Abstract
Pediatric AIDS caused by human immunodeficiency virus type 1 (HIV-1) remains one of the leading worldwide causes of childhood morbidity and mortality. HIV-1 proteins, such as Tat and gp120, are believed to play a crucial role in the neurotoxicity of pediatric HIV-1 infection. Detrimental effects on development, behavior, and neuroanatomy follow neonatal exposure to the HIV-1 viral toxins Tat1-72 and gp120. The present study investigated the neurobehavioral effects induced by the HIV-1 neurotoxic protein Tat1-86, which encodes the first and second exons of the Tat protein. In addition, the potential effects of HIV-1 toxic proteins Tat1-86 and gp120 on inflammatory pathways were examined in neonatal brains. Vehicle, 25 μg Tat1-86 or 100 ng gp120 was injected into the hippocampus of male Sprague-Dawley pups on postnatal day 1 (PD1). Tat1-86 induced developmental neurotoxic effects, as witnessed by delays in eye opening, delays in early reflex development and alterations in prepulse inhibition (PPI) and between-session habituation of locomotor activity. Overall, the neurotoxic profile of Tat1-86 appeared more profound in the developing nervous system in vivo relative to that seen with the first exon encoded Tat1-72 (Fitting et al., 2008b), as noted on measures of eye opening, righting reflex, and PPI. Neither the direct PD1 CNS injection of the viral HIV-1 protein variant Tat1-86, nor the HIV-1 envelope protein gp120, at doses sufficient to induce neurotoxicity, necessarily induced significant expression of the inflammatory cytokine IL-1β or inflammatory factors NFκ-β and Iκ-β. The findings agree well with clinical observations that indicate delays in developmental milestones of pediatric HIV-1 patients, and suggest that activation of inflammatory pathways is not an obligatory response to viral protein-induced neurotoxicity that is detectable with behavioral assessments. Moreover, the amino acids encoded by the second tat exon may have unique actions on the developing hippocampus.
Keywords: Tat1-86, gp120, HIV-1, developmental delay, cytokines, neurotoxicity
1. Introduction
Pediatric human immunodeficiency virus type 1 (HIV-1) infection is a matter of global concern, though it is not equally distributed around the world. As of 2012, ~3.3 million children (under age 15) have been reported to live with HIV-1, with most of these children living in Sub-Saharan Africa (~2.9 million) and less than 1% living in Northern America and Western and Central Europe (UNAIDS, 2013). With the introduction of antiretroviral therapies given to HIV-1 infected women during pregnancy, transmission rate of HIV-1 has decreased dramatically (Suksomboon et al., 2007; Volmink et al., 2007), although in developing countries, the mother-to-child transmission rate of HIV-1 remains very high, ranging from 23% to 34%, with an estimated 260,000 children newly infected in 2012 (UNAIDS, 2013). Because the developing brains of infants and children are extremely vulnerable to this debilitating disease (Mintz, 1994), pediatric HIV-1 is of special interest. The transition of HIV-1 from an acute, lethal disease to a subacute, chronic disease has major implications for the neurocognitive development of children. In the era of combination antiretroviral therapy (cART), vertically acquired HIV-1 infected children that survive to adulthood show high rates of asymptomatic neurocognitive impairment (Paramesparan et al., 2010). Among the neurological abnormalities most commonly seen in HIV-1 infected children on cART are delays in developmental milestones and poor neuropsychological functioning, including attentional, memory and motor dysfunction (Gavin and Yogev, 1999; Gupta et al., 2009; Jeremy et al., 2005; Keller et al., 2004; Le Doare et al., 2012; Paramesparan et al., 2010; Patel et al., 2008; 2009; Willen, 2006).
It is well-established that HIV-1 infected cells release neurotoxic viral proteins, including the transactivating protein Tat and the envelope protein glycoprotein 120 (gp120) (Bansal et al., 2000; Brenneman et al., 1988; Catani et al., 2003; Nath et al., 2000). The nonstructural regulatory protein Tat and the envelope gp120 are likely agents of the observed synaptodendritic alterations (Desplats et al., 2013; Ellis et al., 2007; Gelman and Nguyen, 2010; Moore et al., 2006) and neuronal loss (Del Valle et al., 2000; Jones et al., 2000; Nath et al., 2000) in the brains of HIV-1+ patients and have been studied extensively with in vitro and in vivo animal studies (Aksenov et al., 2001; 2003; 2006; Aksenova et al., 2005; 2006; Bertrand et al., 2013; 2014; Bruce-Keller et al., 2003; Cheng et al., 1998; Corasaniti, et al., 2001a, 2001b, 2001c; Fitting et al., 2006a; 2006b; 2007; 2008a; 2008b; 2010a; Lipton et al., 1995). Tat and gp120 exposure in rats reveals a similar pattern of synaptodendritic damage as seen in patients with HIV-1-associated neurocognitive disorders (HAND) (Tat, Fitting et al., 2010b; 2013; gp120, Gorantla et al., 2012; Kang et al., 2010; Toggas et al., 1994; Roscoe et al., 2014), lending support to the general idea that HIV-1 proteins are pathophysiologically relevant in HIV-1 related neurocognitive abnormalities. In vitro as well as in vivo studies have demonstrated that the HIV-1 Tat protein itself can increase chemokine/cytokine production, and cause astrogliosis, microgliosis and neuronal death (El-Hage et al., 2006; 2008). Specifically, the HIV-1 Tat protein has been shown to increase expression of interleukin-1β in cultures of human fetal astrocytes (Nath et al., 1999).
Tat protein is encoded by two exons producing a clinical isolate with either 86- (Jeang, 1996) or, the more common clinical isolate, 101-amino acids (Jeang et al., 1999). The first 72 amino acids correspond to the first exon of tat and this sequence is essential for efficient viral replication (Frankel et al., 1989); however, the Tat1-72 protein has never been observed in vivo (Campbell & Loret, 2009). The second tat exon encodes the C-terminus, defined by residues 73–101. Relative to the extensive study of the first exon functions in viral pathogenesis (Frankel et al., 1989; Romani et al., 2010), the physiological role of the second exon is less well-defined (Smith et al., 2003). The second exon encodes a RGD sequence in residues 77–79 (Watson and Edwards, 1999). RGD motifs are part of the recognition sequence for cell surface integrin binding (Barczyk et al., 2010), and integrins have a key role in neonatal hippocampal development (Gary et al., 2003; Murase et al., 2011; Wakselman et al., 2008). Further, as documented by in vitro studies, the uptake of extracellular Tat1-86 is 10X more efficient relative to the first exon Tat1-72, suggesting that the region encoded by the second exon is important in mediating efficient Tat internalization (Ma and Nath, 1997). Thus, inclusion of the second tat exon encoded polypeptide, containing the RGD and internalization sequence, in the present studies of Tat1-86 is anticipated to produce specific effects on hippocampal development.
