Abstract
Posterior cingulate cortex (PCC) accumulates amyloid-β (Aβ) early in Alzheimer’s disease (AD). The relative concentrations of full-length Aβ and truncated, pyroglutamate-modified Aβ (NpE3) forms, and their correlations to cognitive dysfunction in AD, are unknown. We quantified AβNpE3-42, AβNpE3-40, Aβ1−42, and Aβ1-40 concentrations in soluble (nonfibrillar) and insoluble (fibrillar) pools in PCC from subjects with an antemortem clinical diagnosis of no cognitive impairment, mild cognitive impairment, or mild-moderate AD. In clinical AD, increased PCC concentrations of Aβ were observed for all Aβ forms in the insoluble pool but only for Aβ1-42 in the soluble pool. Lower Mini-Mental State Exam and episodic memory scores correlated most strongly with higher concentrations of soluble and insoluble Aβ1-42. Greater neuropathology severity by Consortium to Establish a Registry for Alzheimer’s Disease and National Institute on Aging-Reagan pathologic criteria was associated with higher concentrations of all measured Aβ forms, except soluble AβNpE3-40. Low concentrations of soluble pyroglutamate Aβ across clinical groups likely reflect its rapid sequestration into plaques, thus, the conversion to fibrillar Aβ may be a therapeutic target.
Keywords: Pyroglutamate-modified Aβ, Amyloid-β, Alzheimer’s disease, Posterior cingulate cortex, MCI, Episodic memory
1. Introduction
The posterior cingulate cortex (PCC) is a component of the default mode network associated with episodic memory retrieval (Sestieri et al., 2011; Wagner et al., 2005). Functional imaging studies report hypometabolism and fibrillar amyloid-β (Aβ) accumulation in the PCC in mild cognitive impairment (MCI) and early clinical stages of Alzheimer’s disease (AD) (Aizenstein et al., 2008; Klunk et al., 2004; Minoshima et al., 1997) suggesting that dysfunction of this brain region contributes to early cognitive impairment and parallels the development of Aβ plaques. The considerable overlap of Aβ deposition with metabolic impairment and functional disconnection in the default mode network, including the PCC (Sperling et al., 2009), supports the hypothesis that Aβ accumulation in cortical association areas is a source, or a putative marker, of functional impairment in AD. The main goal of the present study was to determine the concentrations of Aβ peptides in the PCC before and in early clinical AD.
Full-length Aβ peptides ending at amino acid residues 42 or 40 (Aβ1-42 and Aβ1-40, respectively) are the predominant Aβ species in brain and concentrations of both insoluble (fibrillar, β-pleated sheet conformed aggregates) and soluble (nonfibrillar, diffusible in physiological solution) Aβ pools increase in AD (McLean et al.,1999; Naslund et al., 2000; Wang et al., 1999). Posttranslational modifications also result in N-terminus truncated forms of Aβ reaching 60% of total Aβ load in AD and aged Down syndrome brains (Masters et al., 1985). Aβ truncated at the third or 11th glutamate residue can be modified by glutaminyl cyclase into pyroglutamate forms NpE3 and NpE11, respectively (Cynis et al., 2008). Compared with unmodified Aβ, AβNpE is more resistant to peptidase cleavage, which may impede clearance resulting in its accumulation in brain (Jawhar et al., 2011), where it might contribute to seeding of amyloid plaques (He and Barrow, 1999; Jawhar et al., 2011; Schilling et al., 2006; Sullivan et al., 2011). However, the relative abundances of AβNpE and unmodified full-length Aβ forms during the earliest clinical stages of AD remain controversial. Several reports indicate that AβNpE3-x is more abundant than Aβ1-x in AD and DS (Frost., 2013; Iwatsubo et al., 1996; Saido et al., 1995). Others reported that AβNpE3-42 and Aβ1-42 species were comparable in aggregation propensity, toxicity, and abundance (Moore et al., 2012; Portelius et al., 2010; Tekirian et al., 1999; Youssef et al., 2008). Higher levels of both pyroglutamate and unmodified Aβ were reported in advanced-stage AD relative to non-AD controls (Wu et al., 2013). By contrast, pyroglutamate Aβ levels during the early stages of cognitive impairment and AD dementia in clinically and pathologically well-characterized cases remain to be evaluated. In this study, we quantified concentrations of soluble and insoluble AβNpE3 and full-length unmodified Aβ using enzyme-linked immunosorbant assay (ELISA) in PCC gray matter from subjects with an antermortem clinical diagnosis of no cognitive impairment (NCI), MCI, and AD. This study tested the hypothesis that compared with full-length Aβ forms, PCC concentrations of AβNpE3-42 and AβNpE3-40 correlate more strongly with cognitive impairment and neuropathology measures during the progression of AD.
2. Methods
2.1. Subjects
This study included 58 cases from the Rush Religious Orders Study (RROS), a longitudinal clinicopathologic study of aging and AD (Bennett et al., 2002; Mufson et al., 1997). Details of clinical evaluation in the RROS cohort were published previously (Wilson et al., 2002). Based on clinical history review, cases examined were classified with NCI (n = 19), MCI (n = 21), and AD (n = 18). AD cases were mild-moderate (mAD) based on Mini-Mental State Examination (MMSE) (Folstein et al., 1975) scores (see Table 1). Final clinical diagnosis was made using previously reported clinical criteria (DeKosky et al., 2002; Ikonomovic et al., 2011; Mufson et al., 1999). Global cognitive score (GCS) and episodic memory tests have been described previously (Wilson et al., 2002). Briefly, GCS is a composite z-score based on 19 cognitive tests; GCS > 0 indicates that, compared with all PROS recruits at baseline, the subject’s cognitive function is above average, whereas GCS < 0 indicates a score below average. Neuropathological diagnosis was based on the recommendations of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), Braak neurofibrillary tangle staging, and the National Institute on Aging-Reagan Institute criteria (Braak and Braak, 1991; Mirra et al., 1991; NIA-Reagan Working Group, 1997). Application of the new National Institute on Aging-Alzheimer’s Association guidelines (Hyman et al., 2012; Montine et al., 2012) to the RROS cohort is currently ongoing. Cases with non-AD pathologies (e.g., stroke or Parkinson’s disease) were excluded from the study. All cases were de-identified and investigators were blinded to demographics and diagnosis. Rush University Institutional Review Board and the University of Pittsburgh’s Committee for Oversight of Research and Clinical Training Involving Decedents approved the study.
Table 1.
