Abstract
Associations between ultraviolet radiation (UVR) exposure and non-Hodgkin lymphoma (NHL) have been inconsistent, but few studies have examined these associations for specific subtypes or across race/ethnicities. We evaluated the relationship between ambient UVR exposure and subtype-specific NHL incidence for whites, Hispanics, and blacks in the United States for years 2001–2010 (n=187,778 cases). Incidence rate ratios (IRRs) and 95% confidence intervals (CIs) were calculated for UVR quintiles using Poisson regression. Incidence was lower for the highest UVR quintile for chronic/small lymphocytic/leukemia (CLL/SLL) (IRR= 0.87, 95% CI: 0.77–0.97), mantle cell (IRR= 0.82, 95% CI: 0.69, 0.97), lymphoplasmacytic (IRR= 0.58, 95% CI: 0.42–0.80), mucosa-associated lymphoid tissue (MZLMALT ) (IRR= 0.74, 95% CI: 0.60–0.90), follicular (FL) (IRR= 0.76, 95% CI: 0.68–0.86), diffuse large B-cell (IRR= 0.84, 95% CI: 0.76–0.94;), peripheral T-cell, other (PTCL) (IRR= 0.76, 95% CI: 0.61–0.95), and PTCL not otherwise specified (PNOS) (IRR= 0.77, 95% CI: 0.61–0.98). Trends were significant for MZLMALT, FL, DLBCL, BNOS and PTCL, with FL and DLBCL still significant after Bonferroni correction. We found interaction by race/ethnicity for CLL/SLL, FL, Burkitt, PNOS, and MF/SS, with CLL/SLL and FL still significant after Bonferroni correction. Some B-cell lymphomas (CLL/SLL, FL, Burkitt) suggested significant inverse relationships in whites and Hispanics, but not blacks. Some T-cell lymphomas suggested the most reduced risk for the highest quintile of UVR among blacks (PNOS and MF/SS), though trends were not significant. These findings strengthen the case for an inverse association of UVR exposure, support modest heterogeneity between NHL subtypes, and suggest some differences by race/ethnicity.
Keywords: ultraviolet radiation, non-Hodgkin lymphoma, race
Introduction
Non-Hodgkin lymphoma (NHL) is the 7th most common cancer in the United States, with approximately 70,000 new cases and 19,000 deaths in 20131. The strongest and most consistently identified risk factors are diseases or treatments causing serious immunodeficiency (e.g., HIV, organ transplants), autoimmune conditions, certain infections (e.g., hepatitis C virus, Epstein Barr virus), and chemical exposures (e.g., PCBs, pesticides, hair dyes)2–5. However, the etiology of most lymphoma subtypes remains unknown.
Exposure to ultraviolet radiation (UVR) has been associated with both increased6, 7 and reduced8–12 risk of NHL in epidemiologic studies. The different approaches used for UVR exposure assessment include estimating personal exposure (e.g., time spent outdoors, number of sunburns, tanning, and occupational exposure) and ambient exposure to solar UVR based on latitude, ground, or satellite-based measurements. Studies measuring personal UVR exposure have typically been small retrospective case-control studies, while those estimating ambient UVR have generally either been confined to small geographic areas (e.g., Connecticut6, United Kingdom7, 13) or used latitude as a surrogate14, 15.
Moreover, NHL is increasingly recognized as a diverse group of cancers with varying etiologies that warrant examination by histological subtype5, 16. Among the studies that have examined the relationship between UVR and specific NHL histological subtypes6, 8, 14, 15, 17–23, only one has comprehensively evaluated an extensive number of subtypes15. No study has examined these relationships across different races or ethnicities. Examining associations by race may provide possible etiologic clues where risks differ by race.
Our objective is to evaluate the associations between ambient UVR exposure at diagnosis and subtype-specific incidence of NHL for non-Hispanic whites, Hispanic whites, and blacks using United States population-based registry data from the Surveillance, Epidemiology, and End Results (SEER) Program for years 2001 to 2010, inclusive. This represents the first study to assess differences in these associations by race/ethnicity. It is the second and largest study of UVR exposure and non-Hodgkin lymphoma subtypes, both common and rare, containing over five times more cases than the previously largest study15. The use of SEER county data linked to NASA satellite-based UVR exposure estimates also provides an opportunity to use more refined ambient UVR exposure data than latitude or state of residence.
Materials and methods
Study population
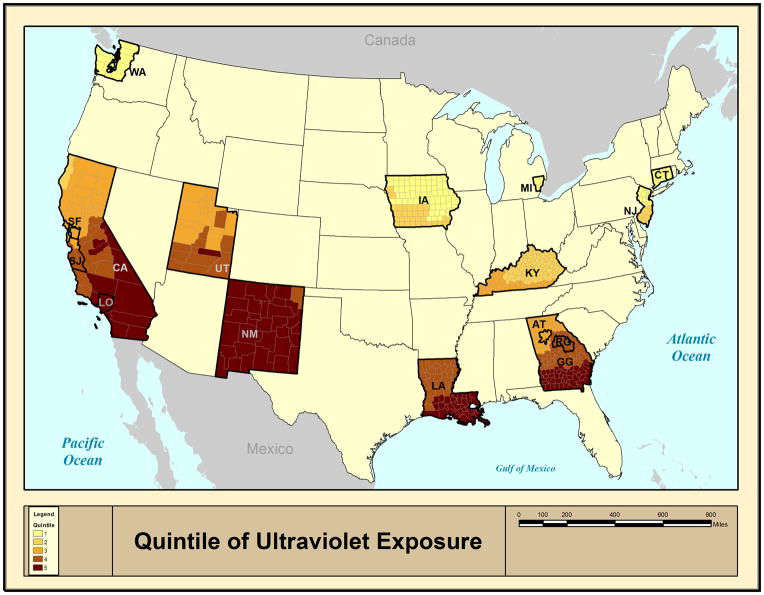
The study population was derived from 15 non-overlapping population-based cancer registries of the SEER 18 database, which contains the widest geographic coverage and represents approximately 26% of the US population (registries include San Francisco, Los Angeles, San Jose-Monterey, greater California, Seattle-Puget Sound, Utah, New Mexico, Detroit, Iowa, Kentucky, Louisiana [cases diagnosed from July – December 2005 are excluded from most statistical analyses due to Hurricanes Katrina and Rita], Connecticut, New Jersey, and Atlanta, rural Georgia, greater Georgia)(Figure 1)24. Hawaii, Alaska Natives, and Arizona Indian registries were excluded because they were outliers for ambient UVR and/or did not collect information on non-Hispanic whites, Hispanic whites, or blacks. SEER cancer registries collect information on patient demographics, year and county of diagnosis, and tumor characteristics (site and morphology). This study comprised patients up to 84 years old who were diagnosed with a first primary NHL between 2001 and 2010, inclusive. In the United States, rates of NHL diagnosed among HIV-uninfected people have been largely stable during the calendar time period included in our study (2001–2010)25. Races/ethnicities other than non-Hispanic white, Hispanic white, and black were excluded due to small numbers.