Using extant preclinical models such as transgenic rats/mice, or direct CNS injection of virotoxins, it is possible to study the direct effects of the HIV-1 proteins independent of any secondary HIV-1 infections or the virus itself. Previous studies in our lab have established that both Tat1-72 and gp120 produce adverse long-term effects on neurocognitive processes involved in sensorimotor gating when injected bilaterally into the hippocampus of postnatal day (PD) 1-old rats (Fitting et al., 2006a; 2006b), and that Tat1-72- and gp120-induced delays in early reflex development are correlated with alterations in hippocampal cell number (Fitting et al., 2008b). The hippocampus is of particular interest due to both the high viral load present in this region (Wiley et al., 1998), and evidence of synaptodendritic injury in the hippocampus in both HIV-1+ humans (Sa et al., 2004; Desplats et al., 2013) and in rats exposed to viral proteins (Fitting et al., 2013).
In addition to the release of different viral proteins by HIV-1 infected cells, elevation of a variety of cytokines may be detected. Cytokines and other inflammatory biomarkers have been clearly associated with HIV-1-associated dementia (HAD) in the pre-cART era (Gartner and Liu, 2002; Glass et al., 1993; Perrella et al. 1992), but they may also play a role in the development of less severe forms of HAND despite viral suppression due to cART. A recent longitudinal study reported significantly higher levels of Interferon-α-2b, IL-6, and sIL-2R in HIV-1+ individuals that became neurocognitively impaired, remained impaired, or worsened one year after the baseline study, compared to HIV-1+ individuals who had either no impairment or improved neurocognitive status (Cassol et al., 2013). Neurocognitive impairment has also been associated with elevated levels of cytokines in perinatally HIV-1-infected children (Foster et al. 2012). The inflammatory response initiated by the immune system in response to the viral presence can damage neurons and disrupt function (Ensoli et al., 1999; Miura et al., 2003; Yadav and Collman, 2009), and could likely account for many clinical and pathological conditions seen in the nervous system in HIV-1+ individuals. Children with HIV-1 may be particularly vulnerable to neuronal damage related to inflammatory cytokine activation, given both an immature CNS and higher levels of cytokines compared to HIV-1+ adults (Jin et al., 2009; Ketlinskii et al., 1992). In particular, cytokines play an important role in hippocampal function (Arisi, 2014) and brain development (Bilbo and Schwarz, 2012).
In the present study, we injected Tat1-86 into the neonatal rat hippocampus on PD1 and investigated two aspects: First, we examined the effects of the HIV-1 neurotoxic protein Tat1-86 on neurobehavioral development. Several early developmental reflexes were used to assess different aspects of neurodevelopment in neonatal rats (i.e. righting reflex, negative geotaxis, eye opening). Preattentive processes were indexed by prepulse inhibition (PPI), a neurodevelopmental assessment of sensorimotor gating (Parisi & Ison, 1979). Locomotor activity measures were employed to determine the presence or absence of motor dysfunction. Second, we investigated the potential effects of Tat1-86 and gp120 on inflammatory responses. The role of inflammatory processes may provide important insights into HIV-1 protein-induced neurotoxicity and is critical in determining the contribution of the viral proteins, Tat1-86 and gp120, to the neurological and neuropsychiatric impairment consequent to early HIV-1 infection. In sum, using Tat1-86, which encodes both the first and second exons, we sought to ascertain HIV-1 viral protein-induced effects on neurobehavioral development and inflammatory activation.
2. Methods
2.1. Animals
Sprague-Dawley pregnant dams (n = 14) were ordered from Harlan Laboratories, Inc. (Indianapolis, IN) and delivered to the vivarium before embryonic day seven. Dams were singly housed with food (Pro-Lab Rat, Mouse Hamster Chow #3000, NIH diet #31) and water available ad libitum. The day the dam gave birth was designated postnatal day 0 (PD0). The animal facility was maintained at 20 ± 2 °C, 50 ± 10 % relative humidity and had a 12-h light: 12-h dark cycle with lights on at 0700h (EST). The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC-accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina, Columbia.
For the behavioral experiment, on PD1, litters (n = 8) were culled to five males and five females. Two males from each litter were assigned to one of the two treatment conditions tested in the present study – vehicle vs. Tat treatment. At 22 days of age, animals were weaned and litters were culled to only treated rats. These two-rat housing arrangements were maintained for the remainder of the experiment.
For the inflammatory biomarker experiment, on PD1, six males from each litter (n = 9) were randomly assigned to one of three treatment groups: vehicle, Tat or gp120. On PD3, 48 h after experimental treatment, the pups were sacrificed and brains were removed.
2.2. HIV-1 Proteins
Purified recombinant Tat1-86 (LA1/Bru strain of HIV-1 clade B, Genbank accession no. K02013, Diatheva, Fano, Italy) and T-tropic gp120 (Protein Sciences Corp., Meriden, CT) were purchased and stored at −80 °C until used for intrahippocampal injections. Recombinant Tat and gp120 proteins were dissolved in sterile buffer to final concentrations of 25 μg/μL and 100 ng/μL, respectively.
2.3. Surgical Techniques and Protein Treatment
Standard stereotaxic surgery techniques, modified for neonates, were used for treatment injection. Individual pups were removed from the dam and cryogenically anesthetized (AVMA, 2001) before being placed in a modified stereotaxic holder for surgery of neonates (Kopf, Inc., Tujunga, CA), which included a chilled base to maintain cryogenic anesthesia. Rubber head bars held the skull in place while microinjections were made directly into the hippocampus using stereotaxic coordinates and a microsyringe [Hamilton Co., Nevada, USA (Microliter #701 RN, 10μL)]. For both the behavioral and inflammatory biomarker studies, bilateral injections were conducted with silanized syringes. The set of coordinates used for the left and right hippocampus were: −0.65 mm anterior to bregma, 1.0 mm lateral to bregma, and −2.2 mm dorsal from dura (Fitting et al., 2008a; 2008b). Hippocampal injection order was alternated across animals, using the same set of coordinates. The injection volume was 1.0 μL released over two min, after a one-min resting period that allowed the tissue to return to its original conformation (Fitting et al., 2006a; 2006b; 2007). Animals were injected bilaterally with sterile buffer (vehicle (VEH); 10 mM Tris HCl, 300 nM NaCl, pH = 7.58, sterile), Tat 25 μg, or 100 ng gp120. These doses were selected on the basis of our previous developmental research (Fitting et al., 2006a; 2006b; 2007; 2008b; 2010a); doses at which no gross growth impairment would be anticipated. The injection needle was withdrawn over two min to prevent reflux. The piercings in the skin of the head were closed with surgical glue and the pups warmed on a heat pad (35 °C) before being returned to the dam, where they were closely monitored for any indications of rejection. No pups were rejected or abused by the dam.
