Abstract
Background
The long allele of DRD4 is associated with greater susceptibility to peer influences on alcohol use in young adulthood, but it is unclear whether this increased susceptibility extends to other developmental periods. This study examined the interactive effects of DRD4 polymorphism and friends' alcohol use from adolescence to adulthood.
Methods
Participants (N=340; 59% female; 98% White) reported on their own and their friends' alcohol use at four time points between mean ages 17 and 33. Autoregressive cross-lagged models evaluated reciprocal relationships between friends' alcohol use and participants' own alcohol use and frequency of heavy drinking over time. Multigroup modeling tested differences in model paths and covariances across high vs. low risk DRD4 polymorphisms.
Results
Alcohol use at age 33 was predicted by previous friends' alcohol use and correlated with current friends' alcohol use only for carriers of the DRD4 long allele. Regardless of DRD4 genotype, friends' alcohol use at age 17 predicted greater alcohol use and more frequent heavy drinking at age 23. Alcohol use and/or heavy drinking predicted greater friends' alcohol use at later time points for both genotype groups across adolescence and adulthood.
Conclusions
The long allele of DRD4 is associated with increased susceptibility to peer influences on alcohol use in young adulthood, but not earlier in development. Adults with the long allele of DRD4 may benefit from interventions educating them about this risk and equipping them with strategies to reduce affiliations with and influence of drinking friends.
Keywords: G × E interaction, peer, alcohol use, DRD4, gene by environment
1. Introduction
Both social environment and genetic factors have long been implicated in alcohol use (Kendler et al., 2008; Zucker, 2006). Recent studies also point to the presence of gene by environment (G × E) interactions, such as specific genetic polymorphisms being associated with increased susceptibility to environmental influences (Belsky and Pluess, 2009). One genetic polymorphism of interest in the alcoholism literature is the number of variable number tandem repeats (VNTR) in the third exon of the DRD4 dopamine receptor gene. The presence of a long DRD4 allele (involving 7+ repeats) decreases dopamine reception efficiency (Asghari et al., 1995) and has been linked with increased craving in response to alcohol cues (McGeary, 2009; Ray et al., 2010), greater alcohol use (Vaughn et al., 2009), and personality correlates of alcohol use such as thrill seeking (Dmitrieva et al., 2011), as well as other types of problem and addictive behaviors such as delinquency (Boutwell and Beaver, 2008) and smoking (Hutchison et al., 2002). This study examines the interaction of DRD4 polymorphism with a key environmental factor in alcohol use – peer drinking (Windle, 2000), across several developmental periods from adolescence through young adulthood.
A sizeable literature also indicates that the DRD4 polymorphism interacts with environmental influences. For instance, young children with the long DRD4 allele are more sensitive to both positive and negative aspects of parental and non-parental care (Bakermans-Kranenburg and Van IJzendoorn, 2011; Belsky and Pluess, 2013). These effects extend to prenatal exposure to maternal stress (Zohsel et al., 2014) and persist developmentally into adolescence (Nikitopoulos et al., 2014). These interactions have also been found in adulthood, with male carriers of the long DRD4 allele being more sensitive to the positive effects of long-term romantic relationships on desisting from delinquency (Beaver et al., 2008).
Since substance use does not emerge developmentally before adolescence, the role of DRD4 in substance use and addiction can be studied only in adolescents and adults. In studies examining alcohol use, peer influences on heavy drinking were stronger among young adults (age 18-28) with the long DRD4 allele (Larsen et al., 2010). Similarly, emerging adults involved in college or Greek organizations who had the long DRD4 allele reported more alcohol dependence symptoms (Park et al., 2011). However, another study with adolescents (ages 13-18) failed to find increased susceptibility to peer influences on alcohol use among those with high risk DRD4 polymorphism (van der Zwaluw et al., 2012), and parenting influences on regular alcohol (or cannabis) use did not vary across DRD4 genotypes in another adolescent study (Creemers et al., 2011). Similarly conflicting results have been reported for smoking outcomes. For instance, adults with the long DRD4 allele smoked more in response to smoking cues and negative affect induction (Hutchison et al., 2002; Perkins et al., 2008), but the effects of smoking-related parenting behaviors on adolescent smoking outcomes did not vary across DRD4 genotypes (Hiemstra et al., 2013).
Applying a developmental perspective to these mixed findings, a relatively consistent picture emerges. Significant interactions between DRD4 and environmental influences are more often found in early childhood and in adulthood, but less so in adolescence. One study with adolescents even found the opposite pattern of interactions, with carriers of the long DRD4 allele being less sensitive to the effects of peer victimization and social well-being on delinquency (Kretschmer et al., 2013). For alcohol use specifically, behavior genetic studies show a clear developmental pattern of primarily environmental influences on alcohol use in adolescence, followed by an increasing role of genetic factors in adulthood (Dick et al., 2006; Kendler et al., 2008). These results suggest that G × E interactions, including those involving DRD4, may not emerge in adolescence when genetic influences on alcohol use are minimal. Together, studies on DRD4 by environment interactions across developmental periods and behavior genetic studies of alcohol use suggest that interactions between DRD4 and peer drinking may manifest developmentally later in adulthood, but not in adolescence.