Figure 1.
Quintiles of ultraviolet radiation exposure for 15 registries participating in the Surveillance, Epidemiology, and End Results Program between 2001 and 2010.
Since HIV is both a major risk factor for several NHL subtypes and HIV cases cluster in specific locations, confounding by HIV infection may substantially bias relative risk estimates. For this reason, self-reported HIV-positive cases which were flagged by SEER were excluded from this study. HIV status was not collected for mycosis fungiodes or Sezary syndrome (MF/SS), so all cases were included. Due to privacy concerns stemming from very small numbers of cases, HIV status was also not reported by the Iowa registry so all Iowa cases were included. A sensitivity analysis excluding the Iowa registry did not substantially affect our results. The epidemiology of NHL in relation to HIV/AIDS has been previously described in detail for this dataset25.
For each county, counts of NHL cases were stratified by calendar year of diagnosis group (2001–2005, 2006–2010), sex, age group (0–44, 45–64, 65–84), race (non-Hispanic white, Hispanic white, black), SEER registry, and ambient annual UVR quintile (219–336, 337–403, 404–486, 487–547, 548–662 mW/m2). Corresponding population counts were obtained using the 2000 United States census. Analyses were restricted to NHL subtypes with a minimum of 50 cases for each racial/ethnic group.
Outcomes
NHL cases consisted of persons diagnosed with B or T-cell lymphoid neoplasm subtypes. B-cell lymphoid neoplasms included chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL), mantle-cell lymphoma (MCL), lymphoplasmacytic lymphoma (LPL), marginal-zone lymphoma mucosa-associated lymphatic tissue (MZL MALT), nodal (NMZL) and splenic (SMZL) types, hairy cell leukemia (HCL), plasmacytoma (Plasma), plasma cell myeloma (PCM), follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (Burk), and the heterogeneous category of B-cell not otherwise specified (BNOS). Neoplasms originating in the T-cells included extranodal natural killer T-cell lymphoma (ENNKTL), peripheral T-cell lymphoma other (PTCL), PTCL not otherwise specified (PNOS), MF/SS, primary cutaneous anaplastic large cell lymphoma (CALCL), and the heterogeneous category of T-cell not otherwise specified (TNOS). Cases were identified using International Classification of Diseases for Oncology third edition (ICD-O-3) morphology codes26. Only patients diagnosed following the introduction of the World Health Organization Classification27 and translation of this into ICDO-328 were included. NHL cases were grouped into lymphoid neoplasm subtypes as defined by the InterLymph hierarchical classification for epidemiologic research29.
Ambient ultraviolet radiation
Ambient UVR exposure was derived using the Total Ozone Mapping Spectrometer (TOMS) database maintained by the National Aeronautics and Space Administration (NASA)30. Cloud-adjusted daily ambient ultraviolet irradiance 305 nm, which is part of the UVB spectrum and therefore related to endogenous vitamin D production31, is provided on a 1 degree latitude by 1 degree longitude grid. In the continental United States, 1 degree latitude by 1 degree longitude grid represents approximately 111 by 85 km (69 by 53 miles), respectively. Satellite-based estimates of UVR have varied little over time since the start of measurements in the late 1970s, aside from relatively small fluctuations due to the 11-year solar cycle32. For this study, daily noon-time estimates over years 1982–1992 were averaged to represent a full 11-year solar cycle. The population centroid of each SEER county was linked to the nearest yearly average UVR estimate using ArcGIS 9.1 software (ESRI 2005). SEER counties were ranked by UVR and assigned quintiles 1 (lowest UVR) thru 5 (highest UVR) (Figure 1).
Statistical analysis
We examined the relationships between ambient UVR and risk of NHL subtypes by calculating incidence rate ratios (IRRs) and 95% confidence intervals (CIs) derived from Poisson regression after adjusting for year of diagnosis group, age group, sex, race, and registry. UVR was treated as categorical (quintile) in main effects regression models. To test for trend in NHL rates with increasing UVR quintile, we regressed the log incident rate ratio parameters for UVR quintiles coded in dummies from the Poisson regression models as the outcome variable on the quintile number (2 thru 5) in a linear regression model and estimated that slope using linear least squares with a known variance-covariance matrix (also obtained from the Poisson models). The p-value for the slope parameter corresponds to the p-trend we report.. We then assessed if the slope parameter was different from zero based on a Wald test. To assess whether these relationships were modified by year of diagnosis, age, sex, or race/ethnicity, tests for statistical interaction were conducted. Interactions were assessed based on a Wald test using continuous coding for UVR quintiles (with values 1 thru 5). Since any systematic geographic difference in NHL ascertainment or diagnosis of the specific subtype could impact our results, SEER registry was included as a random effect in all models. All models contained the natural log of the population size as an offset. Due to overdispersion, we conducted sensitivity analysis using a zero-inflated Poisson model, but this did not affect our results.
Statistical tests were two-sided with a specified type I error of 0.05. Trend and interaction p-values were corrected for multiple comparisons testing using Bonferroni adjustment. Poisson regression was performed with the GLIMMIX procedure using SAS software V9.3 (SAS Institute, Inc.). For models that did not converge using the GLIMMIX procedure (SMZL, Plasma, Burk, and NOSPTCL among combined race groups), the NLMIXED procedure was used33.
Results
A total of 187,778 diagnosed cases of NHL were included in the analysis sample. Of these, 176,596 (94%) were mature B-cell NHL (Table 1) and 11,182 (6%) were mature NK/T-cell NHL subtypes (Table 2). There was no consistent evidence of differences in the relationship between UVR and NHL subtypes by year of diagnosis, age, or sex. Race/ethnicity did significantly modify the association of UVR and risk of several of the subtypes before correction for multiple testing (CLL/SLL, FL, Burk, PNOS, and MF/SS) and afterwards (CLL/SLL, FL), so race/ethnicity-specific estimates are presented for these subtypes (Table 3).
Table 1.