2.4. Behavioral Experimental Design
A randomized block design was employed, with litter as the blocking factor, in which both experimental treatments were represented. Thus, male rats were randomly assigned to one of two treatment groups [VEH (n = 8), or Tat (n = 8)] that received bilateral hippocampal injections on PD1. Weight was taken on the day of surgery, PD1, and subsequent, concurrent weighing started on PD3. Behavioral assessment began on PD3. All rats were tested for (1) early reflex development by assessing righting reflex on PD3-5 and negative geotaxis on PD8-10; (2) developmental milestone by assessing eye opening on PD13-17; (3) preattentive processes by assessing PPI of the acoustic startle response (ASR) on PD18; and (4) motor function by assessing locomotor activity in post-weanling rats on PD26-28.
2.4.1. Health and Somatic Growth
Body Weight
Pups were weighed on a daily basis starting on PD3 for the remainder of the experiment.
Eye opening
Eye opening was examined as a developmental milestone on PD13-16. The first day any opening was witnessed was PD13; all animals’ eyes were fully opened by PD16. Eye opening is a crucial development of sensory processes and vestibular plasticity (Grassi et al., 2004) that critically influences the cellular connectivity of the visual cortex (Heinen et al., 2004; Katz and Shatz, 1996). Eye opening was assessed separately for right and left eye between 0800–1000h (EST). Eye opening was assessed on a two-point scale, with a zero score indicating a closed eye and a score of 1 indicating an open eye.
2.4.2. Early Reflex Development
Righting Reflex
The righting reflex was examined on PD3-5 to assess preweanling alterations in the sensory-motor system very early in development. The goal was to assess the development of the sensory-motor system at a time point when other developmental milestones were still underdeveloped and thus not confounded by secondary variables, such as eye opening and ear opening. The righting reflex is defined as the ability to assume an upright position when there has been a departure from it (Walton et al., 2005). Pups were tested each day between 1600–1800h (EST) on a metallic wire grid. A pup was placed on its back and time in seconds was recorded to return to the upright position by turning over onto its ventral surface.
Negative Geotaxis
Another test to determine the development of the sensory-motor system was the assessment of time in seconds to perform negative geotaxis on PD8-10. Negative geotaxis is defined as an orienting response and movement expressed in opposition to cues of a gravitational vector (Motz and Alberts, 2005). Pups were tested each day between 1600–1800h (EST). The test was conducted by placing the pup oriented facing head down on a 25° tilted plane with a metallic wire grid. The dependent variable was time in sec to turn 180° and orient toward the higher end of the plane.
2.4.3. Sensorimotor function – Preattentive Process (PPI of the ASR)
Apparatus
The startle chamber (SR-Lab Startle Reflex System, San Diego Instruments, Inc.) was enclosed in a 10 cm thick double-walled, 81 × 81 × 116 cm isolation cabinet (external dimensions) (Industrial Acoustic Company, INC., Bronx, NY). Each animal was tested individually in the dark with a high-frequency loudspeaker, that produced a background white noise [70 dB(A)] and was mounted inside the chamber 31 cm above the Plexiglas cylinder. The startle chamber consisted of a Plexiglas cylinder 3.9 cm in internal diameter for preweanling rats. The Plexiglas cylinder was resting on a 12.5 × 20 cm Plexiglas stand. The startle stimulus was 100 dB(A) and the acoustic prepulse stimulus was 85 dB(A) in intensity. Both stimuli had a duration of 20 ms. The animal’s response to the startle stimulus produced deflection of the Plexiglas cylinder, which was converted into an analog signal by a piezoelectric accelerometer. The signals were then digitized (12 bit A to D) and saved to a hard disk on a Pentium class computer. Acoustic stimulus intensities and response sensitivities were calibrated with a sound level meter (Extech Instruments: Waltham, MA) with the microphone placed inside the Plexiglas cylinder.
Testing Procedures
All rats were tested for approximately 20 min on PD18. Animals were first exposed to a five-min acclimation period of 70 dB(A) background of white noise, followed by six single white noise stimuli of 100 dB(A) as adaptation trials, and 36 PPI trials with 0, 8, 40, 80, 120 and 4000 ms interstimulus intervals (ISIs), assigned by a Latin-square design. The stimulus duration was 20 ms. The PPI trials with the 0 and 4000 ms ISIs were defined as control trials in order to provide a reference baseline ASR within the PPI test. Within the constraints of the software, the ISI represents the time from the offset of the prepulse stimulus to the onset of the startle stimulus. For PPI the dependent measure analyzed was peak ASR amplitude.
2.4.4. Motor Function – Locomotor Activity
The activity monitors were square (40 cm × 40 cm) chambers (Flex-Field, San Diego Instruments, San Diego, CA) that detected free movement of animals by infrared photocell interruptions. This equipment used an infrared photocell grid (32 emitter/detector pairs) with locomotor activity being measured by assessing the number and type of photocell interruptions within a 60-min period. Testing occurred on PD26-28 between 1500–1700h (EST) under dim light conditions, in the absence of direct overhead lighting (<10 1x). The dependent variable was the animal’s total ambulation (cm; the sum of x and y photocell interruptions). Total time (s) spent in the center and the periphery of the activity monitor was also recorded.
2.4.5. Data Analyses
The data were expressed as mean (± SEM) or median (± Interquartile Range), as appropriate. Continuous data were analyzed using ANOVA techniques (SYSTAT 11.0 for Windows, SYSTAT Inc.) including treatment (VEH or Tat1-86) as a between-subjects factor. For the within-subjects terms (e.g., test day, trial, ISI), potential violations of sphericity (Winer, 1971) were preferentially handled by the use of the orthogonal decomposition or, if necessary, the use of the Greenhouse-Geisser df correction factor (Greenhouse and Geisser, 1959). The orthogonal decomposition approach for the within-subjects terms also permitted a determination of the shape of the temporal functions (e.g., linear, quadratic, etc.) (Winer, 1971). Simple main effects of test day were also included where appropriate. The non-parametric Mann-Whitney U-Test was performed on the somatic growth index of eye opening. An alpha level of p ≤ 0.05 was considered significant for interpretation of all statistical tests.