The present study examines developmental differences in the interactive effects of DRD4 polymorphism and friends' drinking on alcohol use across four developmental phases: late adolescence (ages 16-18), emerging adulthood (ages 20-25), early young adulthood (ages 26-30), and later young adulthood (ages 30-35). Because the relationship between peer and own alcohol use is dynamic and involves both selection of friends with similar drinking habits and peer influence (Burk et al., 2012), we model both types of mechanisms (peer selection and socialization) across the developmental periods examined. We also include two related, yet distinct alcohol use outcomes -- levels of alcohol use (standard quantity-frequency index) and heavy episodic (or binge) drinking. By examining two alcohol phenotypes across four developmental periods, we are able to determine the developmental and phenotype-related specificity of the DRD4 by peer drinking interactions.
2. Material and Methods
2.1. Study Design and Participants
The sample includes 340 participants (59% female; 98% White, 0.9% Black, 0.6% Hispanic, 0.3% Native American and 0.3% Other) who were assessed four times between mean ages 17 and 33. These participants are a subsample from the Lives Across Time: A Longitudinal Study of Adolescent and Adult Development (LAT; Windle and Wiesner, 2004). The larger study recruited 1,210 high school students (participation rate 76%) from suburban public schools in western New York. The youth were assessed 3-4 times during adolescence (waves 1-4), 6 months apart (retention over 90% at each wave). Participants were followed three more times in adulthood (waves 5-7; mean ages 23.8, 28.9, and 33.5; retention rates 70%, 66%, and 68%). Across the course of the LAT, 46.8% of the young adults participated at all 7 waves, 20.4% at 6 waves, 17.8% at 5 waves, 13% at 4 waves, and less than 2% at less than 4 waves; hence, 85% participated at five or more waves of measurement.
After the last adult assessment (wave 7), a subsample of 349 participants was invited to participate in a supplementary genetics study (sample size was restricted by limited funding). Priority of selection was given to individuals who had participated during both adolescence and young adulthood to maximize the ability to test developmental gene-environment (G × E) prospective relationships. As described below, nine cases were excluded due to invalid genotyping data, yielding a final sample of 340. Statistical comparisons were made between the 340 individuals who were included in this study and those who were not on the major variables used in this study (e.g., alcohol use, heavy drinking friends' alcohol use, age, marital status, racial/ethnic group). The two groups did not differ significantly on any of these variables. Thus, this study used data from waves 4-7 on the 340 participants who had both genetic and interview data (see Table 2 for descriptive statistics of sample).
Table 2. Descriptive statistics for age and all alcohol variables.
| Wave 4 M (SD) | Wave 5 M (SD) | Wave 6 M (SD) | Wave 7 M (SD) | |
|---|---|---|---|---|
| Age | 17.44 (0.64) | 24.27 (1.26) | 29.38 (1.16) | 33.35 (1.39) |
| Friends' alcohol use a | 67.00 (35.46) | 12.34 (19.96) | 10.11 (18.51) | 9.92 (17.45) |
| Alcohol use b | 2.75 (0.61) | 2.93 (0.65) | 2.86 (0.56) | 2.81 (0.53) |
| Heavy drinking c | 0.37 (0.65) | 0.59 (0.73) | 0.39 (0.58) | 0.34 (0.57) |
Assessed as a percentage of close friends drinking alcoholic beverages at Wave 4 and as a percentage of close friends considered to be heavy drinkers at Waves 5-7.
Measured as the log-transformed average number of ounces of ethanol consumed per day in the last month.
Indicates the log-transformed average number of days per month when the participant consumed 6 or more alcoholic drinks in a row in the last 6 months.
2.2. Procedures
At initial recruitment, an information packet was mailed to eligible students at participating high schools. Subsequently, participants were re-recruited through school or contact information they provided. At each wave, participants (and their legal guardians during adolescence) completed informed consent. At waves 1-4, youth completed surveys in a group administration format at school, and their primary caregivers provided information via mail surveys and phone interviews. In waves 5-7, trained interviewers conducted individual interviews with the participants in the participants' homes or at a research site. Sensitive questions, including those on substance use and friends' alcohol use, were answered privately using a self-interview format. For the genetic supplement, participants were mailed a saliva collection kit (DNA Oragene); they returned the sample to the research team by mail. Participants were compensated for their time at each assessment.
2.3. Measures
2.3.1. Friends' alcohol use
At waves 4 to 7, participants were requested to provide the number of close friends they had. At wave 4, a follow up question asked ‘how many of those close friends drink alcoholic beverages?’ In waves 5 to 7, a follow up question inquired how many of those friends ‘do you consider to be heavy drinkers?’ Friends' alcohol use was computed as the percentage of close friends who drank alcohol (wave 4) or were considered heavy drinkers (waves 5-7).