Adjusted incidence rate ratios by quintile of ambient ultraviolet radiation for selected mature B-cell non-Hodgkin lymphoma subtypes, SEER 2001–2010
| B-cell subtype12 | n | IRR3 | 95% CI | P for trend4 | ||
|---|---|---|---|---|---|---|
| Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) | Q1 | 11,837 | Ref | |||
| Q2 | 3,845 | 0.95 | 0.90 | 1.01 | ||
| Q3 | 7,477 | 0.89 | 0.80 | 0.99 | ||
| Q4 | 2,956 | 0.91 | 0.81 | 1.02 | ||
| Q5 | 9,231 | 0.87 | 0.77 | 0.97 | 0.114 | |
| Mantle cell lymphoma (MCL) | Q1 | 1,570 | Ref | |||
| Q2 | 547 | 1.00 | 0.87 | 1.15 | ||
| Q3 | 1,006 | 0.74 | 0.63 | 0.88 | ||
| Q4 | 434 | 0.80 | 0.66 | 0.96 | ||
| Q5 | 1,471 | 0.82 | 0.69 | 0.97 | 0.074 | |
| Lymphoplasmacytic lymphoma (LPL) | Q1 | 1,451 | Ref | |||
| Q2 | 252 | 0.69 | 0.57 | 0.83 | ||
| Q3 | 774 | 0.58 | 0.42 | 0.80 | ||
| Q4 | 269 | 0.53 | 0.38 | 0.75 | ||
| Q5 | 1,105 | 0.58 | 0.42 | 0.80 | 0.208 | |
| Marginal zone lymphoma of mucosa-associated lymphoid tissue (MZLMALT) | Q1 | 2,262 | Ref | |||
| Q2 | 681 | 0.94 | 0.83 | 1.06 | ||
| Q3 | 1,483 | 0.75 | 0.62 | 0.92 | ||
| Q4 | 597 | 0.75 | 0.61 | 0.93 | ||
| Q5 | 2,181 | 0.74 | 0.60 | 0.90 | 0.021 | |
| Nodal marginal zone lymphoma (NMZL) | Q1 | 1,381 | Ref | |||
| Q2 | 306 | 0.80 | 0.68 | 0.94 | ||
| Q3 | 646 | 0.60 | 0.44 | 0.81 | ||
| Q4 | 315 | 0.78 | 0.57 | 1.07 | ||
| Q5 | 1,163 | 0.77 | 0.57 | 1.05 | 0.77 | |
| Splenic marginal zone lymphoma (SMZL) | Q1 | 360 | Ref | |||
| Q2 | 108 | 0.80 | 0.59 | 1.10 | ||
| Q3 | 199 | 0.59 | 0.42 | 0.83 | ||
| Q4 | 72 | 0.58 | 0.39 | 0.87 | ||
| Q5 | 324 | 0.77 | 0.55 | 1.09 | 0.825 | |
| Hairy cell leukemia (HCL) | Q1 | 748 | Ref | |||
| Q2 | 205 | 0.87 | 0.71 | 1.07 | ||
| Q3 | 566 | 0.91 | 0.73 | 1.13 | ||
| Q4 | 212 | 0.85 | 0.66 | 1.09 | ||
| Q5 | 688 | 0.81 | 0.64 | 1.02 | 0.507 | |
| Plasmacytoma (Plasma) | Q1 | 696 | Ref | |||
| Q2 | 239 | 1.02 | 0.81 | 1.28 | ||
| Q3 | 521 | 0.89 | 0.67 | 1.19 | ||
| Q4 | 237 | 0.91 | 0.67 | 1.24 | ||
| Q5 | 741 | 0.90 | 0.67 | 1.20 | 0.408 | |
| Plasma cell myeloma (PCM) | Q1 | 10,637 | Ref | |||
| Q2 | 3,308 | 0.96 | 0.90 | 1.02 | ||
| Q3 | 7,843 | 0.97 | 0.88 | 1.09 | ||
| Q4 | 3,251 | 0.93 | 0.83 | 1.04 | ||
| Q5 | 10,123 | 0.91 | 0.81 | 1.02 | 0.216 | |
| Follicular lymphoma (FL) | Q1 | 8135 | Ref | |||
| Q2 | 2899 | 0.94 | 0.87 | 1.00 | ||
| Q3 | 5591 | 0.77 | 0.69 | 0.87 | ||
| Q4 | 2222 | 0.77 | 0.68 | 0.87 | ||
| Q5 | 7229 | 0.76 | 0.68 | 0.86 | <0.001* | |
| Diffuse large B-cell lymphoma (DLBCL) | Q1 | 12,887 | Ref | |||
| Q2 | 4,217 | 0.98 | 0.93 | 1.04 | ||
| Q3 | 9,556 | 0.90 | 0.81 | 0.99 | ||
| Q4 | 3,647 | 0.84 | 0.76 | 0.93 | ||
| Q5 | 12,429 | 0.84 | 0.76 | 0.94 | <0.001* | |
| Burkitt lymphoma (Burk) | Q1 | 581 | Ref | |||
| Q2 | 155 | 0.78 | 0.60 | 1.00 | ||
| Q3 | 490 | 0.84 | 0.65 | 1.09 | ||
| Q4 | 152 | 0.66 | 0.49 | 0.89 | ||
| Q5 | 673 | 0.82 | 0.62 | 1.07 | 0.851 | |
| B-cell not otherwise specified (BNOS) | Q1 | 3,167 | Ref | |||
| Q2 | 943 | 1.04 | 0.93 | 1.15 | ||
| Q3 | 2,024 | 0.84 | 0.71 | 1.00 | ||
| Q4 | 827 | 0.82 | 0.68 | 0.98 | ||
| Q5 | 2,454 | 0.86 | 0.72 | 1.03 | 0.032 |
Abbreviations: IRR, incidence rate ratio; CI, confidence interval; Ref, reference category; UVR, ultraviolet radiation.
Significant after Bonferroni correction (19 tests).
ICD-O-3 codes: CLL/SLL 9670, 9823; MCL 9673; LPL 9671, 9761; MZL_MALT 9699 (excl. C77.0–C77.9), 9764; NMZL 9699 (C77.0–C77.9); SMZL 9689; HCL 9591 (C42.1–C42.2), 9940; Plasma 9731, 9734; PCM 9732-33; FL 9690-91, 9695, 9698; DLBCL 9678-80, 9684, 9688, 9712, 9735, 9737-38; Burkitt 9687; NOSB 9590-91, 9675, 9820, 9970.
Cutoffs for ambient UVR quintile: 219–336, 337–403, 404–486, 487–547, 548–662 mW/m 2.
Adjusted for sex, age-group (0–44, 45–64, 65–84), year of diagnosis (2001–2005, 2006–2010), race, and a random intercept for SEER registry.