2.5. Inflammatory Biomarker Experimental Design
2.5.1. Tissue collection and cDNA library generation
On PD3, 48 h after viral protein injections, pups were sacrificed by rapid decapitation and the brain was removed from the cranial vault for microdissection. This 48 h interval was selected on the basis of our prior study of Tat protein injection and the temporal relationship to reactive astrocytosis and protein oxidation (Aksenov et al., 2003) and early behavioral sequelae (Fitting et al., 2008b). After removal of obstructing meninges the midbrain was dissected out of the surrounding midbrain tissue to facilitate access to the hippocampus. Both left and right hippocampal tissue were collected from all 6 males of each litter, and pooled within the appropriate treatment groups to ensure sufficient tissue mass for RNA isolation. Total RNA was isolated using the RNAqueous-4PCR kit (Ambion, Austin, TX) and concentration was measured by the 260/280 nm absorbance ratio (DNA Calculator, GeneQuant, Piscataway, NJ). A cDNA library was generated by the reverse transcription PCR (RT-PCR) of 1 μg purified RNA using 50 ng/μL random hexamer primers (Cloned AMV First-Strand cDNA Synthesis kit, Invitrogen, Carlsbad, CA). An additional sample lacking the reverse transcription enzyme was included in the RT-PCR reaction to control for potential DNA contamination.
2.5.2. MPCR
Following the RT-PCR production of cDNAs the samples were probed for the expression of inflammatory cytokine IL-1β, inflammatory factors NFκ-β and Iκ-β, and the internal control, GAPDH. The MPCR reaction was optimized for cycle number and DNA content, and samples were run in a cycle protocol of 94 °C melting, 64 °C annealing and 70 °C elongation. Cytokine primers, a PCR reaction buffer containing dNTPs and a positive control were provided in the MPCR kit (Rat Inflammatory Cytokines–Set 2, Maxim Biotech, San Francisco, CA). Optimal results were achieved with 4 % v/v RT-PCR mixture over 30 cycles at the aforementioned temperatures. Amplicons were resolved on a 10 % TBE gel for 1 h at 200 V. DNA molecular weight ladders, cDNA positive controls and a negative control were included on the gels to provide reference standards.
2.5.3. Data Analysis
PCR amplicons were separated on a 10 % TBE precast gel (Bio-Rad, Hercules, CA) at 200 V for one h, followed by a 30 min gel stain in SYBR Green (Molecular Probes, Carlsbad, CA). Bands were visualized on a ChemiDoc XRS Molecular Imager (Bio-Rad, Hercules, CA) and the data recorded and summarized with the accompanying Quantity One software. Data analysis was then conducted using ANOVA techniques, as with the behavioral data, with treatment as a between-subjects factor and inflammatory biomarker as a within-subjects factor (Winer, 1971). Again, an alpha level of p ≤ 0.05 was considered significant for interpretation of all statistical results.
3. Results
3.1. Behavioral Data
3.1.1. Health and Somatic Growth
Body Weight
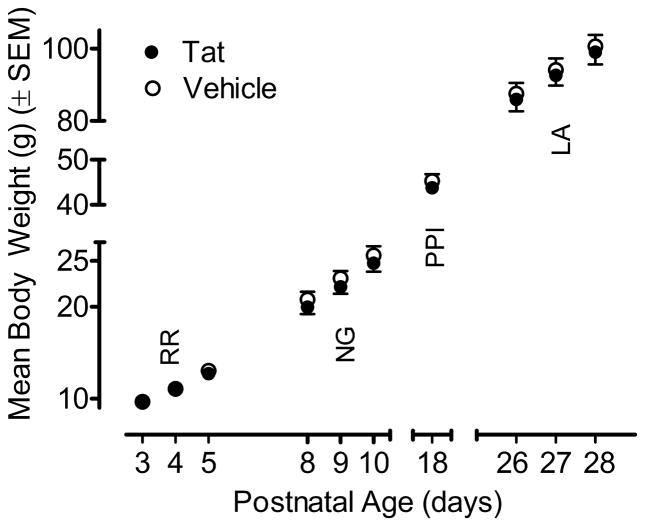
Figure 1 illustrates the mean (± SEM) body weight data across test days for the VEH- and Tat-treated animals. Separate ANOVAs were conducted for each test set with treatment as the between-subjects factor and, where appropriate, test day as a within-subjects factor. Neither a main effect of treatment nor a treatment x day interaction was noted for any of the test sets. As illustrated in Figure 1, body weight increased with age and no detrimental effect of the Tat1-86 protein was detected.
Figure 1.
Mean (± SEM) body weight across the various test days by treatment. No significant treatment effect or interaction between treatment and day was noted. RR = righting reflex; NG = negative geotaxis; PPI = prepulse inhibition; LA= locomotor activity.
Eye Opening
Eye opening started at PD13 for VEH-treated animals, and PD14 for Tat1-86-treated animals. Eyes were fully open for all rats on PD16. Eye opening data were analyzed as ‘eye closed’ (coded as 0) and ‘eye open’ (coded as 1). Collapsing across PD14-15 and computing the sum of left and right eye scores, the maximum rank score was 2 and the minimum rank score was 0.
A Mann-Whitney U-Test on the combined data (collapsed across PD14-15), revealed a Tat-induced deficit in eye opening (U = 14.5, p ≤ 0.05, one-tailed). Separate U-tests conducted for each day suggested an effect of Tat on the second assessment day of eye opening, specifically (PD14: U = 16.0, p ≤ 0.06, one-tailed; Tat, median= 0, IQR=0; VEH, median=0.5, IQR=1). Thus, the analysis of daily observation data indicated significant alterations on PD14 as a function of treatment. No significant treatment effect was noted on PD15.