Demographic and clinical characteristics by clinical diagnosis category
| Clinical diagnosis |
p-value | Pairwise comparison |
||||
|---|---|---|---|---|---|---|
| NCI (n = 19) | MCI (n = 21) | AD (n = 18) | Total (N = 58) | |||
| Age (y) at death: mean ± SD (range) | 85.6 ± 3.9 (78.1–92.8) | 86.3 ± 5.1 (75.4–94) | 90.1 ± 5.4 (76.4–100.9) | 88.1 ± 6.0 (73–100.9) | 0.01 | NCI < AD |
| Number (%) of males | 5 (26.3) | 8 (38.1) | 5 (27.8) | 18 (31.0) | 0.8a | d |
| Years of education: mean ± SD (range) | 17.3 ± 3.6 (10–25) | 18 ± 3.5 (10–25) | 90.1 ± 5.4 (14–26) | 15.9 ± 3.0 (10–26) | 0.7b | d |
| Number (%) with ApoE ε4 allele | 1 (5.3) | 8 (38.1) | 5 (29.4) | 14 (24.6) | 0.04a | d |
| MMSE mean ± SD (range) | 28.1 ± 1.6 (26–30) | 27 ± 2.7 (22–30) | 19.5 ± 5.3 (11–28) | 24.2 ± 6.4 (11–30) | <0.0001b | (NCI, MCI) > AD |
p-value from ANOVA, if not specified otherwise.
Pairwise comparisons reported are considered statistically significant after Bonferroni adjustment (0.05/3 = 0.016).
Key: AD, Alzheimer’s disease; ANOVA, analysis of variance; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; NCI, no cognitive impairment; SD, standard deviation.
Fisher exact test.
Kruskal-Wallis test.
2.2. Tissue samples
Frozen PCC (Brodmann area 23) graymatter harvested at autopsy was homogenized on ice in phosphate-buffered saline (pH 7.4; 300 mg/mL) supplemented with protease inhibitors (Sigma 8340 protease inhibitor cocktail; 10 µL/mL buffer) to final concentration of 150 mg/mL; samples were then centrifuged at 100,000× g for 1 hour at 4 °C. Supernatant was collected as the soluble (nonfibrillar) fraction and divided into aliquots; the pellet was re-homogenized by sonication in 70% formic acid, and centrifuged at 113,000x g for 1 hour at 4 °C. Supernatant was collected as the insoluble (fibrillar) fraction, neutralized to pH 7.4, aliquoted, and frozen at −80°C until assay. There were no differences in tissue sample storage time among clinical groups and ELISAs were run in parallel for each group.
2.3. Quantification of AβNpE3-40, AβNpE3-42 and Aβ1-40, Aβ1-42 concentrations
AβNpE3-40 and AβNpE3-42 concentrations were assayed in triplicates using a chemiluminescence-based ELISA (IBL, Japan) with a capture antibody specific for the neoepitope at carboxy terminal amino acids 40 or 42 of human Aβ and detection antibodies specific for AβNpE3. Values were determined from standard curves using synthetic human AβNpE3-40 or AβNpE3-42 peptides (IBL) and were expressed as picomoles per gram tissue wet weight. Aβ1-40 and Aβ1-42 concentrations were analyzed in triplicates, using chemiluminescent-based ELISA (Invitrogen, Camarillo, CA, USA) (Ikonomovic et al., 2008). ELISA assays were tested for cross-reactivity to Aβ species detection by assaying known concentrations of synthetic AβNpE3-40 and AβNpE3-42 peptides on the Aβ1-40 and Aβ1-42-specific ELISAs, and the converse; in all cases, no signal was detected (data not shown).
2.4. Statistical analyses
Primary outcome measures were concentrations of Aβ1-40, Aβ1-42, AβNpE3-40, and AβpE3-42 in the PCC. Demographic, clinical, and neuropathologic measures were predictor variables. Clinical groups were compared on demographic characteristics, neuropathology scores, and biochemical measures using analysis of variance, Kruskal-Wallis tests (Wilcoxon post hoc tests) or Fisher exact tests, as appropriate. Associations among variables were assessed by Spearman rank correlations. Nonparametric methods were preferred for the primary outcomes because of outliers and non-normality. Results were confirmed using analysis of variance of the transformed data (e.g., using square-root). Overall, the level of statistical significance was set at 0.05 (2-sided). A Bonferroni-corrected significance threshold of 0.0167 (= 0.05/3) was used to account for the possible comparisons between the 3 clinical groups.
3. Results
3.1. Subject demographics, clinical, and neuropathology characteristics
NCI, MCI, and mAD groups did not differ in years of education, sex, postmortem interval, brain tissue pH, or Braak score (Tables 1 and 2). The clinical diagnostic groups differed in MMSE scores and age; mAD subjects were more impaired than NCI and MCI subjects and older than NCI subjects. The groups differed by CERAD and NIA-Reagan neuropathology criteria, with mAD group having a greater pathology burden than the NCI but not the MCI group.
Table 2.
Neuropathological characteristics by clinical diagnosis category
| Clinical diagnosis |
p-value | Pairwise comparison |
||||
|---|---|---|---|---|---|---|
| NCI (n = 19) | MCI (n = 21) | AD (n = 18) | Total (N = 58) | |||
| Postmortem interval, h mean ± SD (range) | 5.2 ±2.1 (1–9.1) | 5.8 ±2.4 (2–11.5) | 4.9 ±2 (1.5–8.7) | 4.2 ±2.0 (1–9.1) | 0.7a | — |
| Tissue pH mean ± SD (range) | 6.5 ± 0.1 (6.3–6.7) | 6.5 ± 0.1 (6.2–6.6) | 6.5 ± 0.1 (6.2–6.8) | 6.5 ± 0.1 (6.2–6.8) | 0.6a | — |
| Distribution of Braak scores | ||||||
| 0 | 0 | 0 | 0 | 0 | 0.1a | — |
| I/II | 4 | 5 | 1 | 10 | ||
| III/IV | 14 | 12 | 12 | 38 | ||
| V/VI | 1 | 4 | 5 | 10 | ||
| CERAD diagnosis | ||||||
| No AD | 4 | 7 | 0 | 11 | 0.02a | AD > NCI |
| Possible | 5 | 2 | 2 | 9 | ||
| Probable | 9 | 8 | 10 | 27 | ||
| Definite | 1 | 4 | 6 | 11 | ||
| NIA-Reagan diagnosis (likelihood of AD) | ||||||
| No AD | 0 | 0 | 0 | 0 | 0.02a | AD > NCI |
| Low | 11 | 10 | 3 | 24 | ||
| Intermediate | 8 | 8 | 12 | 28 | ||
| High | 0 | 3 | 3 | 6 | ||
Pairwise comparisons reported are considered statistically significant after Bonferroni adjustment (0.05/3 = 0.016).