Trend tests use log IRR parameters for UVR quintiles as the outcome variable on the quintile number (2 thru 5) in a linear regression model and estimated that slope using linear least squares with a known variance-covariance matrix.
Table 2.
Adjusted incidence rate ratios by quintile of ambient ultraviolet radiation for mature NK-/T-cell non-Hodgkin lymphoma subtypes, 2001–2010
| T-cell subtype12 | n | IRR3 | 95% CI | P for trend4 | ||
|---|---|---|---|---|---|---|
| Extranodal natural killer T-cell lymphoma/leukemia (ENNKTL) | Q1 | 77 | Ref | |||
| Q2 | 23 | 0.83 | 0.46 | 1.50 | ||
| Q3 | 81 | 0.95 | 0.56 | 1.61 | ||
| Q4 | 39 | 1.26 | 0.70 | 2.26 | ||
| Q5 | 149 | 1.02 | 0.60 | 1.75 | 0.778 | |
| Peripheral T-cell lymphoma, other (PTCL) | Q1 | 1,340 | Ref | |||
| Q2 | 394 | 0.97 | 0.83 | 1.13 | ||
| Q3 | 958 | 0.82 | 0.67 | 1.02 | ||
| Q4 | 362 | 0.73 | 0.58 | 0.92 | ||
| Q5 | 1,210 | 0.76 | 0.61 | 0.95 | 0.05 | |
| Peripheral T-cell lymphoma, not otherwise specified (PNOS) | Q1 | 840 | Ref | |||
| Q2 | 254 | 0.92 | 0.75 | 1.13 | ||
| Q3 | 555 | 0.78 | 0.62 | 0.97 | ||
| Q4 | 211 | 0.69 | 0.53 | 0.90 | ||
| Q5 | 739 | 0.77 | 0.61 | 0.98 | 0.101 | |
| Mycosis fungoides or Sézary syndrome (MF/SS) | Q1 | 895 | Ref | |||
| Q2 | 169 | 0.85 | 0.69 | 1.06 | ||
| Q3 | 924 | 1.42 | 1.00 | 2.03 | ||
| Q4 | 232 | 1.03 | 0.71 | 1.51 | ||
| Q5 | 738 | 0.79 | 0.55 | 1.14 | 0.561 | |
| Primary cutaneous anaplastic large cell lymphoma (CALCL) | Q1 | 227 | Ref | |||
| Q2 | 46 | 0.64 | 0.43 | 0.96 | ||
| Q3 | 146 | 0.76 | 0.55 | 1.06 | ||
| Q4 | 49 | 0.64 | 0.43 | 0.97 | ||
| Q5 | 206 | 0.82 | 0.58 | 1.15 | 0.127 | |
| T-cell lymphoma, not otherwise specified (TNOS) | Q1 | 103 | Ref | |||
| Q2 | 27 | 0.74 | 0.43 | 1.27 | ||
| Q3 | 73 | 1.07 | 0.56 | 2.04 | ||
| Q4 | 23 | 0.74 | 0.35 | 1.58 | ||
| Q5 | 92 | 1.33 | 0.68 | 2.61 | 0.257 |
Abbreviations: IRR, incidence rate ratio; CI, confidence interval; Ref, reference category; UVR, ultraviolet radiation.
ICD-O-3 codes: ENNKTL 9719; PTCL 9705, 9708-09, 9714, 9716-17, 9726; PNOS 9675, 9702, 9724, 9725; MF/SS 9700-01; CALCL 9718; TNOS 9590-91, 9684, 9820, 9970.
Cutoffs for ambient UVR quintile: 219–336, 337–403, 404–486, 487–547, 548–662 mW/m2.
Adjusted for sex, age-group (0–44, 45–64, 65–84), year of diagnosis (2001–2005, 2006–2010), race, and a random intercept for SEER registry.
Trend tests use log IRR parameters for UVR quintiles as the outcome variable on the quintile number (2 thru 5) in a linear regression model and estimated that slope using linear least squares with a known variance-covariance matrix. None withstood adjustment for multiple comparisons using Bonferroni correction (19 tests).
Table 3.
Adjusted incidence rate ratios by quintile of ambient ultraviolet radiation for non-Hodgkin lymphoma subtypes by race, 2001–20101
| Subtype | Whites | Hispanics | Blacks | P for interaction4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IRR2 | 95% CI | P for trend3 | n | IRR1 | 95% CI | P for trend3 | n | IRR2 | 95% CI | P for trend3 | ||||||
| Chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL) | Q1 | 10792 | Ref | 272 | Ref | 773 | Ref | ||||||||||
| Q2 | 3625 | 0.94 | 0.89 | 1.00 | 41 | 1.15 | 0.82 | 1.61 | 179 | 1.01 | 0.82 | 1.23 | |||||
| Q3 | 6480 | 0.89 | 0.79 | 0.99 | 337 | 0.86 | 0.69 | 1.06 | 660 | 1.00 | 0.83 | 1.20 | |||||
| Q4 | 2453 | 0.91 | 0.81 | 1.03 | 166 | 0.74 | 0.58 | 0.94 | 337 | 0.90 | 0.73 | 1.10 | |||||
| Q5 | 7466 | 0.87 | 0.77 | 0.97 | 0.179 | 944 | 0.65 | 0.53 | 0.80 | 0.001 | 821 | 0.93 | 0.77 | 1.12 | 0.346 | <0.001* | |
| Follicular lymphoma (FL) | Q1 | 7378 | Ref | 410 | Ref | 347 | Ref | ||||||||||
| Q2 | 2734 | 0.92 | 0.86 | 0.99 | 47 | 0.87 | 0.64 | 1.18 | 118 | 1.45 | 1.13 | 1.86 | |||||
| Q3 | 4853 | 0.77 | 0.69 | 0.87 | 445 | 0.74 | 0.61 | 0.90 | 293 | 0.90 | 0.71 | 1.15 | |||||
| Q4 | 1863 | 0.79 | 0.70 | 0.89 | 229 | 0.65 | 0.52 | 0.81 | 130 | 0.77 | 0.58 | 1.02 | |||||
| Q5 | 5433 | 0.77 | 0.68 | 0.87 | 0.003 | 1418 | 0.66 | 0.55 | 0.80 | 0.065 | 378 | 0.89 | 0.70 | 1.13 | 0.001 | <0.001* | |
| Burkitt lymphoma (Burk) | Q1 | 478 | Ref | 60 | Ref | 43 | Ref | ||||||||||
| Q2 | 142 | 0.85 | 0.65 | 1.10 | 6 | 0.68 | 0.29 | 1.58 | 7 | 0.66 | 0.29 | 1.50 | |||||
| Q3 | 384 | 0.83 | 0.64 | 1.08 | 69 | 0.78 | 0.55 | 1.11 | 37 | 0.90 | 0.55 | 1.46 | |||||
| Q4 | 111 | 0.68 | 0.50 | 0.93 | 21 | 0.41 | 0.25 | 0.67 | 20 | 0.95 | 0.54 | 1.69 | |||||
| Q5 | 427 | 0.84 | 0.64 | 1.11 | 0.673 | 205 | 0.67 | 0.50 | 0.89 | 0.574 | 41 | 0.87 | 0.54 | 1.39 | 0.506 | 0.016 | |
| Peripheral T-cell lymphoma, not otherwise specified (PNOS) | Q1 | 637 | Ref | 49 | Ref | 154 | Ref | ||||||||||
| Q2 | 214 | 0.97 | 0.78 | 1.20 | 7 | 1.02 | 0.46 | 2.25 | 33 | 0.84 | 0.56 | 1.27 | |||||