3.1.2. Early Reflex Development
Righting Reflex
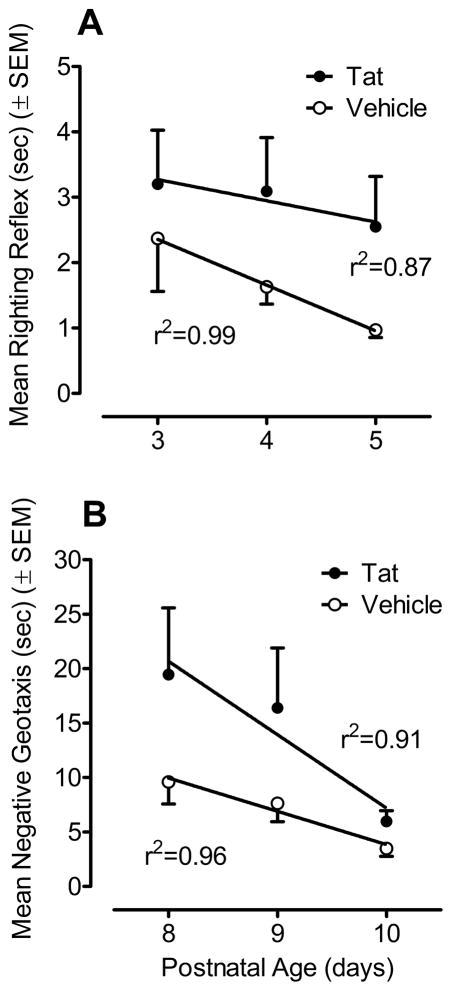
Figure 2A illustrates mean (± SEM) response time for righting reflex of VEH- and Tat-treated animals across the three test days (PD3–5). A 2 (treatment) x 3 (test days) mixed-model ANOVA conducted on the log transformed latency data (time in s) of each day revealed a significant treatment effect [F(1, 14) = 9.8, p ≤ 0.01]. Tat-treated animals were much slower in the righting reflex compared to the VEH-treated group. A significant day effect was found [F(2, 28) = 3.4, p ≤ 0.05] with a prominent linear component [F(1, 14) = 7.1, p ≤ 0.05], indicating that execution of the righting reflex improved across test days. Planned contrast analyses for each treatment indicated significant differences in the progression of the righting reflex across the three testing days. The overall significant day effect was due to a significant linear trend for a decrease in response time for the VEH group [F(1, 7) = 9.1, p ≤ 0.05], which was not evident for the Tat-treated group. Thus, the Tat1-86 treatment appeared to disrupt maturation of the righting reflex across test days.
Figure 2.
Mean (± SEM) response time with the best fit linear regression across three days for each treatment group. (A) A significant treatment effect was detected for righting reflex across all test days (PD3-5) (p ≤ 0.05); Tat1-86 -treated animals were much slower in the righting reflex compared to the VEH-treated group. A significant test day effect was found (p ≤ 0.05) with a prominent linear component (p ≤ 0.05), indicating that execution of the righting reflex improved across test days. Planned contrast analyses for each treatment group revealed a significant linear component for the VEH-treated animals (p ≤ 0.05) which was not found in the Tat1-86-treated group. Thus, the Tat1-86 treatment appeared to disrupt maturation of the righting reflex across test days. (B) A significant overall treatment effect was detected for negative geotaxis across all test days (PD8-10) (p ≤ 0.05); Tat1-86 -treated animals had overall slower response latencies compared to VEH-treated animals. A significant day effect was found (p ≤ 0.05) with a prominent linear component (p ≤ 0.01), indicating that execution of the righting reflex improved across test days. No treatment x test day interaction was detected, suggesting that both groups showed a similar rate of improvement across test days, approximating similar terminal levels of acquisition.
Negative Geotaxis
Figure 2B illustrates the mean (± SEM) response latency for negative geotaxis across the three test days (PD8–10) by treatment group. A 2 (treatment) x 3 (test days) mixed-model ANOVA on latency (time in s) revealed a significant treatment effect [F(1, 14) = 4.7, p ≤ 0.05], indicating overall slower response latencies for animals treated with Tat1-86. Further, the analysis revealed a test day effect [F(2, 28) = 8.3, p ≤ 0.01] with a prominent linear component [F(1, 14) = 4.7, p ≤ 0.05]. No significant treatment x test day interaction was detected, suggesting that both groups showed a similar rate of improvement across test days, approximating similar terminal levels of acquisition.
3.1.3. Sensorimotor Function (PPI of the ASR)
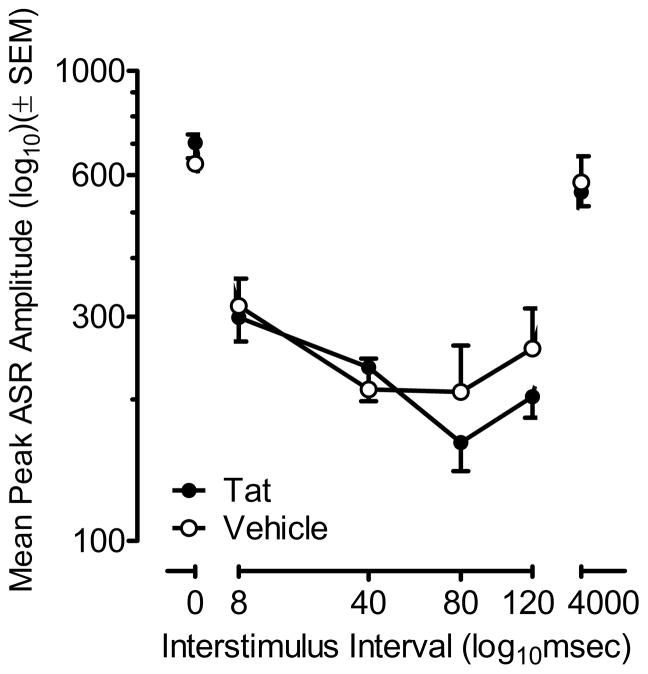
Figure 3 displays (± SEM) peak ASR amplitude for preweanlings (PD18) across ISI (0–4000 ms) by treatment group.
Figure 3.
Mean (± SEM) peak ASR amplitude by treatment across ISIs (0–4000 ms). No significant treatment effect was found on control trials (0 and 4000 ms ISIs), suggesting that Tat1-86 did not affect the animals’ baseline startle. Both groups displayed peak inhibition at the 80 ms ISI. The characteristic quadratic fit of the PPI ISI function for the VEH-treated animals was shifted in the Tat1-86 -treated animals to also include a prominent linear component to the PPI ISI function.
Control Trials (0, 4000 ms ISI combined)
A one-way ANOVA on peak ASR amplitude revealed no significant treatment effects, suggesting that Tat1-86 did not affect the animals’ baseline startle response.
PPI Trials (8–120 ms ISI)
Response amplitude for the PPI trials was decreased by 61.8 % compared to the ASR control trials, demonstrating effective sensorimotor gating across groups. A 2 (treatment) x 4 [ISI (8, 40, 80, 120 ms)] mixed-model ANOVA conducted on peak ASR amplitude demonstrated a significant ISI effect [F(3,42) = 10.3, p ≤ 0.001] with a most prominent quadratic component [F(1,14) = 36.1, p ≤ 0.001]. Although the treatment x ISI interaction failed to achieve statistical significance, the functional relationship describing the inhibition curve appeared to be altered as a function of treatment. Specifically, the ISI function for the VEH-treated animals was exclusively represented by a significant quadratic relationship [F(1,7) = 28.7, p ≤ 0.001], whereas the ISI function for the Tat-treated animals displayed both linear and quadratic components [F(1,7) = 6.2, p ≤ 0.05 and F(1,7) = 10.8, p ≤ 0.01, respectively]. Both treatment groups displayed maximal prepulse inhibition at the 80 ms ISI.