2.3.2. DRD4 genotyping
Genotyping was conducted at the Georgia Genomics Facility (GGF) at the University of Georgia (http://dna.uga.edu) where DNA was extracted from saliva samples according to the manufacturer's specifications by a technician blind to other sources of data for the research project. Genotyping was performed on the DRD4 polymorphism of 48-base pair variable number of tandem repeat (VNTR) in exon 3 as described by LaHoste et al. (1996). The genotype at DRD4 was determined for each participant using the primers forward 5'-CGA CTA CGT GGT CTA CTC G-3' and reverse 5'-/56-FAM/AGG ACC CTC ATG GCC TTG-3', ABI 360 PCR master mix plus 10% GC enhancer. Determination of the allele length was performed by analysis on an automated capillary sequencer (AB13730, Applied Biosystems). Genotype was then called using GeneMapper 4.0 software (Applied Biosystems) by an individual blind to the study hypotheses and other information about the participants. Quality control procedures included a genotype call rate of GE 98%, subject filtering call rates of GE 95%, non-significant departures of Hardy-Weinberg equilibrium, and outlier screening (using a criterion of 5 SDs). None of the alleles deviated from Hardy-Weinberg equilibrium (p = .87, ns). Applying these quality control procedures resulted in nine participants (2.6%) being excluded from further analyses. Consistent with previous research (Hutchison et al., 2003; van der Zwaluw et al., 2012), DRD4 polymorphism was dichotomized as low risk (two short alleles, 2-6 repeats) or high risk (one or two long alleles, 7-11 repeats; see Table 1 for frequencies of alleles and genotypes).
Table 1. Allele and genotype frequencies for the DRD4 variable number tandem repeat polymorphism (n=340).
| Allele/genotype | Number | Frequency (%) |
|---|---|---|
| Allele | ||
| 2 | 79 | 11.6 |
| 3 | 46 | 6.7 |
| 4 | 408 | 60.0 |
| 5 | 14 | 2.0 |
| 6 | 3 | < 1 |
| 7 | 124 | 18.2 |
| 8 | 6 | < 1 |
| Total | 680 | |
| Genotype | ||
| 2/2 | 12 | 3.5 |
| 2/3 | 8 | 2.3 |
| 2/4 | 31 | 9.1 |
| 2/5 | 1 | <1 |
| 2/6 | 1 | <1 |
| 2/7* | 13 | 3.8 |
| 2/8* | 1 | <1 |
| 3/4 | 25 | 7.3 |
| 3/5 | 6 | 1.8 |
| 3/6 | 1 | <1 |
| 3/7* | 4 | 1.2 |
| 3/8* | 2 | < 1 |
| 4/4 | 133 | 39.1 |
| 4/5 | 7 | 2.0 |
| 4/6 | 1 | <1 |
| 4/7* | 77 | 22.6 |
| 4/8* | 1 | <1 |
| 7/7* | 14 | 4.1 |
| Genotype classification | ||
| Short (< 7 repeats) | 228 | 67.1 |
| Long (≥ 7 repeats) | 112 | 32.9 |
| Total | 340 | 100.0 |
These genotypes were classified as long (high risk); all remaining genotypes were classified as short (low risk).
2.3.3. Alcohol use
At each wave, alcohol consumption was measured with a standard quantity–frequency index assessing beer, wine, and hard liquor consumption in the last 30 days. Respondents were asked how often they usually had each beverage in the last 30 days (using a 7-point scale ranging from 1 - ‘never’ to 7 – ‘every day’) and, when they had the beverage, on average how much they usually drank (using a 10-point scale ranging from 1 - ‘none’ to 10 -‘more than 8 drinks’). The quantity–frequency index, based on all three beverages, provides a measure of the average number of ounces of ethanol consumed per day in the last month (Armor and Polich, 1982). Consistent with prior research (Windle and Windle, 2012), a logarithmic transformation was applied to the index to improve normality.
2.3.4. Heavy alcohol use
At each wave, participants were asked an open-ended question of how many times they had 6 or more drinks of beer, wine, or liquor in a row in the last 6 months. The numbers of days reported for each beverage were summed and divided by 6, yielding an average number of heavy drinking days per month. The positive skew of this variable was improved by applying logarithmic transformation.
2.3.5. Covariates
Demographic variables included participants' age at each assessment, sex, race (dichotomized as minority vs. white), and family income (parent-report at wave 4, self-report at waves 5-7). In adulthood (waves 5-7), participants' marital status was also included (married vs. not).
2.4. Statistical Analyses
Prior to main analyses, univariate distributions and bivariate associations among variables were examined. The reciprocal relationships between friends' alcohol use and participants' own alcohol use and heavy drinking were evaluated with autoregressive cross-lagged models conducted in Mplus version 7. To reduce the complexity of the models and presentation of the results, alcohol use and heavy drinking were analyzed separately (the results were identical when these variables were analyzed together). The models included autoregressive paths modeling the continuity in each construct over time, covariances between friends' and own alcohol use within each time point, and cross-lagged paths linking friends' alcohol use and own alcohol use or heavy drinking across adjacent time points (see Figures 1 and 3). All paths were adjusted for participants' sex and racial/ethnic minority status, as well as age, family income and marital status at the time each predicted variable was measured. Because some variables were not normally distributed, the MLR estimator which is robust to violations of normality was used. The role of DRD4 genotype as a moderator of the relationships between friends' and own alcohol use was tested with multigroup modeling. Specifically, we compared the fit of a model where all paths and covariances were constrained to be equal for those with low and high risk genotypes to a model where the paths and covariances were free to vary across the two genotype groups. Significant results were followed up with multigroup testing of each single path and covariance, comparing a fully constrained model to a model where single path or covariance could vary by genotype. Due to the large number of comparisons made for each model, the Bonferroni correction was used with a critical cutoff of p=.003 (05/16).