Key: AD, Alzheimer’s disease; ANOVA, analysis of variance; MCI, mild cognitive impairment; NCI, no cognitive impairment; SD, standard deviation.
Kruskal-Wallis test.
3.2. PCC pyroglutamate-modified and full-length Aβ concentrations
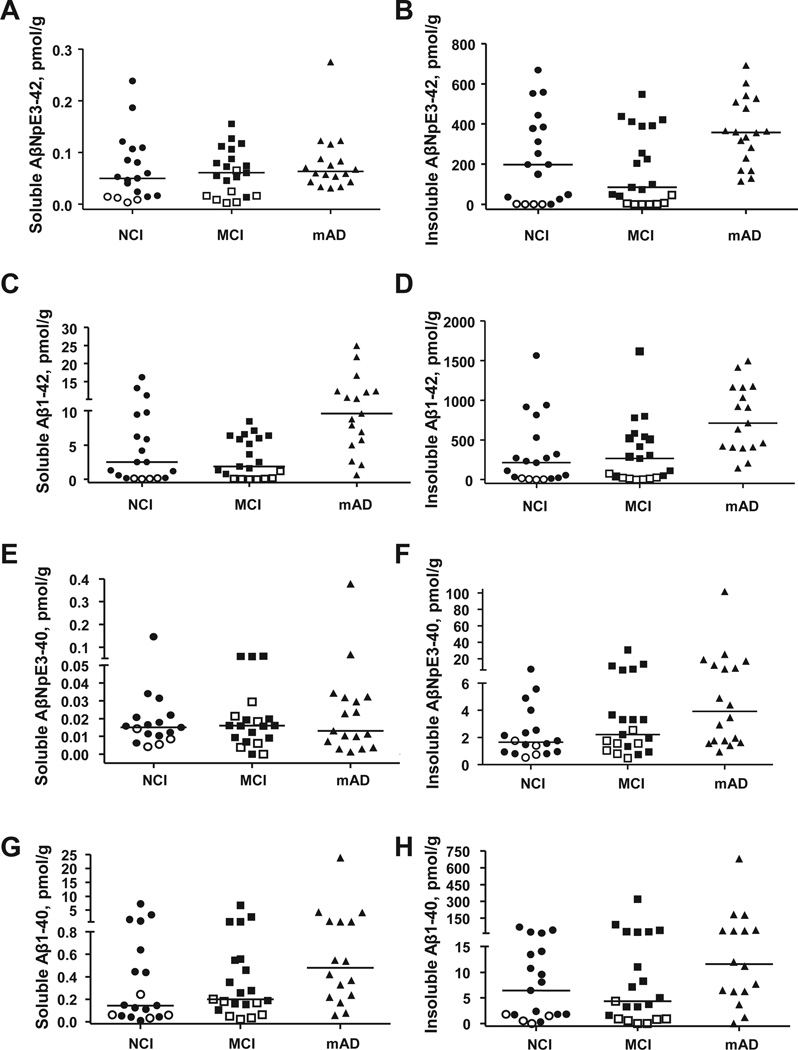
PCC concentrations of all Aβ forms were higher in the insoluble compared with the soluble Aβ pool. Insoluble AβNpE3-42 and Aβ1-42 exhibited the highest concentrations (median values: 227.6 and 317.4 pmol/g, respectively; Fig. 1B and D, Table 3). Insoluble AβNpE3-40 and Aβ1-40 were in the lower picomolar range (median values: 2.2 and 6.8 pmol/g, respectively; Fig. 1F and H, Table 3). In the soluble Aβ pool, Aβ1-42 was also the most abundant Aβ form, followed by Aβ1-40; both in low picomolar range. Concentrations of soluble AβNpE3-42 and AβNpE3-40 were the lowest of all measured Aβ forms, consistently below 0.3 pmol/g (Fig. 1).
Fig. 1.
Concentrations of soluble (A, C, E, and G) and insoluble (B, D, F, and H) forms of AβNpE3-42 (A, B), Aβ1-42 (C, D), AβNpE3-10 (E, F), and Aβ1-40 (G, H) in the PCC across the RROS clinical groups. In the pool of soluble Aβ, only Aβ1-42 concentrations are significantly different in mAD compared with the NCI and MCI (p < 0.001) (C), although the three clinical groups do not differ by AβNpE3-12, Aβl-40, or AβNpE3-10 concentrations (p = 0.17, 0.66, and 0.39, respectively). Insoluble AβNpE3-42, Aβ1-42, and AβNpE3-40 concentrations are significantly higher in mAD compared with NCI and MCI (p < 0.05), whereas concentrations of insoluble Aβl-40 are not different among the clinical groups (p = 0.08). Open symbols represent pathology free cases (not AD by CERAD criteria). Horizontal bars depict median concentration. Abbreviations: Aβ, amyloid-β; AD, Alzheimer’s disease; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; mAD, mild-moderate Alzheimer’s disease; MCI, mild cognitive impairment; NCI, no cognitive impairment; PCC, posterior cingulate cortex; RROS, Rush Religious Orders Study.
Table 3.
Summary of soluble and insoluble pyroglutamate-modified and full length Aβ concentrations by clinical diagnosis category
| Clinical diagnosis, median (range) | ||||||
|---|---|---|---|---|---|---|
| Aβ species, pmol/g | NCI (n = 19) | MCI (n = 21) | AD (n = 18) | Total (N = 58) | p-valuea | Pairwise comparison |
| Insoluble AβNpE3-42 | 197.9 (0.01 –669.0) | 84.8 (0.01–548.3) | 358.5 (115.1–692.1) | 227.6 (0.01–692.1) | 0.005 | NCI, MCI < AD |
| Insoluble Aβ1-42 | 212.8 (0.01–1564.1) | 266.0 (0.01–1615.3) | 710.7 (141.9–1495.1) | 317.4 (0.01–1615.3) | 0.0009 | NCI, MCI < AD |
| Insoluble AβNpE3-40 | 1.7 (0.5–7.1) | 2.2 (0.5–30.6) | 3.9 (0.9–101.7) | 2.2 (0.5–101.7) | 0.03 | NCI < AD |
| Insoluble Aβ1-40 | 6.5 (0.01–69.5) | 4.4 (0.01–319.3) | 11.6 (0.01–681.2) | 6.8 (0.01–681.2) | 0.08 | — |
| Soluble AβNpE3-42 | 0.05 (0.004–0.2) | 0.06 (0.003–0.2) | 0.06 (0.03–0.3) | 0.06 (0.003–0.3) | 0.4 | — |
| Soluble Aβ1-42 | 2.5 (0.06–16.2) | 1.9 (0.01–8.5) | 9.6 (0.6–24.9) | 5 (0.01–24.9) | 0.0003 | NCI, MCI < AD |
| Soluble AβNpE3-40 | 0.02 (0.004–0.1) | 0.02 (0.0001–0.06) | 0.01 (0.001–0.4) | 0.02 (0.0001–0.4) | 0.7 | — |
| Soluble Aβ1-40 | 0.1 (0.009–7.3) | 0.2 (0.02–6.7) | 0.5 (0.06–23.9) | 0.2 (0.009–23.9) | 0.2 | — |
Pairwise comparisons reported are considered statistically significant after Bonferroni adjustment (0.05/3 = 0.016). Key: Aβ, amyloid-β; AD, Alzheimer’s disease; MCI, mild cognitive impairment; NCI, no cognitive impairment.
