Abstract
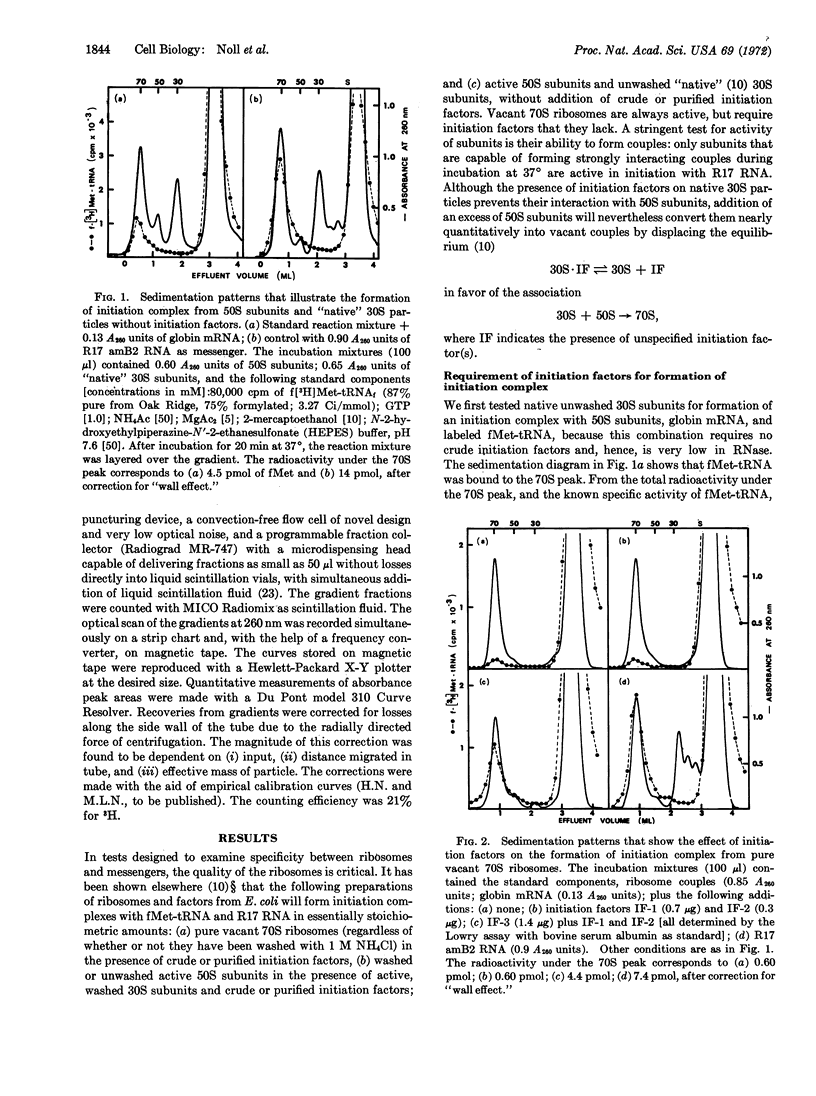
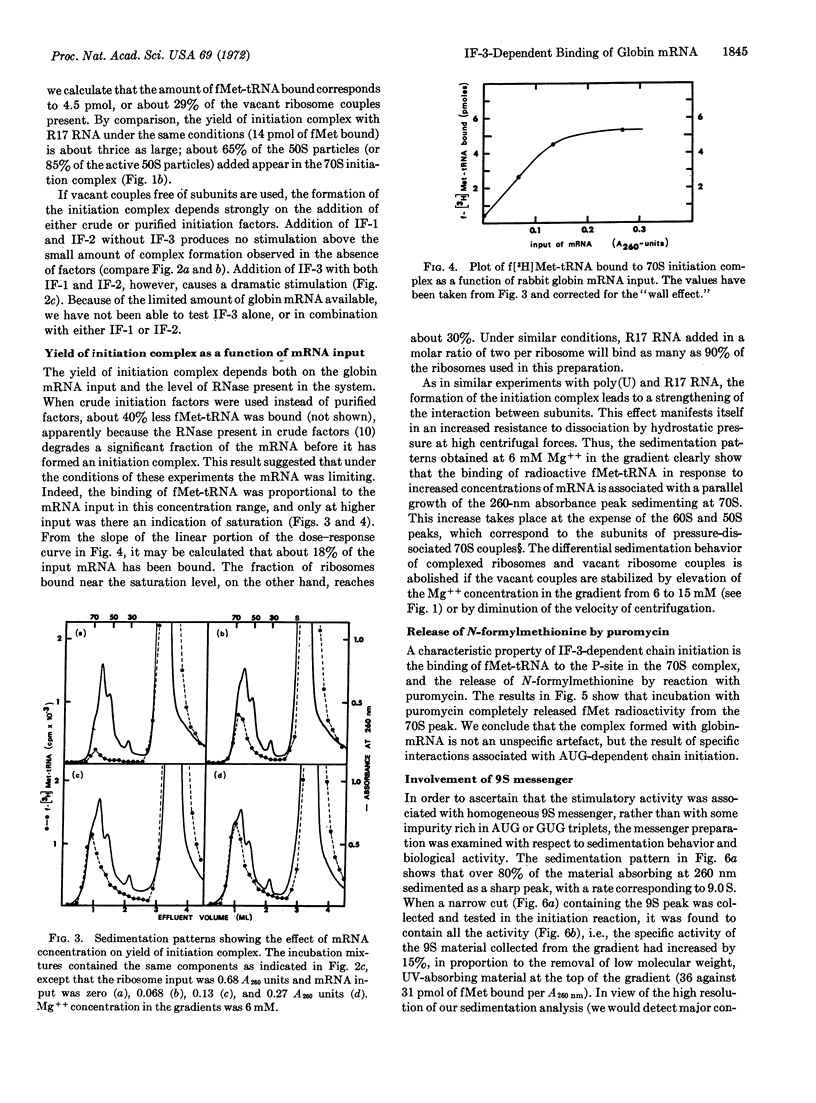
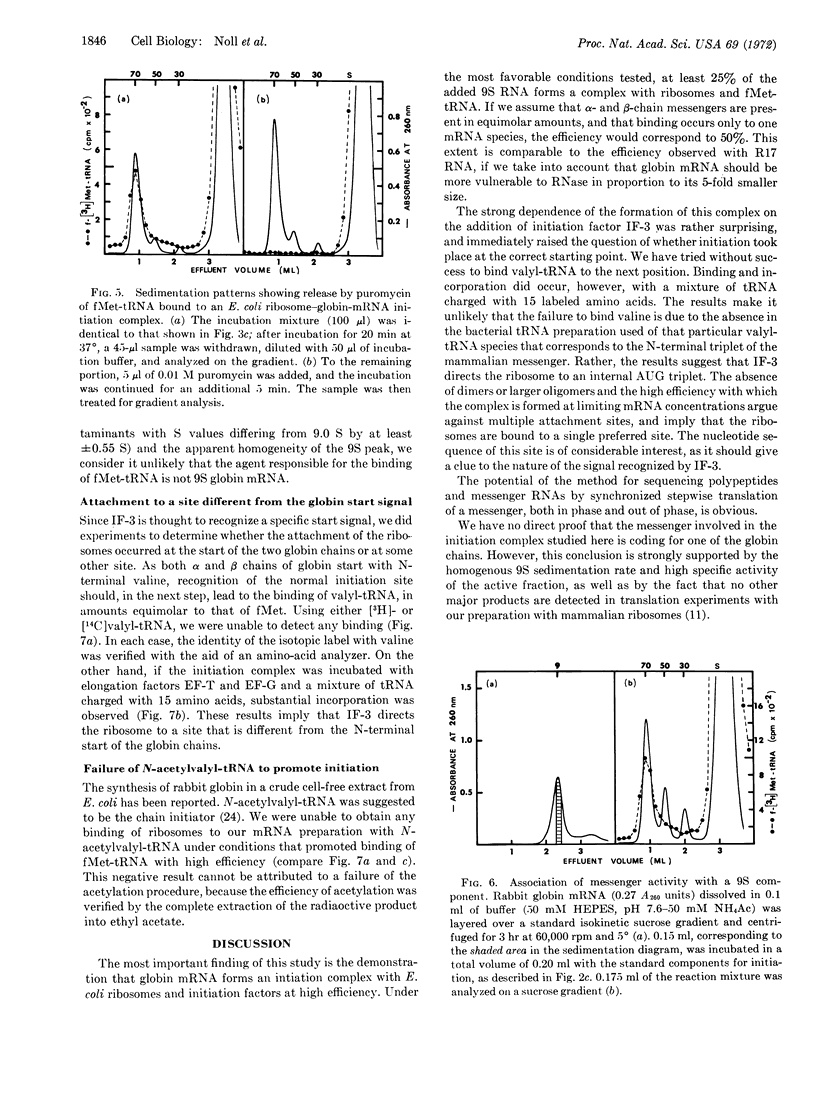
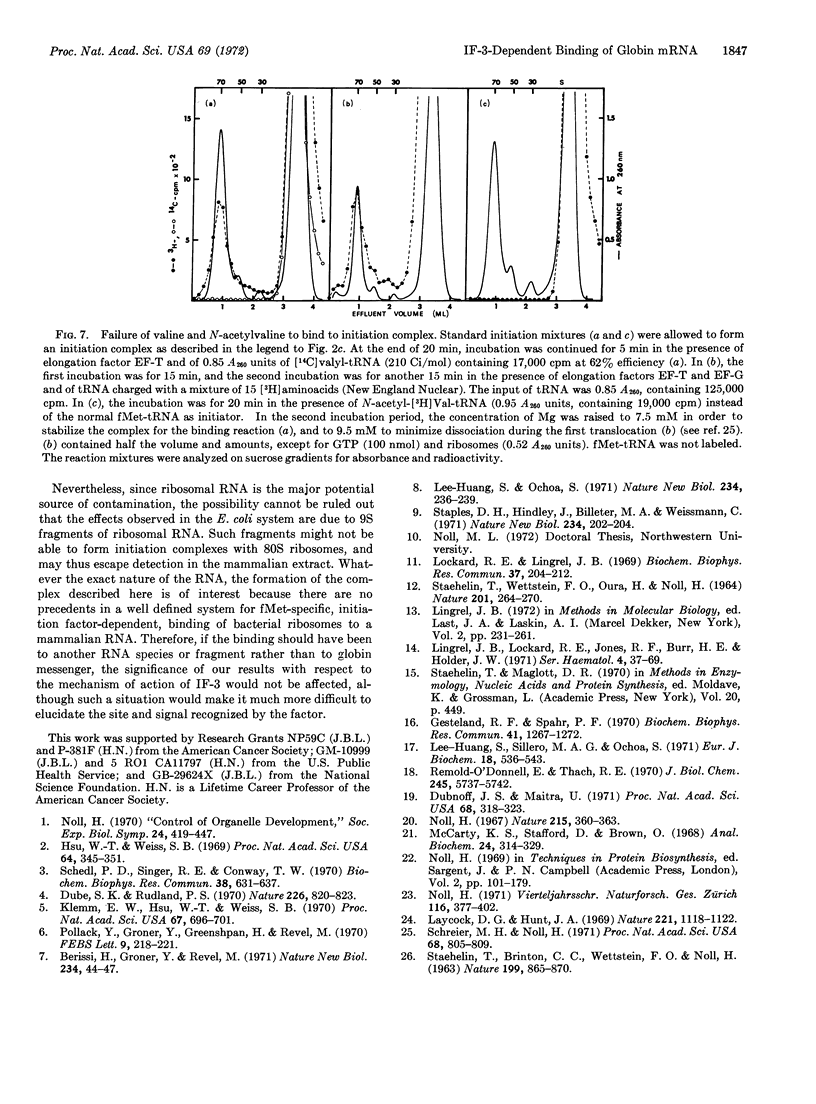
An initiation complex has been formed in high yields from E. coli ribosomes, 9S messenger RNA for rabbit hemoglobin, and N-formylmethionine-tRNA. Initiation factor IF-3 is required for the binding and puromycin is required for the release of fMet. Valyl-tRNA fails to bind to the second codon, whereas a mixture of 15 aminoacyl-tRNAs promotes incorporation. Together with quantitative data, the findings suggest that IF-3 directs the ribosomes to an AUG codon on one of the two globin messengers, at a site that is different from the normal starting point for globin synthesis.