Figure 1.
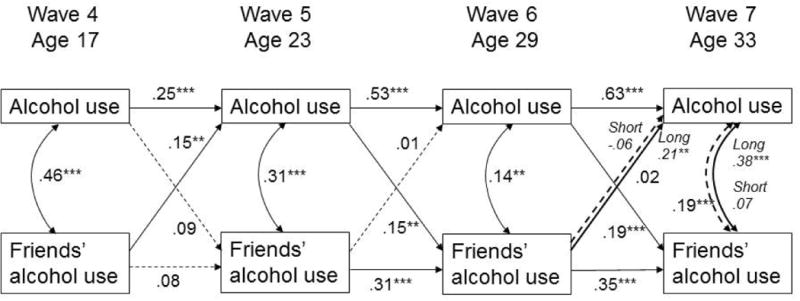
Autoregressive cross-lagged model of reciprocal relationships between alcohol use and friends' alcohol use.
Note: Coefficients are standardized. Dashed lines are not significant. All paths are adjusted for sex, racial/ethnic minority status, and current age, family income, and marital status. Long – 1 or 2 long DRD4 alleles; Short – two short DRD 4 alleles.
Alcohol use is measured as the log-transformed average number of ounces of ethanol consumed per day in the last month; friends' alcohol use is assessed as a percentage of close friends drinking alcoholic beverages at Wave 4 and as a percentage of close friends considered to be heavy drinkers at Waves 5-7.
*p<.05; **p<.01; ***p<.001.
Figure 3.
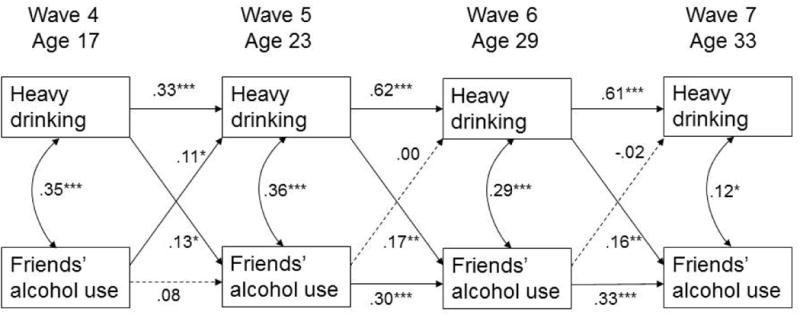
Autoregressive cross-lagged model of reciprocal relationships between heavy drinking and friends' alcohol use.
Note: Coefficients are standardized. Dashed lines are not significant. All paths are adjusted for sex, racial/ethnic minority status, and current age, family income, and marital status. Heavy drinking is measured as the long-transformed average number of days per month when the participant consumed 6 or more alcoholic drinks in a row in the last 6 months; friends' alcohol use is assessed as a percentage of close friends drinking alcoholic beverages at Wave 4 and as a percentage of close friends considered to be heavy drinkers at Waves 5-7.
*p<.05; **p<.01; ***p<.001.
3. Results
The allele and genotype frequencies for DRD4 are provided in Table 1. The distributions of the allele and genotype frequencies, and the short-long genotype classification are consistent with prior research with Caucasian and European samples (Hutchison et al., 2003; van der Zwaluw et al., 2012) as well as with European samples reported in the Allele Frequency Database (Rajeevan et al., 2012). We did not collect data on ancestry informative markers and relied on self-reports of ethnicity. Because the frequency of the DRD4 7-repeat allele may vary considerably across world populations (Kidd et al., 2014), and a small percentage of our sample was of non-European origin, we conducted the subsequent data analyses with and without the small subsample; results were not altered and therefore the results subsequently reported on are based on the full sample size of 340.
Average levels of alcohol use and heavy drinking increased from age 17 to age 24 and then decreased. Friends' alcohol use decreased throughout adulthood (Table 2). Correlations between alcohol use and heavy drinking within each wave ranged from .47 to .79 (all p<.001), with lower correlations at older ages. At each wave, friends' alcohol use was positively related to own alcohol use and heavy drinking (r = .18 to .45, all p<.001). DRD4 was not correlated with alcohol use, heavy drinking, or friends' alcohol use at any wave.