3.1.4. Motor Function (Locomotor Activity)
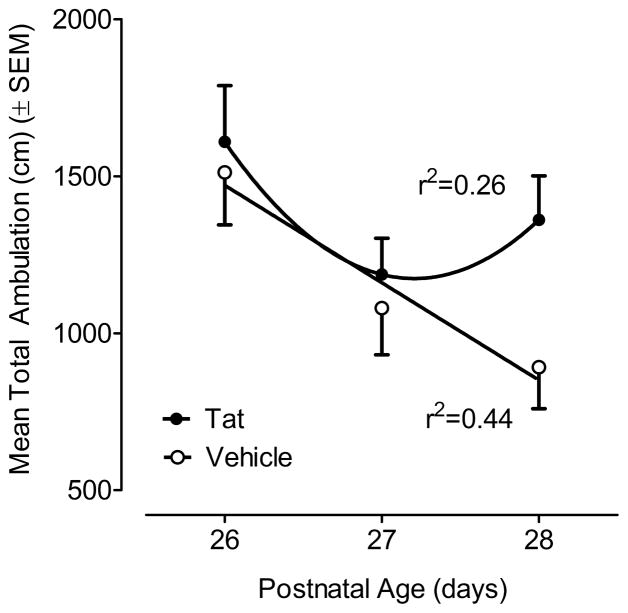
Mean total ambulation over a 60-min period on PD26-28 is illustrated in Figure 4. A 2 (treatment) x 3 (test days) mixed-model ANOVA revealed a significant test day effect [F(2, 28) = 9.4, p ≤ 0.002] with significant linear and quadratic orthogonal components [F(1, 14) = 11.2, p ≤ 0.005 and F(1, 14) = 6.2, p ≤ 0.03, respectively]. A significant test day effect was confirmed in the VEH-treated group [F(2,28)=7.68, p<0.002], with a prominent linear component [F(1,14)=11.5, p ≤ 0.005], indicating that the VEH-treated animals displayed prominent consistent between-session habituation. In contrast, the significant test day effect for the Tat-treated group [F(2,28)=3.41, p<0.05] contained only a significant quadratic component [F(1,14)=6.19, p<0.03], suggesting a failure to habituate across the daily test sessions. Tests of simple main effects further confirmed that the Tat1-86 -treated group demonstrated significantly greater ambulation on the third session compared to the VEH-treated group [F(1,14)=5.9, p ≤ 0.05]. Temporal measures of activity revealed a similar pattern of results with a parallel profile of statistically significant outcomes (data not shown).
Figure 4.
Mean (± SEM) total ambulation for each treatment group across the three test days (PD26-28). Between-session habituation was confirmed by a significant effect of test day with a significant linear decrease across test day (p ≤ 0.005) for the VEH-treated animals; whereas the between-session habituation of the Tat-treated animals was best represented by a quadratic orthogonal component (p ≤ 0.03). The Tat1-86 -treated group demonstrated significantly greater ambulation on the third session compared to the VEH-treated group (p ≤ 0.05).
3.2. Inflammatory Biomarker Data
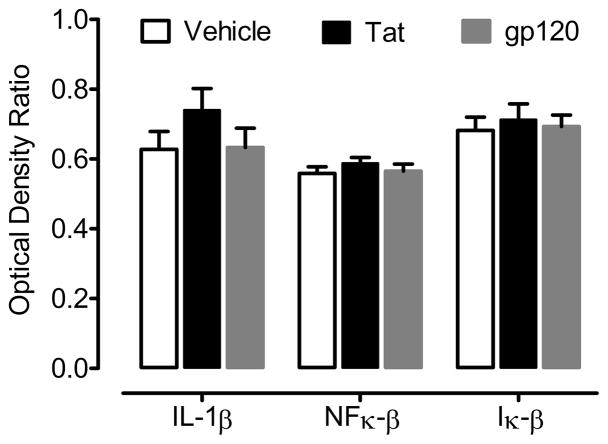
Figure 5 illustrates the mean (± SEM) inflammatory biomarker expression by treatment group. Expression of IL-1β, NFκ-β, and Iκ-β was detected across all three treatment groups. Neither an overall ANOVA nor one-way ANOVAs for each of the three genes expressed revealed a significant treatment effect of Tat or gp120 on biomarker expression.
Figure 5.
Mean (± SEM) optical density ratio values of biomarkers reflecting the potential activation of inflammatory pathways as a function of in vivo neonatal Tat or gp120 treatment. Statistical analysis failed to detect any significant increase in the expression of IL-1β, NFκ-β, or Iκ-β in Tat1-86 -treated (non-significant % increases of 17.9 %, 4.5 %, and 5.3 %, respectively) or gp120-treated (all values within 2 % of control) animals.
4. Discussion
Neurobehavioral assessments revealed significant, perhaps transient, adverse effects of hippocampal HIV-1 protein Tat1-86 injection in male rats, whereas an absence of significant expression of inflammatory cytokine IL-1β and related factors NFκ-β and Iκ-β was observed following Tat1-86 or gp120 injection. Notably, no significant effects on body weight were detected, indicating that the Tat1-86 dose used in the present study produced no general growth deficits, similar to previous findings when injecting Tat1-72 (Fitting et al., 2008b). The protein variant, Tat1-86, has been shown to induce neuronal cell death in vitro (Pocernich et al., 2005), and its uptake is ten times more efficient relative to the first exon Tat1-72 (Ma et al., 1997). The results of the present study provide greater relevance of this model by demonstrating the effects of the Tat1-86.
The significant Tat-induced alterations detectable during the neonatal period included measures of both somatic (eye opening) and reflex development. Our results suggest that the viral HIV-1 protein Tat1-86 acts differently on the developing CNS compared to the short HIV-1 protein variant Tat1-72. Whereas Tat1-86 delayed the onset of eye opening in the current study, alterations in eye opening following treatment with Tat1-72 (same dose) were never large enough within a reasonable sample size to be confirmed as a delay in development (Fitting et al., 2008b). The two Tat proteins also appeared to induce a different profile of effects on the righting reflex, an early reflex developmental milestone; specifically, Tat1-86 produced a significant overall impairment in the execution of the righting reflex across test days, whereas Tat1-72 produced a significant deficit only on PD3, with normal latency displayed on PD4 and 5 (Fitting et al., 2008b). In contrast, regarding negative geotaxis, the outcome from either Tat variant appeared quite similar. With Tat1-86, there was over a 100 % slower latency to right on P8; with Tat1-72, there was a 70 % slower latency to right on P8 (Fitting et al., 2008b); on P10, righting latencies for either Tat-treated group were not distinguishable from VEH-treated controls. Thus, negative geotaxis data suggested an approximate two-day delay in reflexive development, albeit not differentially as a function of Tat variant.