Kruskal-Wallis test.
In the insoluble pool of Aβ in the PCC, there were strong associations among the measured Aβ forms (correlations ranged from rs = 0.54 [for AβNpE3-40 vs. Aβ1-40] to rs = 0.89 [for AβNpE3-42 vs. Aβl-42], all p < 0.0001). In the soluble pool of Aβ in the PCC, significant associations were between Aβ1-42 and Aβ1-40 (rs = 0.65, p < 0.00001) and AβNpE3-40 and Aβ1-40 (rs = 0.4, p = 0.003). There were significant correlations between insoluble and soluble Aβ forms except between insoluble Aβ1-42 and soluble AβNpE3-40.
3.3. PCC pyroglutamate-modified and full-length Aβ concentrations by clinical diagnosis
The three clinical diagnostic groups differed significantly in PCC concentrations of insoluble Aβ1-42 (p = 0.0009) and AβNpE3-42 (p = 0.005), with higher levels in mAD compared with both NCI and MCI groups. NCI, MCI, and mAD cases also differed in insoluble AβNpE3-40 (p = 0.03) with mAD higher than the NCI group. Difference in insoluble Aβ1-40 among the clinical groups was not significant (p = 0.08). Of all measured Aβ forms in the soluble pool only Aβ1 -42 concentration differed significantly among the clinical groups (p = 0.0003) with the mAD group higher than both NCI and MCI groups (Fig. 1). An exploratory analysis of a small group of severe AD cases from the same cohort (n = 6; mean age: 93.62 ± 4.14, range: 88.18–98.69 years; mean MMSE: 5.67 ± 3.67, range: 2–10) demonstrated that none of the Aβ measures differed statistically when comparing these severe AD with moderate AD (MMSE range 11–20) and mild AD (MMSE range 21–28) cases.
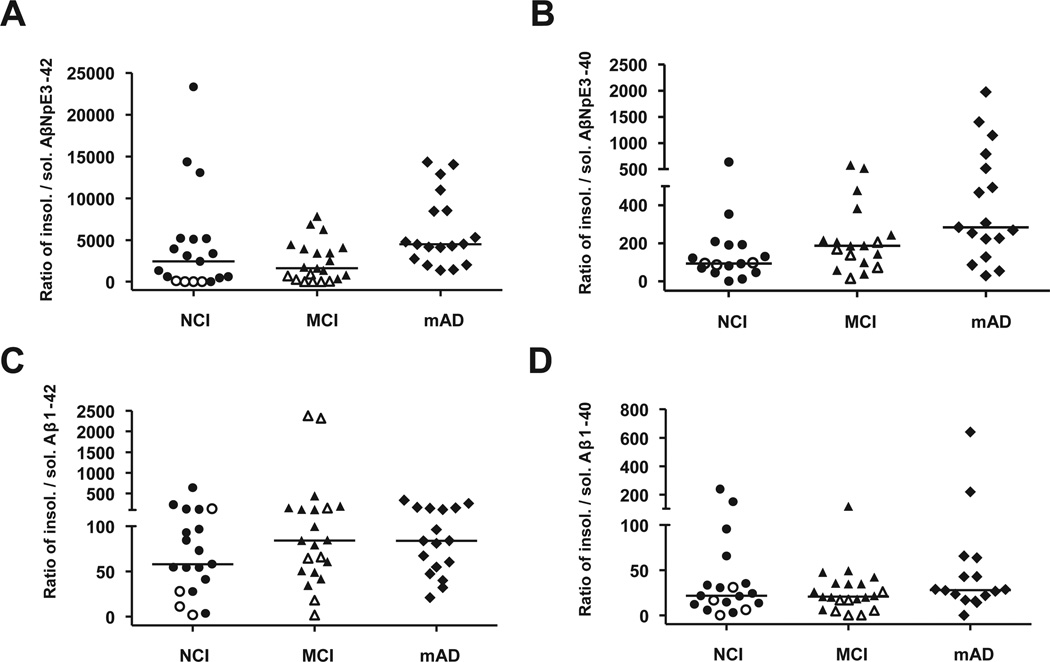
When the ratios of insoluble to soluble Aβ concentrations were compared across the clinical groups, only pyroglutamate Aβ forms displayed significant differences (insoluble to soluble AβNpE3-42: p = 0.008, mAD > NCI, MCI; insoluble to soluble AβNpE3-40: p = 0.006, mAD > NCI) (Fig. 2), due entirely to increases in insoluble pyroglutamate Aβ.
Fig. 2.
Ratios of insoluble to soluble forms of pyroglutamate and full-length Aβ across RROS clinical groups. Ratios of insoluble to soluble Aβ are significantly increased in mAD for AβNpE3-42 (mAD > NCI, MCI; p = 0.008; A) and AβNpE3-40 (mAD > NCI; p = 0.006; B), but not for Aβ1-42 (p = 0.47; C) and Aβ1-40 (p = 0.35; D). Open symbols represent pathology-free cases (not AD by CERAD criteria). Horizontal bars depict median concentration. Abbreviations: Aβ, amyloid-β; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; mAD, mild-moderate Alzheimer’s disease; MCI, mild cognitive impairment; NCI, no cognitive impairment; RROS, Rush Religious Orders Study.
3.4. Associations between PCC Aβ concentrations and demographics
Across all cases, changes in Aβ species were not associated with sex or postmortem interval (Table 4). Greater concentrations of insoluble and soluble full-length Aβ, but not pyroglutamate Aβ, were weakly associated with a greater age at death (Table 4). Higher soluble AβNpE3-42 was associated with more years of education, and higher soluble and insoluble Aβl-40 was weakly associated with lower brain pH levels (Table 4). Greater concentration of insoluble AβNpE3-40 was associated with the presence of APOE4 (p = 0.018).
Table 4.