Overall, the fit of the model with alcohol use (Figure 1) was acceptable [χ2(69)=138.15, p<.001; CFI=.91, RMSEA=.05, SRMR=.05]. There was significant continuity in own alcohol use, with stronger coefficients at older ages, as well as significant continuity in friends' alcohol use in adulthood, although not between adolescence and emerging adulthood (age 17 to age 23). Friends' and own alcohol use were positively correlated within each wave. In addition, friends' alcohol use at age 17 predicted higher levels of own alcohol use at age 23, indicating a peer socialization effect, and own alcohol use at ages 23 and 29 predicted higher proportion of heavy drinking friends at the subsequent wave, indicating friend selection effects (see Figure 1). Multigroup modeling indicated significant model differences between low and high risk DRD4 genotype groups [Δχ2(16)=35.61, p<.01]. Follow up analyses using Bonferroni correction revealed differences in one path and one covariance. Having more heavy drinking friends at age 29 predicted greater alcohol use at age 33 for those with the high risk DRD4 genotype (β=.21, p<.0l) but not those with the low risk genotype (β=.06, ns) [Δχ2(l)=9.44, p=.002]. This difference is depicted visually in Figure 2. Additionally, friends' alcohol use at age 33 was correlated with own concurrent alcohol use only among those with the high risk DRD4 genotype but not those with the low risk variants (r=.38, p<.001, vs. r=.07, ns) [Δχ2(l)=10.28, p=.001].
Figure 2.
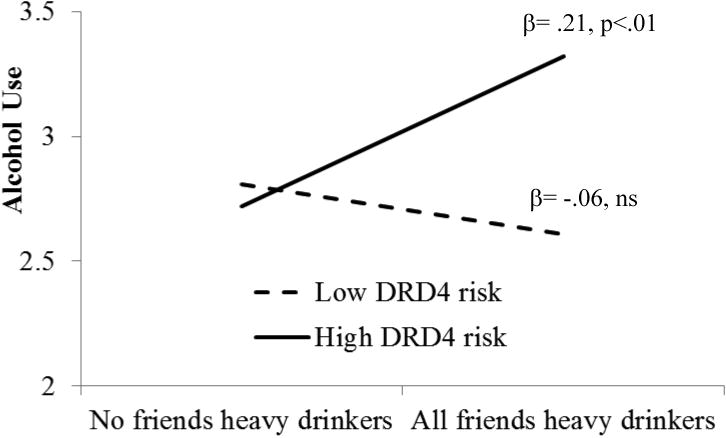
Heavy drinking friends at age 29 predict greater alcohol use at age 33 for those with high risk DRD4 genotype, but not those with low risk genotype.
The fit of the model with heavy drinking (Figure 3) also was acceptable [χ2(69)=l 57.26, p<.00l; CFI=.89, RMSEA=.06, SRMR=.06]. With the exception of friends' alcohol use between adolescence and emerging adulthood, there was significant continuity in both heavy drinking and friends' alcohol use across adjacent time points; continuity was stronger during adulthood compared to the adolescence to adulthood transition. Friends' alcohol use and heavy drinking were positively correlated within each wave. Heavy drinking predicted higher proportion of heavy drinking friends at the next time point across all ages, indicating developmentally consistent friend selection effects. In addition, friends' alcohol use at age 17 predicted more frequent heavy drinking at age 23, suggesting a peer socialization effect developmentally specific to the transition from adolescence to emerging adulthood (see Figure 3). Multigroup modeling indicated significant model differences between the low and high risk genotype groups [Δχ2(16)=28.99, p<.05]. However, none of the follow up tests reached significance level after the Bonferroni correction was applied.
4. Discussion
This study used a prospective design to examine G × E interaction between friends' alcohol use and DRD4 genotype in predicting alcohol use and heavy drinking from adolescence to later young adulthood. The analyses modeled the reciprocal processes of peer selection and socialization over time, as well as continuity in own and friends' alcohol use and any differences in these relationships by DRD4 polymorphism. The results revealed the presence of an interaction between DRD4 and friends' alcohol use in young adulthood, but not earlier in adolescence or emerging adulthood. Specifically, higher levels of alcohol use in early 30's were predicted by having more friends who drank heavily in late 20's among those with the high risk (long) DRD4 allele, but not among those with the low risk genotype. Similarly, alcohol use in early 30's was correlated with current friends' heavy use only among those with the high risk DRD4 allele, but not in those with two low risk alleles. Regardless of DRD4 genotype, the results revealed a developmental pattern of peer influence on both alcohol use and heavy drinking from late adolescence to emerging adulthood, accompanied by peer selection for similar levels of alcohol use and/or heavy drinking from adolescence throughout later young adulthood. Finally, the results indicated lower continuity in friends' alcohol use, as well as own alcohol use and heavy drinking during the transition from adolescence to emerging adulthood, followed by greater continuity in all constructs throughout emerging and young adulthood.
Together, the findings are consistent with behavior genetic studies reporting stronger environmental influences on alcohol use in adolescence (as reflected by significant peer socialization effects from adolescence to emerging adulthood), with the role of genetic factors increasing in adulthood (as evidenced by the G × E interaction not emerging before young adulthood; Kendler et al., 2008). The results also help reconcile previous findings of no DRD4 by environment interactions for substance use outcomes in adolescence (Creemers et al., 2011; Hiemstra et al., 2013; van der Zwaluw et al., 2012), but the presence of such interactions in samples containing adults (Hutchison et al., 2002; Larsen et al., 2010; Park et al., 2011; Perkins et al., 2008). Thus, our findings and the literature suggest that the presence of some G × E interactions is dependent on developmental timing and may not appear during all stages of development. For substance use outcomes specifically, G × E interactions that are found among adults may not replicate for adolescents. Future studies of G × E interactions in alcohol and other substance use outcomes should explicitly examine age as a possible moderator of these interactive effects to further elucidate the role of developmental timing.