The preweanling/weanling age neurobehavioral assessments also supported the perspective that the two Tat proteins may produce a different profile of neurobehavioral alterations. With respect to the auditory startle response and PPI, the Tat1-86 treatment appeared to alter the prepulse inhibition function relative to VEH controls whereas no significant effects of Tat1-72 were detectable during the preweaning period (Fitting et al., 2008b). However, neonatal Tat1-72 treatment-induced alterations in PPI were detectable once the animals reached adulthood (PD91). Regarding the weanling assessments of locomotor activity, both the Tat1-86- and the Tat1-72-treated (Fitting et al., 2008) animals displayed less between-session habituation across days relative to the VEH-treated controls. One possible explanation for the reduction in habituation is a Tat-induced altered response to novelty. Dopamine systems are particularly vulnerable to the neurotoxic effects of Tat (Aksenov et al. 2008; Ferris et al. 2009; Midde et al. 2013; Zhu et al. 2009; Zhu et al. 2011), and variations in dopamine systems have been associated with differences in locomotor responses to novel environments (Chefer et al., 2003; Hooks et al., 1992, 1994; Zhu et al., 2007). Based on the Tat1-86-induced delays in eye opening, delays in righting reflex development, and alterations in PPI, the present data suggest that Tat1-86 is more neurotoxic in the developing nervous system in vivo compared to Tat1-72. The Tat1-72 isolate protein has never been observed in vivo, whereas Tat1-86 and Tat1-101 represent clinical isolates (Campbell and Loret, 2009), and may represent more fully the physiological actions of the Tat protein.
The sequences of both Tat1-86 and Tat1-72 differ mainly in the presence of the second exon encoded region of the protein. Both versions of the HIV-1 Tat protein have been shown to induce synaptodendritic injury (Bertrand et al., 2013) and neuronal cell death in vitro (Pocernich et al., 2005); however, the uptake of extracellular Tat into brain cells by Tat1-86 is 10 times more efficient relative to the first exon Tat1-72 (Ma and Nath, 1997). Moreover, the second exon region of the Tat protein contains an RGD sequence; RGD motifs are part of the recognition sequence for cell surface integrin binding (Barczyk et al., 2010). Integrins have a key role in neonatal hippocampal development (Gary et al., 2003; Wakselman et al., 2008), perhaps regulating the survival of hippocampal neurons and the formation of appropriate connectivity (Murase et al., 2011). Thus, the presence of the second exon in Tat1-86 protein might be expected to produce different effects on hippocampal development, relative to Tat1-72.
A design-based stereological study from our laboratory demonstrated long-term neurotoxicity in response to intrahippocampal injection of Tat1-72 (Fitting et al., 2008a). Tat1-72 decreased the number of neurons and increased glial cells in specific hippocampal subfields, and these alterations were correlated with delays in early reflex development (Fitting et al., 2008b). Astrogliosis is frequently reported in pediatric HIV-1 infection (Saito et al., 1994; Tornatore et al., 1994; Vallat et al., 1998) and is suggested to occur only if the insult includes the activation of microglia and the production of inflammatory cytokines in the perinatal CNS (Corasaniti et al., 2001a). HIV-1 infection of microglia/monocytes has been shown to lead to CNS pathology through the release of toxic cytokines (Dickson et al., 1993; Nath et al., 1999; Philippon et al., 1994). Further, perinatal inflammatory cytokine challenges can result in distinct neurobehavioral alterations; e.g., IL-1α treated rats displaying abnormal startle response and prepulse inhibition after puberty (Tohmi et al., 2004) and IL-6 overexpressing transgenic mice showing a progressive decline in avoidance learning (Heyser et al., 1997). The important role of cytokines in pediatric CNS disease following HIV-1 infection is suggested by previous research that reported significant elevation of inflammatory cytokines, including IL-1β and TNF-α (Foster et al., 2012; Jin et al., 2009; Ketlinskii et al., 1992; Torre et al., 1996). Neither the presence of HIV-1 protein Tat1-86 nor gp120, at least at the doses our studies typically employ, was sufficient to induce significant gene expression of the inflammatory cytokine IL-1β or inflammatory factors NFκ-β and Iκ-β. As such, it is unlikely that the induction of an inflammatory response by the HIV-1 Tat1-86 protein provides any significant contribution to the observed neurobehavioral alterations seen in the present study. Overall, while increased cytokine production may be associated with diffuse gliosis in pediatric AIDS (Saito et al., 1994; Tornatore et al., 1994; Vallat et al., 1998), the present data suggest that activation of inflammatory pathways is not an obligatory response to viral protein induced neurotoxicity that is detectable with behavioral assessments. Nevertheless, the chronic overexpression of inflammatory cytokines in the mammalian CNS, as via the transgenic model approach, does suggest that expression of specific cytokines alone in the intact CNS results in unique neuropathological alterations and functional impairments (Heyser et al., 1997). Given that the present study examined a limited profile of inflammatory indicators at a specific time point, it remains possible that other cytokines might be elevated at different time points post-treatment. As the gene expression reported here does not directly correlate with protein expression, further experiments that include measures of cytokine protein expression may provide more information on inflammatory pathways that may be involved in HIV-1 infection.
In the absence of an overt inflammatory response, a likely cause of the observed developmental and neurobehavioral alterations is Tat-induced injury to synaptodendritic networks. Synaptic dysfunction has been reported in HIV-1+ patients with latent viral infection (Desplats et al., 2013; Gelman and Nguyen, 2010), and, along with dendritic injury (Ellis et al., 2007; Moore et al., 2006), has also been correlated with severity of neurocognitive decline (Everall et al., 1999; Masliah et al., 1997). Single protein (Tat or gp120) transgenic mice studies confirm decreased spine density and/or synaptic dysfunction (Tat, Fitting et al., 2010b; 2013; gp120, Gorantla et al., 2012; Kang et al., 2010); moreover, synaptic alterations are found in HIV-1 transgenic rats expressing both Tat and gp120 proteins throughout development (Roscoe et al., 2014). Tat-induced synaptodendritic damage is highly specific and dependent upon the presence of the cysteine region of the Tat protein (Bertrand et al., 2013). Moreover, Tat-induced synaptodendritic damage occurs prior to cell death, at very low concentrations, and may be reversible (Bertrand et al., 2014).