| Q3 | 401 | 0.82 | 0.65 | 1.04 | 44 | 0.64 | 0.42 | 0.96 | 110 | 0.76 | 0.53 | 1.08 | |||||
| Q4 | 127 | 0.67 | 0.51 | 0.89 | 28 | 0.69 | 0.43 | 1.10 | 56 | 0.75 | 0.50 | 1.11 | |||||
| Q5 | 454 | 0.85 | 0.66 | 1.08 | 0.175 | 181 | 0.71 | 0.52 | 0.98 | 0.405 | 104 | 0.60 | 0.42 | 0.86 | 0.181 | 0.039 | |
| Mycosis fungoides or Sézary syndrome (MF/SS) | Q1 | 686 | Ref | 38 | Ref | 171 | Ref | ||||||||||
| Q2 | 141 | 0.81 | 0.64 | 1.03 | 7 | 1.39 | 0.61 | 3.17 | 21 | 0.68 | 0.39 | 1.17 | |||||
| Q3 | 673 | 1.37 | 0.92 | 2.03 | 86 | 1.49 | 0.90 | 2.48 | 165 | 1.29 | 0.83 | 2.02 | |||||
| Q4 | 160 | 1.06 | 0.69 | 1.62 | 29 | 1.00 | 0.55 | 1.82 | 43 | 0.72 | 0.42 | 1.21 | |||||
| Q5 | 482 | 0.81 | 0.53 | 1.22 | 0.661 | 148 | 0.81 | 0.49 | 1.34 | 0.127 | 108 | 0.63 | 0.39 | 1.01 | 0.416 | 0.033 | |
Abbreviations: IRR, incidence rate ratio; CI, confidence interval; Ref, reference category; UVR, ultraviolet radiation.
Significant after Bonferroni correction (included 57 tests for trend p-values and 19 tests for interaction p-values).
Cuttoffs for ambient annual UVR quintile: 219–336,337–403,404–486,487–547, 548–662 mW/m2.
Adjusted for sex, age-group (0–44, 45–64, 65–84), year of diagnosis (2001–2005, 2006–2010), and a random intercept for SEER registry.
Trend tests use log IRR parameters for UVR quintiles as the outcome variable on the quintile number (2 thru 5) in a linear regression model and estimated that slope using linear least squares with a known variance-covaria
Interaction tests treat UVR quintile as a continuous variable (coded 1 thru 5).
Among B-cell NHL subtypes, for whites, Hispanics, and blacks combined, we observed reduced risk for at least one of the two highest quintiles for the following B-cell subtypes: CLL/SLL, MCL, LPL, MZL MALT, SMZL, FL, DLBCL, Burk, and BNOS(Table 1). We found a significantly decreasing trend between UVR and risk of MZLMALT, FL, DLBCL, and BNOS, with FL and DLBCL still significant after Bonferroni correction.. There was little evidence of a UVR association for Plasma or PCM.
Among T-cell NHL subtypes for all races combined, we observed a significantly reduced risk at least one of the two highest quintiles for PTCL, PNOS, and CALCL (Table 2). There was no evidence of a UVR association for ENNKTL, MF/SS, or TNOS for all races combined. A significant trend was found for PTCL, but this did not withstand Bonferroni correction.
Interaction by race was significant for the following subtypes: CLL/SLL (p for interaction < 0.001), FL (p for interaction < 0.001), Burk (p for interaction = 0.016), PNOS (p for interaction = 0.039) and MF/SS (p for interaction = 0.033), with CLL/SLL and FL race interactions withstanding correction for multiple comparisons (19 tests). Of these subtypes, the B-cell lymphomas (CLL/SLL, FL, Burk) demonstrated significant inverse relationships in both whites and Hispanic whites for at least one of the two highest quintiles, but not in blacks. For example, UVR was most protective in the highest quintile for CLL/SLL (IRR=0.65; 95% CI: 0.53, 0.80) and FL (IRR=0.66; 95% CI: 0.55, 0.80) among Hispanics. In contrast, the T-cell lymphomas (PNOS, MF/SS) suggested the most protective relationship for the highest quintile of UVR among blacks (IRR for PNOS=0.60; 95% CI: 0.42, 0.86 and IRR for MF/SS=0.63; 95% CI: 0.39, 1.01), though MF/SS was not significantly reduced.
Discussion
In this United States population-based study of ambient UVR and NHL subtypes, we report a reduction in risk of most NHL subtypes (CLL/SLL, MCL, LPL, MZLMALT, SMZL, FL, DLBCL, Burk, BNOS, PTCL, PNOS, and CALCL) associated with high levels of ambient UVR, with trends for FL and DLBCL still significant after Bonferroni correction for all races combined. The following subtypes, however, were not associated with ambient UVR for all races combined: Plasma, PCM, ENNKTL, MF/SS, and TNOS. These results strengthen the evidence for UVR involvement in many NHL subtypes and support the case for modest etiologic heterogeneity among NHL subtypes with regard to UVR exposure. This is the only study we are aware of to assess the relation between UVR and NHL subtypes by race and ethnicity. Our results suggest little evidence of a reduced risk of CLL/SLL and Burk associated with UVR exposure in blacks, whereas IRRs were reduced for these subtypes in Hispanics and whites, though trends were only significant for CLL/SLL in Hispanics. There was evidence of reduced risk of FL associated with UVR for each racial group. We also observed a greater reduction in risk for PNOS and MF/SS in relation to UVR in blacks compared with the risks of these types of NHL in other racial groups, though tests of interaction did not withstand Bonferroni correction.