Multiple studies provide several clues to the mechanisms through which the long allele of DRD4 may increase susceptibility to peer alcohol use in adulthood. This polymorphism is associated with increased self-reported cravings for alcohol in response to alcohol cues (Ray et al., 2010), perhaps as a result of greater neural response to alcohol cues in the mesocorticolimbic reward-craving pathway (Filbey et al., 2008). In turn, greater craving increases the perceived value of alcohol and is associated with maladaptive patterns of drinking (MacKillop et al., 2007, 2010). Similarly, increased neural activation in the reward-craving pathway is related to higher alcohol use (Filbey et al., 2008). The DRD4 high risk genotype has also been linked with poorer response inhibition and lower neural activation in related neural networks (Filbey et al., 2012), so these individuals may be more likely to act on their alcohol cravings. Finally, individuals with the long DRD4 allele experience greater social bonding when using alcohol with others (Creswell et al., 2012), which may further reinforce patterns of social drinking.
Thus, these studies paint a picture of greater vulnerability to peer alcohol use among adults with the high risk DRD4 genotype -- heavy drinking friends provide alcohol cues that increase the activation of neural reward-craving circuits and subjective craving for alcohol, which, aided by reduced behavioral inhibition and greater social reinforcement for drinking, increases alcohol consumption. By contrast, individuals with the low risk DRD4 genotype may be more immune to alcohol cues provided by heavy drinking friends, better able to inhibit impulses to drink, and less dependent on drinking for social reinforcement. Individual differences in these mechanisms related to DRD4 may become more prominent in adulthood, when the normative development of impulse control is complete, compared to adolescence which is characterized by greater impulsivity and sensation seeking (Steinberg et al., 2008). More research is needed to verify these developmentally-specific mechanisms in the context of the interaction between peer drinking and DRD4 polymorphism.
Interestingly, DRD4 polymorphism moderated the effect of heavy drinking friends on alcohol use, but not on frequency of heavy drinking. This discrepancy suggests that overall alcohol use may be a more sensitive outcome measure of G × E effects than the frequency of heavy drinking which required participants to meet the cutoff of 6 or more drinks in a row. Together, the results indicate that heavy drinking friends may contribute to higher overall alcohol intake among those with a long DRD4 allele, but not necessarily to more frequent binge drinking. Incorporating multiple alcohol use phenotypes in future studies will be important to provide a more nuanced understanding of environmental and genetic effects on specific aspects of substance use.
The additional findings of developmental differences in peer selection and socialization are consistent with other studies finding diminished magnitude of peer and other social influences on alcohol use between adolescence and emerging adulthood (Scholte et al., 2008). These findings indicate that adolescents are more susceptible to peer influences on alcohol use than adults, perhaps due to the incomplete maturation of the prefrontal cortex at this time (Crews et al., 2007). By contrast, in adulthood the similarity between friends' and own drinking may be due primarily to the selection of friends based on similar drinking habits. The weaker continuity in both friends' and own alcohol use between adolescence to young adulthood is likely a function of the life events and transitions associated with this developmental period, such as transition to employment, involvement in serious long-term romantic relationships, marriage, and childbearing; these transitions typically place limits on social drinking and alcohol use in general that endure throughout young adulthood (Dawson et al., 2006; Duncan et al., 2006).
While this study advances the burgeoning literature on G × E interactions in alcohol use, the results are limited by several factors. Participants' heavy drinking was defined as 6 drinks in a row for all participants, which is a more stringent cutoff than the commonly used 4 drinks for females and 5 for males, and it does not account for sex differences in alcohol use. Thus, the results for heavy drinking may have been attenuated by these differences and may not replicate across studies using lower and sex-specific thresholds. Friends' alcohol use was measured with items that did not provide specific definitions of ‘drinking’ or ‘heavy drinking,’ so the answers may have been affected by subjective interpretations of these terms. Friends' alcohol use was also measured differently in adolescence and adulthood, with a focus on any alcohol use among friends in adolescence but heavy use among friends in adulthood. This difference could serve as an alternative explanation for the developmental differences in findings regarding the moderating role of DRD4 genotype. It will be important to replicate the present results with identical measures of peer alcohol use over time. Self-reports of friends' alcohol use also represent a limitation, as they tend to be confounded by own levels of alcohol use; future studies should replicate these findings with different methodologies (e.g., experimental manipulation of peer drinking, friends' self-reports of own alcohol use). The sample size used in this study is relatively small (Duncan and Keller, 2011) and may not have been sufficient to detect smaller G × E effects or to expand the model by including additional moderators of the studied G × E interactions (e.g., sex, other genetic polymorphisms, or other personal or environmental characteristics). Finally, although the distributions of allele and genotype frequencies were similar to those of other Caucasian and European samples, ancestry informative markers were not collected and would be of benefit in future studies. Also of benefit would be to conduct genotyping in duplicate and to include concordance genotyping to increase the quality of the genotyping data.