Associations of the pyroglutamate-modified and full-length Aβ species with demographic, clinical, and postmortem diagnosis data
| Aβ species, Spearman correlations (p) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Insoluble Pyro42 | Soluble Pyro42 | Insoluble Pyro40 | Soluble Pyro40 | Insoluble Aβ 1-42 | Soluble Aβ 1-42 | Insoluble Aβ 1–40 | Soluble Aβ 1–40 | |
| Age at death, y | 0.3 (0.05) | −0.02 (0.9) | 0.2 (0.1) | 0.1 (0.3) | 0.3 (0.03) | 0.3 (0.01) | 0.3 (0.02) | 0.3 (0.02) |
| Education, y | 0.2 (0.2) | 0.3 (0.01) | 0.2 (0.08) | 0.04 (0.8) | 0.2 (0.2) | 0.1 (0.4) | 0.2 (0.07) | 0.2 (0.09) |
| Postmortem interval, h | −0.06 (0.7) | −0.1 (0.4) | −0.1 (0.3) | 0.2 (0.3) | −0.1 (0.3) | −0.1 (0.4) | −0.1 (0.4) | −0.2 (0.07) |
| Brain tissue pH | 0.03 (0.8) | −0.05 (0.7) | −0.15 (0.2) | −0.24 (0.06) | 0.03 (0.8) | 0.03 (0.8) | −0.26 (0.04) | −0.27 (0.03) |
| Braak stage | 0.5 (<0.001) | 0.3 (0.05) | 0.4 (0.005) | 0.09 (0.5) | 0.4 (<0.001) | 0.4 (0.001) | 0.4 (0.002) | 0.4 (0.006) |
| CERAD diagnosis | −0.6 (<0.001) | −0.5 (<0.001) | −0.5 (0.0003) | −0.1 (0.4) | −0.6 (<0.001) | −0.5 (<0.001) | −0.5 (<0.001) | −0.4 (0.003) |
| NIA-Reagan diagnosis | −0.6 (<0.001) | −0.4 (0.001) | −0.5 (<0.001) | −0.2 (0.3) | −0.6 (<0.001) | −0.5 (<0.001) | −0.5 (<0.001) | −0.3 (0.01) |
| MMSE score | −0.3 (0.04) | −0.08 (0.6) | −0.2 (0.1) | 0.1 (0.3) | −0.3 (0.02) | −0.4 (0.006) | −0.1 (0.4) | −0.1 (0.3) |
| Global cognition score | −0.4 (0.002) | −0.1 (0.4) | −0.3 (0.04) | 0.04 (0.8) | −0.5 (<0.001) | −0.5 (<0.001) | −0.2 (0.1) | −0.3 (0.03) |
| Episodic memory score | −0.5 (<0.001) | −0.2 (0.07) | −0.4 (0.003) | −0.03 (0.9) | −0.5 (<0.001) | −0.5 (<0.001) | −0.3 (0.02) | −0.4 (0.006) |
Key: Aβ, amyloid-β; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; MMSE, Mini-Mental State Examination.
3.5. Associations of PCC Aβ concentrations with clinical and neuropathological data
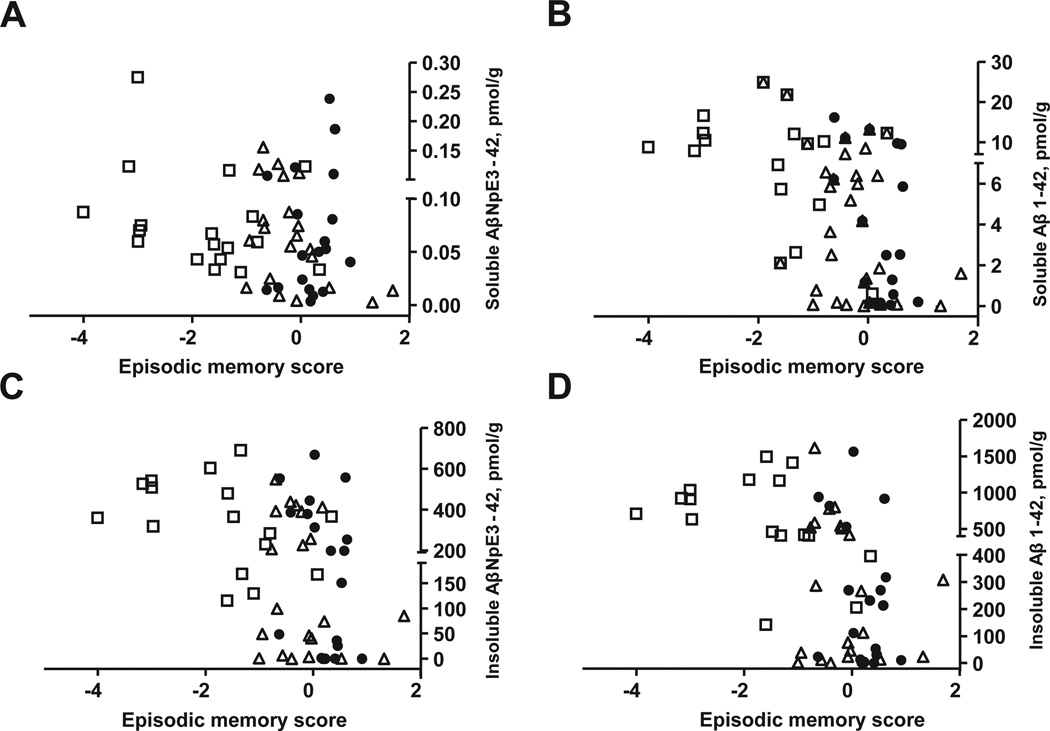
Aβ1-42 concentrations were the strongest correlates of impaired performance on neuropsychological tests; higher concentrations of both insoluble and soluble Aβ1-42 were associated with greater impairments on GCS (rs = −0.46 and rs = −0.48, respectively; p < 0.001) and episodic memory (rs = −0.52 and rs = −0.47, respectively; p < 0.001) (Fig. 3, Table 4). Insoluble and soluble Aβ1-40 concentrations correlated less strongly with cognitive measures (Table 4). Increased concentrations of insoluble, but not soluble, AβNpE3-42 and AβNpE3-40 correlated with worse episodic memory score (Fig. 3) and GCS (Table 4). With the exception of soluble AβNpE3-40, increased concentrations of all Aβ forms in both pools correlated significantly with neuropathology by CERAD and NIA-Reagan criteria (Table 4), across the entire study cohort. Most of the NCI and MCI cases had neuropathological diagnosis of possible or probable AD by CERAD criteria and exhibited a wide range of Aβ concentrations (Fig. 1, Table 2). NCI and MCI cases classified as “Not AD” by CERAD criteria had very low Aβ concentrations in both insoluble and soluble pools (Fig. 1, open symbols). Elevations in all measured Aβ species, except the two soluble pyroglutamate forms, correlated with more advanced Braak stages (Table 4).