Exposure to UVR has been hypothesized to influence the risk of NHL because UVR is associated with a number of immunological changes34, 35. A protective role for UVR in NHL has been proposed via immune modulation, specifically through depressing T-helper 1-mediated immune response and enhancing T-helper 2 activity34, 36. UVR exposure also contributes to vitamin D production, which has been linked to lower risk of some cancers, although the few epidemiologic studies of NHL and circulating vitamin D have found limited or no evidence of an overall association37–39. In addition, there are racial differences in incidence of several subtypes of NHL40, measures of immune function4, 41, relationships between immune system–related gene polymorphisms and NHL42, and circulating vitamin D levels43.
Overall, epidemiologic studies have found UVR to be inversely associated with diffuse large B-cell lymphoma (DBCL) 8, 15, 17, 18, 20–22, as they were in this study. In contrast, the associations with the other two main B-cell subtypes, FL and CLL/SLL have been inconsistent13, 15, 17, 18, 22. In this study, UVR was inversely related to risk of both CLL/SLL and Burk in whites and Hispanics, but not in blacks, while UVR was inversely associated with FL in all three race/ethnicities. The largest and most comprehensive study preceding the current study is a registry-based analysis in Australia which used latitude as a surrogate for ambient UVR exposure15. In that study, van Leeuwen and colleagues suggest that their failure to find an association with FL, which has no established relationship to infection or immune dysfunction, provides evidence that UVR plays a protective role specifically in those NHL subtypes where infection or immune dysfunction are involved. Our finding that UVR is inversely related to FL does not support the hypothesis that UVR reduces risk of specific NHL subtypes only associated with immune dysfunction or infection-related mechanisms.
As noted, we also found a significant inverse trend of CLL/SLL risk by increasing level of UVR quintile in Hispanics and significant protective association in whites with the highest level of UVR, but not in blacks. In one of the few studies of NHL and circulating vitamin D, Lim and colleagues found CLL/SLL was more inversely related to 25(OH)D, the major circulating form of vitamin D, than other common subtypes of NHL38. Luczynska and colleagues also found that circulating vitamin D was statistically significantly inversely related to CLL, but not other subytpes39. A recent genome-wide study found that regions of the genome associated with CLL also contain a disproportionately large number of vitamin D receptor binding sites, providing additional evidence of an etiological role of 25(OH)D for this subtype44.
In the two previous studies that have examined T-cell lymphomas, UVR associations have been generally inverse, but the two studies use different classification systems for NHL and thus can’t be easily compared15, 18. In our study, statistically significant inverse associations with UVR include PTCL, PNOS, and CALCL, with the inverse association for PNOS largely driven by the association in blacks, though trends were not significant. As noted, race is significantly related to circulating vitamin D, with blacks having lower levels of circulating mean serum 25(OH) than whites41, 43. Experimental evidence has also demonstrated that a low dose of UVB radiation increases serum vitamin D3 concentrations in whites, but not in blacks45. Our finding of stronger inverse relationships for PNOS and MF/SS in blacks argues against a biologic mechanism involving vitamin D in this group. However, racial differences in immune-related conditions and risk of non-Hodgkin lymphoma have been observed, possibly due to differences in immune system regulatory genes4. Also, experimental evidence involving whole body irradiation with low levels of UVB in blacks and whites has been associated with differences in immunological responses consisting of increased B-cells (CD19) and helper T-cells (CD4 and CD29) in whites and decreased CD3 T-cells in blacks45. While whites have higher overall incidence of NHL5, blacks have had notably higher incidence rates of peripheral T-cell lymphomas and mycosis fungoides40. In addition, race is a well-established modifier of the relationship between UVR and skin cancer risk.
Our findings should be interpreted within the context of several limitations. Our study lacked data on personal UVR exposure; however, ambient UVR based on TOMS satellites, as estimated here, has been significantly positively associated with UVR exposure measured by personal dosimeter46. Misclassification of exposure may also have resulted because ambient UVR was linked to location of residence only at time of diagnosis. The direction of this potential bias is difficult to ascertain, but could be particularly relevant to NHL subtypes with longer latency periods, such as CLL/SLL. Residual confounding due to unmeasured confounders may also bias our results; however, there are few strong personal risk factors that have been established for NHL47. In addition, since most of the observed associations were not significantly different by race and race is known to be related to a number of lifestyle factors, there is additional evidence that residual confounding by these lifestyle factors did not play a large role in the UVR NHL relationships we observed.
One of the major advantages of this study is a sample size large enough to look at many NHL subtypes by racial/ethnic groups. To our knowledge, this analysis is the largest to date of UVR and NHL subtypes, comprising over five times the number of cases as the previous largest study15. The large population included in the United States’ SEER registries has both a substantial number of Hispanics and blacks and a broad range of ambient UVR exposures. Although incidence of NHL subtypes varies by race48, race/ethnicity has not been previously examined in terms of its role in the relationship between UVR and NHL. Racial/ethnic differences may reflect differences in genes, other physiological characteristics, behaviors, and sun sensitivity.
In summary, this United States population-based study of ambient UVR and NHL, the largest reported to date, showed a statistically significant reduction in risk of most NHL subtypes by all racial groups combined for increasing ambient UVR exposure, with trends for FL and DLBCL withstanding Bonferroni correction for multiple comparisons. There was evidence of differences in risk for CLL/SLL, FL, Burk, PNOS, and MF/SS associated with UVR by race/ethnicity, though only CLL/SLL and FL withstood Bonferroni correction. Our results support and expand previous findings of a protective association of UVR on NHL subtypes and serve as a starting point for understanding the differences in the relationship between UVR and specific NHL subtypes by race/ethnicity. Epidemiological studies with personal UVR exposure are needed to confirm these findings and investigate potential roles of UVR on immunologic mechanisms. Experimental studies of the effect of UVR exposure are also needed to elucidate mechanistic differences by race/ethnicity.
Novelty.
This U.S. population-based study of ambient UVR and NHL, the largest reported to date, showed a reduction in risk of most NHL subtypes for increasing ambient UVR exposure and differences in relative risk for CLL/SLL, FL, Burk, PNOS, and MF/SS by race/ethnicity. Our results support and expand previous findings of a protective association of UVR on NHL subtypes and serve as a starting point for understanding the differences in this relationship by race/ethnicity.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH and the National Cancer Institute.