In conclusion, this study provides further evidence for the long allele of DRD4 increasing susceptibility to peer influences on alcohol use in adulthood. It suggests that this G × E interaction emerges developmentally in young adulthood, but not in adolescence or emerging adulthood, and is more prominent for total alcohol consumption compared to heavy episodic drinking. The findings suggest that young adults with the long allele of DRD4 may benefit from interventions designed to help them reduce affiliations with heavy drinking friends or learn strategies to resist peer influences on alcohol use.
Highlights.
Alcohol use in early 30's was related to previous and current friends' alcohol use only among carriers of the DRD4 long allele
Friends' drinking in adolescence predicted more alcohol use and heavy drinking in emerging adulthood regardless of DRD4 genotype
Through adolescence and adulthood, alcohol use and/or heavy drinking predicted having more heavily drinking friends at later time points regardless of DRD4 genotype
Acknowledgments
Role of Funding Source: Funding for this study was provided by NIAAA Grant AA07861. NIAAA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Contributions: Sylvie Mrug reviewed the literature, conceptualized the study, conducted statistical analyses and drafted and revised the manuscript. Michael Windle directed that LAT study and contributed to the conceptualization, drafting and revisions of the manuscript.
Conflict of Interest: No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armor DJ, Polich JM. Measurement of alcohol consumption. In: Pattison EM, Kaufman E, editors. Encyclopedic Handbook Of Alcoholism Gardner. New York: 1982. pp. 72–81. [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: new evidence and a meta-analysis. Dev Psychopathol. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Wright JP, DeLisi M, Vaughn MG. Desistance from delinquency: the marriage effect revisited and extended. Soc Sci Res. 2008;37:736–752. doi: 10.1016/j.ssresearch.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Genetic moderation of early child-care effects on social functioning across childhood: a developmental analysis. Child Dev. 2013;84:1209–1225. doi: 10.1111/cdev.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutwell BB, Beaver KM. A biosocial explanation of delinquency abstention. Crim Behav Ment Health. 2008;18:59–74. doi: 10.1002/cbm.678. [DOI] [PubMed] [Google Scholar]
- Burk WJ, Van der Vorst H, Kerr M, Stattin H. Alcohol use and friendship dynamics: selection and socialization in early-, middle-, and late-adolescent peer networks. J Stud Alcohol Drugs. 2012;73:89–98. doi: 10.15288/jsad.2012.73.89. [DOI] [PubMed] [Google Scholar]
- Creemers HE, Harakeh Z, Dick DM, Meyers J, Vollebergh WAM, Ormel H, Verhulst FC, Huizink AC. DRD2 and DRD4 in relation to regular alcohol and cannabis use among adolescents: does parenting modify the impact of genetic vulnerability? The TRAILS Study Drug Alcohol Depend. 2011;115:35–42. doi: 10.1016/j.drugalcdep.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell KG, Sayette MA, Manuck SB, Ferrell RE, Hill SY, Dimoff JD. DRD4 polymorphism moderates the effect of alcohol consumption on social bonding. PLoS One. 2012;7:e28914. doi: 10.1371/journal.pone.0028914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS. Maturing out of alcohol dependence: the impact of transitional life events. J Stud Alcohol. 2006;67:195–203. doi: 10.15288/jsa.2006.67.195. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–90. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dmitrieva J, Cheng C, Greenberger E, Oguneitan E, Ding YC. Gender-specific expression of the DRD4 gene on adolescent delinquency, anger, and thrill-seeking. Soc Cogn Affect Neurosci. 2011;6:82–89. doi: 10.1093/scan/nsq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Wilkerson B, England P. Cleaning up their act: the effects of marriage and cohabitation on licit and illicit drug use. Demography. 2006;43:691–710. doi: 10.1353/dem.2006.0032. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin Exp Res. 2008;32:1113–23. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus ED, Morgan M, Forester GR, Hutchison K. Dopaminergic genes modulate response inhibition in alcohol abusing adults. Addict Biol. 2012;17:1046–56. doi: 10.1111/j.1369-1600.2011.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemstra M, Engels RCME, Barker ED, van Schayck OCP, Otten R. Smoking-specific parenting and smoking onset in adolescence: the role of genes from the dopaminergic system (DRD2, DRD4, DAT1 genotypes) PLoS One. 2013;8:e61673. doi: 10.1371/journal.pone.0061673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, La Chance H, Naiura R, Bryan A, Smolen A. The DRD4 VNTR polymorphism influences reactivity to smoking cues. J Abnorm Psychol. 2002;111:134–143. doi: 10.1037//0021-843x.111.1.134. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Wooden A, Swift RM, Smolen A, McGeary J, Adler L, Paris L. Olanzapine reduces craving for alcohol: a DRD4 VNTR polymorphism by pharmacotherapy interaction. Neuropsychopharmacology. 2003;28:1882–8. doi: 10.1038/sj.npp.1300264. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt BS, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65:674–82. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd KK, Pakstis AJ, Libing Y. An historical perspective on “The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus”. Hum Genet. 2014;133:431–433. doi: 10.1007/s00439-013-1386-0. [DOI] [PubMed] [Google Scholar]