Fig. 3.
Correlations of insoluble and soluble AβNpE3-42 and Aβ1-42 forms with episodic memory scores. Soluble AβNpE3-42 concentrations do not correlate with episodic memory scores (A; rs = −0.24, p = 0.07). Higher concentrations of soluble Aβ1-42 (B), insoluble AβNpE3-42 (C), and insoluble Aβl-42 (D) significantly correlate with worse episodic memory scores (rs = −0.47, −0.44, −0.52, respectively; p ≤ 0.001). Solid circles, open triangles, and open squares represent NCI, MCI, and mAD cases, respectively. Abbreviations: mAD, mild-moderate Alzheimer’s disease; MCI, mild cognitive impairment; NCI, no cognitive impairment.
4. Discussion
The present study found that pyroglutamate and full-length Aβ42 dominated the insoluble Aβ pool in the PCC across clinical groups, which is consistent with the hypothesis that C-terminal amino acid residue 42 (alanine) in Aβ plays a major role in amyloid plaque formation (Iwatsubo et al., 1994,1995). In the PCC soluble Aβ pool (diffusible Aβ oligomers and monomers), concentrations of Aβ1-42 and Aβ1-40 were substantially higher than concentrations of AβNpE3-42 and AβNpE3-40. Concentrations of insoluble Aβ1-42 and AβNpE3-42, and only Aβ1-42 in the soluble pool, were elevated in mAD compared with NCI and MCI and were the strongest correlates of cognitive impairment. Therefore, altered Aβ metabolism in AD involves changes in both modified and unmodified Aβ42 forms in the insoluble pool, but only in the unmodified Aβ1-42 in the soluble pool in PCC.
The current findings support observations that most of the Aβ found in the AD brain is in the insoluble and/or fibrillar pool (Naslund et al., 2000; Wang et al., 1999), with over 90% of Aβ consisting of longer, Aβ42 (43) including N-terminus truncated and/or modified forms (Gravina et al., 1995). Similar to our results, prior studies using ELISA technique found that AβNpE3-42 (43) comprised a substantial portion of total Aβ42 (43) in AD temporal cortex (Harigaya et al., 2000) and the concentration of cortical Aβ1-42 (43) was approximately 10-fold higher than Aβ1-40 (Harigaya et al., 2000). On the other hand, AβNpE3-42 (43) or AβNpE3-40 concentrations were higher than Aβ1-42 (43) or AβNpE3-40 concentrations in AD frontal cortex (Harigaya et al., 2000; Hosoda et al., 1998). This contrasts our findings of lower AβNpE3-42 or AβNpE3-40 compared with Aβ1-42 or Aβ1-40 concentrations in the insoluble Aβ pool in the PCC, independent of clinical diagnosis. Another ELISA analysis, using the same pyroglutamate antibody (AβNpE3, IBL) but a different pan-Aβ (clone 4G8) capture antibody (Wu et al., 2013), examined unspecified brain region (s) and reported that both unmodified and pyroglutamate forms of soluble Aβ are higher in advanced-stage AD than in non-AD controls (Wu et al., 2013). This contrasts with our results that soluble pyroglutamate Aβ42 and Aβ40 are stable in AD PCC. These discrepancies could be because of different subject cohorts, disease severity, brain regions examined, or other methodological differences.
In line with the current observations, mass spectrometry revealed that Aβ1-42 was a major form found in brain, however, AβNpE3-42 and other pyroglutamate forms were reported in the insoluble (Moore et al., 2012; Portelius et al., 2010) but not in the soluble Aβ pools (Moore et al., 2012). Here, concentrations of soluble AβNpE3-42 and AβNpE3-40 were detectable but consistently low in the PCC across clinical groups, suggesting that pyroglutamate Aβ forms are more prone to oligomerization and fibrillization (Harigaya et al., 2000; Pike et al., 1995; Schilling et al., 2006). Supporting this concept, immunohistochemical studies identified pyroglutamate Aβ as a major component of amyloid plaques, with either comparable or greater levels of AβNpE3-x compared with Aβ1-x (Frost., 2013; Saido et al., 1995), suggesting that pyroglutamate Aβ is an early form in AD plaque genesis. We found that both AβNpE3-42 and Aβ1-42 are major components of the insoluble Aβ pool, with Aβ1-42 concentrations clearly dominating the spectrum of Aβ peptides assayed. In the soluble Aβ fraction, AβNpE3-42 and AβNpE3-40 levels were low, and Aβ1-42 was the dominant form, correlating best with cognitive impairment. These data support the hypothesis that Aβ42 oligomers are detrimental to memory function and perhaps are an important therapeutic target and/or biomarker (Klein, 2013; Selkoe, 2008). We found that the ratios of insoluble to soluble AβNpE3-42 and AβNpE3-40, but not Aβ1-42 and Aβ1-40 were greatest in the PCC in mAD because of increases in insoluble and stability of soluble AβNpE3-42 and AβNpE3-40, possibly reflecting higher hydrophobicity and aggregation kinetics of pyroglutamate-modified Aβ compared with their full-length unmodified equivalents (He and Barrow, 1999; Sullivan et al., 2011). Our detection of soluble pyroglutamate-modified forms, albeit in low concentrations, indicates that at least some Aβ may undergo N-terminus modifications before fibril formation and deposition into amyloid plaques. Although it is likely that most of the soluble Aβ pool in the brain consists of diffusible oligomers, our ELISA assay could not distinguish between monomers and oligomers of Aβ.
4.1. Clinical correlates of pyroglutamate-modified and unmodified Aβ during AD progression
Oligomeric Aβ is linked to impaired memory in AD (Klyubin et al., 2012), but it remains unclear if modified Aβ is similarly detrimental to cognitive function. Pyroglutamate Aβ affects memory function in transgenic AD mice (Wittnam et al., 2012), and greater plasma levels of autoantibodies against pyroglutamate Aβ correlate with AD cognitive decline (Marcello et al., 2011). However, we observed that PCC soluble AβNpE3-42 and AβNpE3-40 concentrations were in the low picomolar concentration range and were stable across clinical diagnostic groups, although soluble Aβ1-42 increased significantly and correlated with changes in global cognitive performance and, even more strongly, with episodic memory impairment (Table 4). This suggests that soluble pyroglutamate Aβ forms are less informative regarding clinical AD progression than soluble Aβ1-42. This observation, together with the reported inability to detect pyroglutamate Aβ in cerebrospinal fluid or plasma (Wu et al., 2013), compared with Aβ1-42 (Fagan et al., 2006), argues against pyroglutamate Aβ as a potential AD biomarker.