Abbreviations
- CLL/SLL
chronic lymphocytic leukemia or small lymphocytic lymphoma
- MCL
mantle-cell lymphoma
- LPL
lymphoplasmacytic lymphoma
- MZL MALT
marginal-zone lymphoma of mucosa-associated lymphoid tissue
- NMZL
nodal marginal-zone lymphoma
- SMZL
splenic marginal-zone lymphoma
- HCL
hairy cell leukemia
- Plasma
plasmacytoma
- PCM
plasma cell myeloma
- FL
follicular lymphoma
- DLBCL
diffuse large B-cell lymphoma
- Burk
Burkitt lymphoma
- BNOS
B-cell not otherwise specified
- ENNKTL
extranodal natural killer/T-cell lymphoma, nasal-type/aggressive natural killer leukemia
- PTCL
peripheral T-cell lymphoma other
- PNOS
PTCL not otherwise specified
- MF/SS
mycosis fungoides or Sezary syndrome
- CALCL
primary cutaneous anaplastic large cell lymphoma
- TNOS
T-cell lymphoma not otherwise specified
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Grulich AE, Vajdic CM, Kaldor JM, Hughes AM, Kricker A, Fritschi L, Turner JJ, Milliken S, Benke G, Armstrong BK. Birth order, atopy, and risk of non-Hodgkin lymphoma. Journal of the National Cancer Institute. 2005;97:587–94. doi: 10.1093/jnci/dji098. [DOI] [PubMed] [Google Scholar]
- 3.Grulich AE, Vajdic CM. The epidemiology of non-Hodgkin lymphoma. Pathology. 2005;37:409–19. doi: 10.1080/00313020500370192. [DOI] [PubMed] [Google Scholar]
- 4.Koshiol J, Lam TK, Gridley G, Check D, Brown LM, Landgren O. Racial differences in chronic immune stimulatory conditions and risk of non-Hodgkin’s lymphoma in veterans from the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:378–85. doi: 10.1200/JCO.2010.30.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skrabek P, Turner D, Seftel M. Epidemiology of Non-Hodgkin Lymphoma. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2013 doi: 10.1016/j.transci.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Holford TR, Leaderer B, Boyle P, Zhu Y, Wang R, Zou K, Zhang B, Wise JP, Sr, Qin Q, Kilfoy B, Han J, et al. Ultraviolet radiation exposure and risk of non-Hodgkin’s lymphoma. American journal of epidemiology. 2007;165:1255–64. doi: 10.1093/aje/kwm020. [DOI] [PubMed] [Google Scholar]
- 7.Bentham G. Association between incidence of non-Hodgkin’s lymphoma and solar ultraviolet radiation in England and Wales. BMJ. 1996;312:1128–31. doi: 10.1136/bmj.312.7039.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boffetta P, van der Hel O, Kricker A, Nieters A, de Sanjose S, Maynadie M, Cocco PL, Staines A, Becker N, Font R, Mannetje A, Goumas C, et al. Exposure to ultraviolet radiation and risk of malignant lymphoma and multiple myeloma--a multicentre European case-control study. International journal of epidemiology. 2008;37:1080–94. doi: 10.1093/ije/dyn092. [DOI] [PubMed] [Google Scholar]
- 9.Hartge P, Devesa SS, Grauman D, Fears TR, Fraumeni JF., Jr Non-Hodgkin’s lymphoma and sunlight. Journal of the National Cancer Institute. 1996;88:298–300. doi: 10.1093/jnci/88.5.298. [DOI] [PubMed] [Google Scholar]
- 10.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu S, Ma F, Collado-Mesa F, Kirsner RS. Ultraviolet radiation and incidence of non-Hodgkin’s lymphoma among Hispanics in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13:59–64. doi: 10.1158/1055-9965.epi-03-0187. [DOI] [PubMed] [Google Scholar]
- 12.Freedman DM, Kimlin MG, Hoffbeck RW, Alexander BH, Linet MS. Multiple indicators of ambient and personal ultraviolet radiation exposure and risk of non-Hodgkin lymphoma (United States) Journal of photochemistry and photobiology B, Biology. 2010;101:321–5. doi: 10.1016/j.jphotobiol.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane EV, Painter D, Roman E, Allan J, Law G, Lightfoot T. Melanocortin 1 receptor (MC1R), pigmentary characteristics and sun exposure: findings from a case-control study of diffuse large B-cell and follicular lymphoma. Cancer epidemiology. 2010;34:136–41. doi: 10.1016/j.canep.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Adami J, Gridley G, Nyren O, Dosemeci M, Linet M, Glimelius B, Ekbom A, Zahm SH. Sunlight and non-Hodgkin’s lymphoma: a population-based cohort study in Sweden. International journal of cancer Journal international du cancer. 1999;80:641–5. doi: 10.1002/(sici)1097-0215(19990301)80:5<641::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.van Leeuwen MT, Turner JJ, Falster MO, Meagher NS, Joske DJ, Grulich AE, Giles GG, Vajdic CM. Latitude gradients for lymphoid neoplasm subtypes in Australia support an association with ultraviolet radiation exposure. International journal of cancer Journal international du cancer. 2013 doi: 10.1002/ijc.28081. [DOI] [PubMed] [Google Scholar]
- 16.Morton LM, Wang SS, Cozen W, Linet MS, Chatterjee N, Davis S, Severson RK, Colt JS, Vasef MA, Rothman N, Blair A, Bernstein L, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes. Blood. 2008;112:5150–60. doi: 10.1182/blood-2008-01-133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang ET, Canchola AJ, Cockburn M, Lu Y, Wang SS, Bernstein L, Clarke CA, Horn-Ross PL. Adulthood residential ultraviolet radiation, sun sensitivity, dietary vitamin D, and risk of lymphoid malignancies in the California Teachers Study. Blood. 2011;118:1591–9. doi: 10.1182/blood-2011-02-336065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kricker A, Armstrong BK, Hughes AM, Goumas C, Smedby KE, Zheng T, Spinelli JJ, De Sanjose S, Hartge P, Melbye M, Willett EV, Becker N, et al. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. International journal of cancer Journal international du cancer. 2008;122:144–54. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 19.Soni LK, Hou L, Gapstur SM, Evens AM, Weisenburger DD, Chiu BC. Sun exposure and non-Hodgkin lymphoma: a population-based, case-control study. European journal of cancer. 2007;43:2388–95. doi: 10.1016/j.ejca.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Smedby KE, Eloranta S, Duvefelt K, Melbye M, Humphreys K, Hjalgrim H, Chang ET. Vitamin D receptor genotypes, ultraviolet radiation exposure, and risk of non-Hodgkin lymphoma. American journal of epidemiology. 2011;173:48–54. doi: 10.1093/aje/kwq340. [DOI] [PubMed] [Google Scholar]
- 21.Weihkopf T, Becker N, Nieters A, Mester B, Deeg E, Elsner G, Blettner M, Seidler A. Sun exposure and malignant lymphoma: a population-based case-control study in Germany. International journal of cancer Journal international du cancer. 2007;120:2445–51. doi: 10.1002/ijc.22492. [DOI] [PubMed] [Google Scholar]