- Kretschmer T, Dijkstra JK, Ormel J, Verhulst FC, Veenstra R. Dopamine receptor D4 gene moderates the effect of positive and negative peer experiences on later delinquency: the Tracking Adolescents' Individual Lives Survey study. Dev Psychopathol. 2013;25:1107–1117. doi: 10.1017/S0954579413000400. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, Kennedy JL. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry. 1996;1:121–124. [PubMed] [Google Scholar]
- Larsen H, van der Zwaluw CS, Overbeek G, Franke B, Engels RCME. A variable-number-of-tandem-repeats polymorphism in the dopamine D4 receptor gene affects social adaptation of alcohol use: investigation of a gene-environment interaction. Psychol Sci. 2010;21:1064–1068. doi: 10.1177/0956797610376654. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Menges DP, McGeary JE, Lisman SA. Effects of craving and DRD4 VNTR genotype on the relative value of alcohol: an initial human laboratory study. Behav Brain Funct. 2007;3:11. doi: 10.1186/1744-9081-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Monti PM, Ray LA, Murphy JG, Rohsenow DJ, McGeary JE, Swift RM, Tidey JW, Gwaltney CJ. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J Abnorm Psychol. 2010;119:106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeary J. The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review. Pharmacol Biochem Behav. 2009;93:222–229. doi: 10.1016/j.pbb.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitopoulos J, Zohsel K, Blomeyer D, Buchmann AF, Schmid B, Jennen-Steinmetz C, Becker K, Schmidt MH, Esser G, Brandeis D, Banaschewski T, Laucht M. Are infants differentially sensitive to parenting? Early maternal care, DRD4 genotypes and externalizing behavior during adolescence. J Psychiatr Res. 2014 doi: 10.1016/j.jpsychires.2014.08.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Park A, Sher KJ, Todorov AA, Heath AC. Interaction between the DRD4 VNTR polymorphism and proximal and distal environments in alcohol dependence during emerging and young adulthood. J Abnorm Psychol. 2011;120:585–595. doi: 10.1037/a0022648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Grottenthaler A, Ciccocioppo MM, Milanak M, Conklin CA, Bergen AW, Benowitz NL. Dopamine and opioid gene variants are associated with increased smoking reward and reinforcement owing to negative mood. Behav Pharmacol. 2008;19:641–649. doi: 10.1097/FBP.0b013e32830c367c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeevan H, Soundararajan U, Kidd JR, Pakstis AJ, Kidd KK. ALFRED: an allele frequency resource for research and teaching. Nucleic Acids Res. 2012;40(D1):D1010–D1015. doi: 10.1093/nar/gkr924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Scholte RHJ, Poelen EAP, Willemsen G, Boomsma DI, Engels RCME. Relative risks of adolescent and young adult alcohol use: the role of drinking fathers, mother, siblings and friends. Addict Behav. 2008;33:1–14. doi: 10.1016/j.addbeh.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Vaughn MG, Beaver KM, DeLisi M, Howard MO, Perron BE. Dopamine D4 receptor gene exon III polymorphism associated with binge drinking attitudinal phenotype. Alcohol. 2009;43:179–84. doi: 10.1016/j.alcohol.2009.02.001. [DOI] [PubMed] [Google Scholar]
- van der Zwaluw CS, Larsen H, Engels RC. Best friends and alcohol use in adolescence: the role of the dopamine D4 receptor gene. Addict Biol. 2012;17:1036–1045. doi: 10.1111/j.1369-1600.2010.00305.x. [DOI] [PubMed] [Google Scholar]
- Windle M. Parental, sibling, and peer influences on adolescent substance use and alcohol problems. Appl Dev Sci. 2000;4:98–110. [Google Scholar]
- Windle M, Wiesner M. Trajectories of marijuana use from adolescence to young adulthood: predictors and outcomes. Dev Psychopathol. 2004;16:1007–1027. doi: 10.1017/s0954579404040118. [DOI] [PubMed] [Google Scholar]
- Windle M, Windle RC. Testing the specificity between social anxiety disorder and drinking motives. Addict Behav. 2012;37:1003–1008. doi: 10.1016/j.addbeh.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohsel K, Buchmann AF, Blomeyer D, Hohm E, Schmidt MH, Esser G, Brandeis D, Banaschewski T, Laucht M. Mothers' prenatal stress and their children's antisocial outcomes – a moderating role for the Dopamine D4 Receptor (DRD4) gene. J Child Psychol Psychiatry. 2014;55:69–76. doi: 10.1111/jcpp.12138. [DOI] [PubMed] [Google Scholar]
- Zucker RA. Alcohol use and the alcohol use disorders: a developmental-biopsychosocial systems formulation covering the life course. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Risk, Disorder and Adaptation. 2nd. Vol. 3. Wiley; New York: 2006. pp. 620–656. [Google Scholar]


