15.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The chronic renal insufficiency cohort (cric) study: Design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 16.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic renal insufficiency cohort (cric) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP Cric, Groups HCS. Ckd in hispanics: Baseline characteristics from the cric (chronic renal insufficiency cohort) and hispanic-cric studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, de Jong PE, Coresh J, Nahas ME, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: A kdigo controversies conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 20.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O'Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT., Jr Hypertension awareness, treatment, and control in adults with ckd: Results from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: A report from the american society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG Chronic Renal Insufficiency Cohort Study G. Associations between kidney function and subclinical cardiac abnormalities in ckd. J Am Soc Nephrol. 2012;23:1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal N, Keane M, Delafontaine P, Dries D, Foster E, Gadegbeku CA, Go AS, Hamm LL, Kusek JW, Ojo AO, Rahman M, Tao K, Wright JT, Xie D, Hsu CY. A longitudinal study of left ventricular function and structure from ckd to esrd: The cric study. Clin J Am Soc Nephrol. 2013;8:355–362. doi: 10.2215/CJN.06020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foley RN. Cardiac disease in chronic uremia: Can it explain the reverse epidemiology of hypertension and survival in dialysis patients? Semin Dial. 2004;17:275–278. doi: 10.1111/j.0894-0959.2004.17326.x. [DOI] [PubMed] [Google Scholar]
- 25.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. 1996;49:1379–1385. doi: 10.1038/ki.1996.194. [DOI] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Lu JL, Molnar MZ, Ma JZ, Canada RB, Streja E, Kalantar-Zader K, Bleyer AJ. Observational modeling of strict vs conventional blood pressure control in patients with chronic kidney disease. JAMA internal medicine. doi: 10.1001/jamainternmed.2014.3279. ePub August 4, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, Lefevre ML, Mackenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 28.Robinson BM, Tong L, Zhang J, Wolfe RA, Goodkin DA, Greenwood RN, Kerr PG, Morgenstern H, Li Y, Pisoni RL, Saran R, Tentori F, Akizawa T, Fukuhara S, Port FK. Blood pressure levels and mortality risk among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. 2012;82:570–580. doi: 10.1038/ki.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55:762–768. doi: 10.1161/HYPERTENSIONAHA.109.144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, Teehan BP, Levey AS. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 32.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2:1228–1234. doi: 10.2215/CJN.02250507. [DOI] [PubMed] [Google Scholar]
- 33.Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, Gallagher M, Roberts MA, Cass A, Neal B, Perkovic V. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: A systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373:1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tepel M, Hopfenmueller W, Scholze A, Maier A, Zidek W. Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant. 2008;23:3605–3612. doi: 10.1093/ndt/gfn304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gul A, Miskulin D, Gassman J, Harford A, Horowitz B, Chen J, Paine S, Bedrick E, Kusek JW, Unruh M, Zager P. Design of the blood pressure in dialysis pilot study. Am J Med Sci. 2014;347:125–130. doi: 10.1097/MAJ.0b013e31827daee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman M, Griffin V, Kumar A, Manzoor F, Wright JT, Jr, Smith MC. A comparison of standardized versus "usual" blood pressure measurements in hemodialysis patients. Am J Kidney Dis. 2002;39:1226–1230. doi: 10.1053/ajkd.2002.33395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.