- 22.Bertrand KA, Chang ET, Abel GA, Zhang SM, Spiegelman D, Qureshi AA, Laden F. Sunlight exposure, vitamin D, and risk of non-Hodgkin lymphoma in the Nurses’ Health Study. Cancer causes & control : CCC. 2011;22:1731–41. doi: 10.1007/s10552-011-9849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin SW, Wheeler DC, Park Y, Cahoon EK, Hollenbeck AR, Freedman DM, Abnet CC. Prospective study of ultraviolet radiation exposure and risk of cancer in the United States. International journal of cancer Journal international du cancer. 2012;131:E1015–23. doi: 10.1002/ijc.27619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surveillance Research Program NCI. SEER *Stat software, ed. version 8.0.2. 2013. [Google Scholar]
- 25.Shiels MS, Engels EA, Linet MS, Clarke CA, Li J, Hall HI, Hartge P, Morton LM. The epidemic of non-Hodgkin lymphoma in the United States: disentangling the effect of HIV, 1992–2009. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:1069–78. doi: 10.1158/1055-9965.EPI-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, Jack A, Cozen W, Maynadie M, Spinelli JJ, Costantini AS, Rudiger T, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffe ES, Stein HN, Vardiman HJS. World Health Organization Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 28.Fritz A, PC, Jack A, Shanmugaratnam K, Sobin LH, Parkin MD. International Classification of Diseases for Oncology. 3. Geneva, Switzerland: World Health Organization; [Google Scholar]
- 29.Turner JJ, Morton LM, Linet MS, Clarke CA, Kadin ME, Vajdic CM, Monnereau A, Maynadie M, Chiu BC, Marcos-Gragera R, Costantini AS, Cerhan JR, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116:e90–8. doi: 10.1182/blood-2010-06-289561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Aeronautics and Space Administration. Total Ozone Mapping Spectrometer data product: erythemal UV exposure. Greenbelt, MD: Goddard Space Flight Center; 2004. [Google Scholar]
- 31.Fioletov VE, McArthur LJ, Mathews TW, Marrett L. On the relationship between erythemal and vitamin D action spectrum weighted ultraviolet radiation. Journal of photochemistry and photobiology B, Biology. 2009;95:9–16. doi: 10.1016/j.jphotobiol.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Lean JL, Rottman GJ, Kyle HL, Woods TN, Hickey JR, Puga LC. Detection and parameterization of variations in solar mid- and near-ultraviolet radiation (200–400 nm) Journal of Geophysical Research: Atmospheres. 1997;102:29939–56. [Google Scholar]
- 33.Iddi S, Molenberghs G. A combined overdispersed and marginalized multilevel model. Comput Stat Data An. 2012;56:1944–51. [Google Scholar]
- 34.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunology and cell biology. 2001;79:547–68. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 35.Ullrich SE, Byrne SN. The immunologic revolution: photoimmunology. The Journal of investigative dermatology. 2012;132:896–905. doi: 10.1038/jid.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duthie MS, Kimber I, Norval M. The effects of ultraviolet radiation on the human immune system. The British journal of dermatology. 1999;140:995–1009. doi: 10.1046/j.1365-2133.1999.02898.x. [DOI] [PubMed] [Google Scholar]
- 37.Purdue MP, Freedman DM, Gapstur SM, Helzlsouer KJ, Laden F, Lim U, Maskarinec G, Rothman N, Shu XO, Stevens VL, Zeleniuch-Jacquotte A, Albanes D, et al. Circulating 25-hydroxyvitamin D and risk of non-hodgkin lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. American journal of epidemiology. 2010;172:58–69. doi: 10.1093/aje/kwq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim U, Freedman DM, Hollis BW, Horst RL, Purdue MP, Chatterjee N, Weinstein SJ, Morton LM, Schatzkin A, Virtamo J, Linet MS, Hartge P, et al. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. International journal of cancer Journal international du cancer. 2009;124:979–86. doi: 10.1002/ijc.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luczynska A, Kaaks R, Rohrmann S, Becker S, Linseisen J, Buijsse B, Overvad K, Trichopoulou A, Valanou E, Barmpitsioti A, Masala G, Agnoli C, et al. Plasma 25-hydroxyvitamin D concentration and lymphoma risk: results of the European Prospective Investigation into Cancer and Nutrition. The American journal of clinical nutrition. 2013;98:827–38. doi: 10.3945/ajcn.112.054676. [DOI] [PubMed] [Google Scholar]
- 40.Wu XC, Andrews P, Chen VW, Groves FD. Incidence of extranodal non-Hodgkin lymphomas among whites, blacks, and Asians/Pacific Islanders in the United States: anatomic site and histology differences. Cancer epidemiology. 2009;33:337–46. doi: 10.1016/j.canep.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Archives of internal medicine. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skibola CF, Bracci PM, Nieters A, Brooks-Wilson A, de Sanjose S, Hughes AM, Cerhan JR, Skibola DR, Purdue M, Kane E, Lan Q, Foretova L, et al. Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA) polymorphisms and risk of non-Hodgkin lymphoma in the InterLymph Consortium. American journal of epidemiology. 2010;171:267–76. doi: 10.1093/aje/kwp383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freedman DM, Cahoon EK, Rajaraman P, Major JM, Doody MM, Alexander BH, Hoffbeck RW, Kimlin MG, Graubard BI, Linet MS. Sunlight and other determinants of circulating 25-hydroxyvitamin D levels in black and white participants in a nationwide U.S. study. American journal of epidemiology. 2013;177:180–92. doi: 10.1093/aje/kws223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, Watson CT, Morahan JM, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome research. 2010;20:1352–60. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuoka LY, McConnachie P, Wortsman J, Holick MF. Immunological responses to ultraviolet light B radiation in Black individuals. Life sciences. 1999;64:1563–9. doi: 10.1016/s0024-3205(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 46.Cahoon EK, Wheeler DC, Kimlin MG, Kwok RK, Alexander BH, Little MP, Linet MS, Freedman DM. Individual, environmental, and meteorological predictors of daily personal ultraviolet radiation exposure measurements in a United States cohort study. PloS one. 2013;8:e54983. doi: 10.1371/journal.pone.0054983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:401–4. doi: 10.1158/1055-9965.EPI-06-1056. [DOI] [PubMed] [Google Scholar]
- 48.Muller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiology. Annals of hematology. 2005;84:1–12. doi: 10.1007/s00277-004-0939-7. [DOI] [PubMed] [Google Scholar]