Keywords: puromycin, AUG codon, valyl-tRNA binding, protein synthesis, hemoglobin
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berissi H., Groner Y., Revel M. Effect of a purified initiation factor F3 (B) on the selection of ribosomal binding sites on phage MS2 RNA. Nat New Biol. 1971 Nov 10;234(45):44–47. doi: 10.1038/newbio234044a0. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Rudland P. S. Control of translation by T4 phage: altered binding of disfavoured messengers. Nature. 1970 May 30;226(5248):820–823. doi: 10.1038/226820a0. [DOI] [PubMed] [Google Scholar]
- Dubnoff J. S., Maitra U. Isolation and properties of polypeptide chain initiation factor FII from Escherichia coli: evidence for a dual function. Proc Natl Acad Sci U S A. 1971 Feb;68(2):318–323. doi: 10.1073/pnas.68.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F., Spahr P. F. A new procedure for the preparation of highly labeled intact 32P RNA from bacteriophage R-17. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1267–1272. doi: 10.1016/0006-291x(70)90224-x. [DOI] [PubMed] [Google Scholar]
- Hsu W. T., Weiss S. B. Selective translation of T4 template RNA by ribosomes from T4-infected Escherichia coli. Proc Natl Acad Sci U S A. 1969 Sep;64(1):345–351. doi: 10.1073/pnas.64.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem E. B., Hsu W. T., Weiss S. B. The selective inhibition of protein initiation by T4 phage-induced factors. Proc Natl Acad Sci U S A. 1970 Oct;67(2):696–701. doi: 10.1073/pnas.67.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock D. G., Hunt J. A. Synthesis of rabbit globin by a bacterial cell free system. Nature. 1969 Mar 22;221(5186):1118–1122. doi: 10.1038/2211118a0. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S., Ochoa S. Messenger discriminating species of initiation factor F3. Nat New Biol. 1971 Dec 22;234(51):236–239. doi: 10.1038/newbio234236a0. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S., Sillero M. A., Ochoa S. Isolation and properties of crystalline initiation factor F1 from Escherichia coli ribosomes. Eur J Biochem. 1971 Feb;18(4):536–543. doi: 10.1111/j.1432-1033.1971.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Lingrel J. B., Lockard R. E., Jones R. F., Burr H. E., Holder J. W. Biologically active messenger-RNA for hemoglobin. Ser Haematol. 1971;4(3):37–69. [PubMed] [Google Scholar]
- Lockard R. E., Lingrel J. B. The synthesis of mouse hemoglobin beta-chains in a rabbit reticulocyte cell-free system programmed with mouse reticulocyte 9S RNA. Biochem Biophys Res Commun. 1969 Oct 8;37(2):204–212. doi: 10.1016/0006-291x(69)90720-7. [DOI] [PubMed] [Google Scholar]
- McCarty K. S., Stafford D., Brown O. Resolution and fractionation of macromolecules by isokinetic sucrose density gradient sedimentation. Anal Biochem. 1968 Aug;24(2):314–329. doi: 10.1016/0003-2697(68)90185-1. [DOI] [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]
- Noll H. Organelle integration and the evolution of ribosome structure and function. Symp Soc Exp Biol. 1970;24:419–447. [PubMed] [Google Scholar]
- Pollack Y., Groner Y., Aviv(Greenshpan) H., Revel M. Role of initiation factor B (F3) in the preferential translation of T4 late messenger RNA in T4 infected E. Coli. FEBS Lett. 1970 Aug 17;9(4):218–221. doi: 10.1016/0014-5793(70)80359-3. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Thach R. E. A new method for the purification of initiation factor F2 in high yield, and an estimation of stoichiometry in the binding reaction. J Biol Chem. 1970 Nov 10;245(21):5737–5742. [PubMed] [Google Scholar]
- STAEHELIN T., BRINTON C. C., WETTSTEIN F. O., NOLL H. STRUCTURE AND FUNCTION OF E. COLI ERGOSOMES. Nature. 1963 Aug 31;199:865–870. doi: 10.1038/199865a0. [DOI] [PubMed] [Google Scholar]
- STAEHELIN T., WETTSTEIN F. O., OURA H., NOLL H. DETERMINATION OF THE CODING RATIO BASED ON MOLECULAR WEIGHT OF MESSENGER RIBONUCLEIC ACID ASSOCIATED WITH ERGOSOMES OF DIFFERENT AGGREGATE SIZE. Nature. 1964 Jan 18;201:264–270. doi: 10.1038/201264a0. [DOI] [PubMed] [Google Scholar]
- Schedl P. D., Singer R. E., Conway T. W. A factor required for the translation of bacteriophage f2 RNA in extracts of T4-infected cells. Biochem Biophys Res Commun. 1970 Feb 20;38(4):631–637. doi: 10.1016/0006-291x(70)90627-3. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Noll H. Conformational changes in ribosomes during protein synthesis. Proc Natl Acad Sci U S A. 1971 Apr;68(4):805–809. doi: 10.1073/pnas.68.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples D. H., Hindley J., Billeter M. A., Weissmann C. Localization of Q-beta maturation cistron ribosome binding site. Nat New Biol. 1971 Sep 15;234(50):202–204. doi: 10.1038/newbio234202a0. [DOI] [PubMed] [Google Scholar]