4.2. Neuropathological correlates of pyroglutamate-modified and full-length Aβ during AD progression
Several reports suggest that pyroglutamate Aβ contributes to Aβ plaque formation (Gunn et al., 2010; He and Barrow, 1999; Jawhar et al., 2011; Schilling et al., 2006; Wirths et al., 2010; Wittnam et al., 2012). However, studies in transgenic AD mice suggest that pyroglutamate Aβ deposition is preceded by “general” Aβ deposition (Frost et al., 2013) and that plaques are composed primarily of Aβ1-42 (Guntert et al., 2006; Iwatsubo et al., 1996). Our data suggest that both pyroglutamate and full-length Aβ contribute to plaque genesis, based on correlations with disease stage by both CERAD criteria and NIA-Reagan diagnosis (Table 4). Interestingly, greater concentrations of Aβ forms (except soluble pyroglutamate Aβ40 and Aβ42) were also associated with advanced Braak pathology stage (Table 4).
4.3. Study considerations
Based on CERAD criteria and Braak staging some of our NCI and MCI cases had AD pathology, consistent with previous reports in the RROS cohort where higher level of education may reduce the effect of neuropathology on the odds of developing AD dementia (Bennett et al., 2003). Pathology-burdened NCI cases might be preclinical AD or “pathological aging” (Dickson et al., 1992) consistent with observations of amyloid pathology in approximately 30% of NCI and approximately 70% MCI postmortem and in positron emission tomography imaging studies (Aizenstein et al., 2008; Crystal et al., 1988; Dickson et al., 1992; Mufson et al., 1999; Rowe et al., 2010; Schneider et al., 2009). Regardless of their pathology burden, some NCI subjects may never develop AD, whereas pathology-free MCI may have other causes of cognitive impairment (e.g., vascular disease, depression), which were not present in our cases. We observed that pathology-free cases had low levels of insoluble and soluble pyroglutamate and unmodified Aβ (Fig. 1). In contrast, pathology-burdened cases across clinical groups displayed unmodified Aβ concentrations many-fold higher in the insoluble than in the soluble pools as reported in advanced AD (Wang et al., 1999). Future investigations will examine other cortical areas and other forms of pyroglutamate (e.g., AβNpE11-x) (Vassar et al., 1999) to determine regional and peptide specific changes in clinical diagnostic groups. The unique fibril promoting nature of pyroglutamate Aβ might be important for sequestering neurotoxic oligomeric Aβ into relatively inert plaque deposits. Therefore, reducing or preventing brain pyroglutamate Aβ modification as a potential therapeutic strategy in patients with prodromal or early AD (Frost et al., 2012; Morawski et al., 2014) should be regarded with caution.
Acknowledgements
This study was supported by grants PO1AG014449, PO1AG025204, RO1AG043375 and P30AG10161 from the National Institute on Aging, National Institutes of Health. The authors are indebted to the Catholic nuns, priests, and lay brothers who participated in the Rush Religious Orders Study. They are grateful to Dr Ana W. Capuano (Rush University Medical Center) for assistance with statistical analyses and to Mr. William Paljug for expert technical assistance.
Footnotes
Disclosure statement
Violetta Pivtoraiko, Eric Abrahamson, Sue Leurgans, and Elliott Mufson have no conflicts of interest to disclose. Steven DeKosky discloses consultant fees at Merck, Lilly, Janssen, Helicon Therapeutics, Genzyme, and AstraZeneca, involvement in experimental therapeutic trials at Pfizer, Elan Corporation and/or Wyeth, Novartis, Janssen, Baxter Pharmaceuticals, and editorial of Dementia section in “Up To Date.” Milos Ikonomovic discloses consultant fees at GE Healthcare.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Education modifies the relation of AD pathology to cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L. Clinico-pathologic studies in dementia: non-demented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- Cynis H, Scheel E, Saido TC, Schilling S, Demuth HU. Amyloidogenic processing of amyloid precursor protein: evidence of a pivotal role of glutaminyl cyclase in generation of pyroglutamate-modified amyloid-beta. Biochemistry. 2008;47:7405–7413. doi: 10.1021/bi800250p. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren S, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann. Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied non-demented elderly humans. Neurobiol. Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann. Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frost JL, Liu B, Kleinschmidt M, Schilling S, Demuth HU, Lemere CA. Passive immunization against pyroglutamate-3 amyloid-beta reduces plaque burden in Alzheimer-like transgenic mice: a pilot study. Neurodegener. Dis. 2012;10:265–270. doi: 10.1159/000335913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JL, Le KX, Cynis H, Kleinschmidt M, Palmour RM, Ervin FR, Snigdha S, Cotman CW, Saido TC, Vassar RJ, St George-Hyslop P, Ikezu T, Schilling S, Demuth HU, Lemere CA. Pyroglutamate-3 amyloid-beta deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am. J. Pathol. 2013;183:369–381. doi: 10.1016/j.ajpath.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Jr, Younkin LH, Suzuki N, Younkin SG. Amyloid beta protein (A beta) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42 (43) J. Biol. Chem. 1995;270:7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- Gunn AP, Masters CL, Cherny RA. Pyroglutamate-Aβ: role in the natural history of Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2010;42:1915–1918. doi: 10.1016/j.biocel.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Guntert A, Dobeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience. 2006;143:461–475. doi: 10.1016/j.neuroscience.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Harigaya Y, Saido TC, Eckman CB, Prada CM, Shoji M, Younkin SG. Amyloid beta protein starting pyroglutamate at position 3 is a major component of the amyloid deposits in the Alzheimer’s disease brain. Biochem. Biophys. Res. Commun. 2000;276:422–427. doi: 10.1006/bbrc.2000.3490. [DOI] [PubMed] [Google Scholar]
- He W, Barrow CJ. The A beta 3-pyroglutamyl and 11-pyroglutamyl peptides found in senile plaque have greater beta-sheet forming and aggregation propensities in vitro than full-length A beta. Biochemistry. 1999;38:10871–10877. doi: 10.1021/bi990563r. [DOI] [PubMed] [Google Scholar]
- Hosoda R, Saido TC, Otvos LJ, Arai T, Mann DM, Lee VM, Trojanowski JQ, Iwatsubo T. Quantification of modified amyloid beta peptides in Alzheimer disease and Down syndrome brains. J. Neuropathol. Exp. Neurol. 1998;57:1089–1095. doi: 10.1097/00005072-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Demen. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Wuu J, Mathis CA, Scheff SW, Mufson EJ, DeKosky ST. Precuneus amyloid burden is associated with reduced cholinergic activity in Alzheimer disease. Neurology. 2011;77:39–47. doi: 10.1212/WNL.0b013e3182231419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Aβ42 (43) and Aβ40 in senile plaques with end-specific Aβ mono-clonals: evidence that an initially deposited species is Aβ42 (43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Mann D, Odaka A, Suzuki N, Ihara Y. Amyloid protein (Aβ) deposition: aβ 42 (43) precedes Aβ 40 in Down syndrome. Ann. Neurol. 1995;37:294–299. doi: 10.1002/ana.410370305. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Saido TC, Mann DM, Lee VM, Trojanowski JQ. Full-length amyloid-beta (1-42 (43)) and amino-terminally modified and truncated amyloid-beta 42 (43) deposit in diffuse plaques. Am. J. Pathol. 1996;149:1823–1830. [PMC free article] [PubMed] [Google Scholar]
- Jawhar S, Wirths O, Bayer TA. Pyroglutamate amyloid-β (Aβ): a hatchet man in Alzheimer disease. J. Biol. Chem. 2011;286:38825–38832. doi: 10.1074/jbc.R111.288308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL. Synaptotoxic amyloid-β oligomers: a molecular basis for the cause, diagnosis, and treatment of Alzheimer’s disease? J. Alzheimers Dis. 2013;33(Suppl 1):S49–S65. doi: 10.3233/JAD-2012-129039. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Cullen WK, Hu NW, Rowan MJ. Alzheimer’s disease Abeta assemblies mediating rapid disruption of synaptic plasticity and memory. Mol. Brain. 2012;5:25. doi: 10.1186/1756-6606-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcello A, Wirths O, Schneider-Axmann T, Degerman-Gunnarsson M, Lannfelt L, Bayer TA. Reduced levels of IgM autoantibodies against N-truncated pyroglutamate Abeta in plasma of patients with Alzheimer’s disease. Neurobiol. Aging. 2011;32:1379–1387. doi: 10.1016/j.neurobiolaging.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann. Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT National Institute on Aging; Alzheimer’s Association. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BD, Chakrabarty P, Levites Y, Kukar TL, Baine AM, Moroni T, Ladd TB, Das P, Dickson DW, Golde TE. Overlapping profiles of Abeta peptides in the Alzheimer’s disease and pathological aging brains. Alzheimers Res. Ther. 2012;4:18. doi: 10.1186/alzrt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawski M, Schilling S, Kreuzberger M, Waniek A, Jäger C, Koch B, Cynis H, Kehlen A, Arendt T, Hartlage-Rubsamen M, Demuth HU, Roβner S. Glutaminyl cyclase in human cortex: correlation with (pGlu)-amyloid-beta load and cognitive decline in Alzheimer’s disease. J. Alzheimers Dis. 2014;39:385–400. doi: 10.3233/JAD-131535. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Lavine N, Jaffar S, Kordower JH, Quirion R, Saragovi HU. Reduction in pl40-TrkA receptor protein within the nucleus basalis and cortex in Alzheimer’s disease. Exp. Neurol. 1997;146:91–103. doi: 10.1006/exnr.1997.6504. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp. Neurol. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- NIA-Reagan Working Group. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol. Aging. 1997;18:SI–S2. [PubMed] [Google Scholar]
- Pike CJ, Cummings BJ, Cotman CW. Early association of reactive astrocytes with senile plaques in Alzheimer’s disease. Exp. Neurol. 1995;132:172–179. doi: 10.1016/0014-4886(95)90022-5. [DOI] [PubMed] [Google Scholar]
- Portelius E, Bogdanovic N, Gustavsson MK, Volkmann I, Brinkmalm G, Zetterberg H, Winblad B, Blennow K. Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer’s disease. Acta Neuropathol. 2010;120:185–193. doi: 10.1007/s00401-010-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, Kawashima S. Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3 (pE), in senile plaques. Neuron. 1995;14:457–166. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- Schilling S, Lauber T, Schaupp M, Manhart S, Scheel E, Böhm G, Demuth HU. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro) Biochemistry. 2006;45:12393–12399. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J. Neurosci. 2011;31:4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CP, Berg EA, Elliott-Bryant R, Fishman JB, McKee AC, Morin PJ, Shia MA, Fine RE. Pyroglutamate-Aβ 3 and 11 colocalize in amyloid plaques in Alzheimer’s disease cerebral cortex with pyroglutamate-Aβ 11 forming the central core. Neurosci. Lett. 2011;505:109–112. doi: 10.1016/j.neulet.2011.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekirian TL, Yang AY, Glabe C, Geddes JW. Toxicity of pyroglutaminated amyloid beta-peptides 3 (pE)−40 and −42 is similar to that of A betal−40 and −42., J. Neurochem. 1999;73:1584–1589. doi: 10.1046/j.1471-4159.1999.0731584.x. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn. Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Aβ distinguish Alzheimer’s disease from normal and pathologic aging. Exp. Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol. Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wirths O, Erck C, Martens H, Harmeier A, Geumann C, Jawhar S, Kumar S, Multhaup G, Walter J, Ingelsson M, Degerman-Gunnarsson M, Kalimo H, Huitinga I, Lannfelt L, Bayer TA. Identification of low molecular weight pyroglutamate A{beta} oligomers in Alzheimer disease: a novel tool for therapy and diagnosis. J. Biol. Chem. 2010;285:41517–41524. doi: 10.1074/jbc.M110.178707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittnam JL, Portelius E, Zetterberg H, Gustavsson MK, Schilling S, Koch B, Demuth HU, Blennow K, Wirths O, Bayer TA. Pyroglutamate amyloid beta (Abeta) aggravates behavioral deficits in transgenic amyloid mouse model for Alzheimer disease. J. Biol. Chem. 2012;287:8154–8162. doi: 10.1074/jbc.M111.308601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Miller RA, Connolly B, Marcus J, Renger J, Savage MJ. Pyroglutamate-modified amyloid-beta protein demonstrates similar properties in an Alzheimer’s disease familial mutant knock-in mouse and Alzheimer’s disease brain. Neurodegener. Dis. 2013 doi: 10.1159/000353634. http://dx.doi.org/10.1159/000353634. [DOI] [PubMed] [Google Scholar]
- Youssef I, Florent-Bechard S, Malaplate-Armand C, Koziel V, Bihain B, Olivier JL, Leininger-Muller B, Kriem B, Oster T, Pillot T. N-truncated amyloid-beta oligomers induce learning impairment and neuronal apoptosis. Neurobiol Aging. 2008;29:1319–1333. doi: 10.1016/j.neurobiolaging.2007.03.005. [DOI] [PubMed] [Google Scholar]