Another central mechanism of HIV-1 viral protein-induced neurotoxicity is dopamine transporter (DAT) impairment. In vitro studies have shown that DAT is targeted by Tat1-86 (Aksenov et al. 2008; Ferris et al. 2009; Midde et al. 2013; Zhu et al. 2009; Zhu et al. 2011), which induces DAT impairment with direct protein-protein interactions (Zhu et al. 2009) involving an allosteric modulation of DAT by the Tat1-86 protein (Zhu et al. 2011). In addition, DA-dependent signaling has been identified as a mechanism of HIV-1 protein neurotoxicity (Aksenova et al. 2006; Silvers et al. 2007; Wallace et al. 2006). DA systems are indeed particularly vulnerable to HIV-1-induced neurotoxic insults that may underlie HAND. Reduced DAT levels in the putamen and ventral striatum (Chang et al., 2008; Wang et al., 2004), reductions in DA levels in the substantia nigra (Kumar et al. 2011), and decreases in homovanillic acid (di Rocco et al. 2000) have been associated with neurocognitive deficits in HIV-1+ individuals.
Collectively, there were three main findings: (1) Direct PD1 CNS injection of the HIV-1 protein variant Tat1-86 induced developmental neurotoxic effects, as witnessed by delays in eye opening, delays in early reflex development and alterations in PPI and between-session habituation of locomotor activity, in the absence of any gross growth deficits. (2) The neurotoxic profile of Tat1-86 appeared more profound in the developing nervous system in vivo relative to that seen with Tat1-72 (Fitting et al., 2008b) as noted on measures of eye opening, righting reflex, and PPI. (3) Neither the direct PD1 CNS injection of the full-length viral HIV-1 protein variant Tat1-86, nor the HIV-1 envelope protein gp120, at doses sufficient to induce neurotoxicity, induced significant expression of the inflammatory cytokine IL-1β or inflammatory factors NFκ-β and Iκ-β. Additionally, it is of interest that Tat1-86 contains integrin binding sequences, whereas Tat1-72 does not, suggesting further investigation of the biological actions of the second exon-encoded polypeptide region of Tat on hippocampal development and plasticity is warranted.
Highlights.
The neurotoxic profile of Tat1-86 appeared more profound relative to that of Tat1-72.
Tat1-86 did not induce significant expression of inflammatory biomarkers.
The second tat exon may uniquely perturb hippocampal development.
Acknowledgments
The authors regretfully acknowledge the untimely passing of Dr. Michael Aksenov, who contributed much to our research program and to the present work. This research was partially supported by grants from the National Institutes of Health: HD043680 (CFM), DA13137 and DA14401 (RMB). The authors would like to thank Carly Leahey for technical assistance she provided while she was a visiting undergraduate student in the Summer Research Institute at the University of South Carolina, funded by the NSF grant 0649249. SF collected and analyzed the behavioral data, SF, CFM and RMB wrote the early drafts of the manuscript, KMW and MA collected and analyzed the inflammatory biomarker data, and LMM and CFM updated and revised the final draft of the manuscript and figures and refined the statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Silvers JM, Mactutus CF, Booze RM. Different effects of selective dopamine uptake inhibitors, GBR 12909 and WIN 35428, on HIV-1 Tat toxicity in rat fetal midbrain neurons. Neurotoxicology. 2008;29:971–977. doi: 10.1016/j.neuro.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Cell culture models of oxidative stress and injury in the central nervous system. Curr Neurovasc Res. 2005;2:73–89. doi: 10.2174/1567202052773463. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Arisi GM. Nervous and immune systems signals and connections: Cytokines in hippocampus physiology and pathology. Epilepsy Behav. 2014 doi: 10.1016/j.yebeh.2014.01.017. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- AVMA. 2000 Report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc. 2001;218:669–696. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Aksenova MV, Mactutus CF, Booze RM. HIV-1 Tat protein variants: Critical role for the cysteine region in synaptodendritic injury. Exp Neurol. 2013;248:228–235. doi: 10.1016/j.expneurol.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Mactutus CF, Aksenova MV, Espensen-Sturges TD, Booze RM. Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens. J Neurochem. 2014;128:140–51. doi: 10.1111/jnc.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Frontiers Neuroendocrin. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Loret EP. What does the structure-function relationship of the HIV-1 Tat protein teach us about developing an AIDS vaccine? Retrovirology. 2009;6:50. doi: 10.1186/1742-4690-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassol E, Misra V, Morgello S, Gabuzda D. Applications and limitations of inflammatory biomarkers for studies on neurocognitive impairment in HIV infection. J Neuroimmune Pharmacol. 2013;8:1087–1097. doi: 10.1007/s11481-013-9512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani MV, Corasaniti MT, Ranalli M, Amantea D, Litovchick A, Lapidot A, Melino G. The Tat antagonist neomycin B hexa-arginine conjugate inhibits gp-120-induced death of human neuroblastoma cells. J Neurochem. 2003;84:1237–1245. doi: 10.1046/j.1471-4159.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Zakharova I, Shippenberg TS. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: quantitative microdialysis in rats under transient conditions. J Neurosci. 2003;23:3076–3084. doi: 10.1523/JNEUROSCI.23-07-03076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, Magnuson DS. Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuroscience. 1998;82:97–106. doi: 10.1016/s0306-4522(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Maccarrone M, Nistico R, Malorni W, Rotiroti D, Bagetta G. Exploitation of the HIV-1 coat glycoprotein, gp120, in neurodegenerative studies in vivo. J Neurochem. 2001a;79:1–8. doi: 10.1046/j.1471-4159.2001.00537.x. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Nistico R, Costa A, Rotiroti D, Bagetta G. The HIV-1 envelope protein, gp120, causes neuronal apoptosis in the neocortex of the adult rat: a useful experimental model to study NeuroAIDS. Funct Neurol. 2001b;16:31–38. [PubMed] [Google Scholar]
- Corasaniti MT, Piccirilli S, Paoletti A, Nistico R, Stringaro A, Malorni W, Finazzi-Agro A, Bagetta G. Evidence that the HIV-1 coat protein gp120 causes neuronal apoptosis in the neocortex of rat via a mechanism involving CXCR4 chemokine receptor. Neurosci Lett. 2001c;312:67–70. doi: 10.1016/s0304-3940(01)02191-7. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Ellis R, Cherner M, Grant I, Masliah E. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80:1415–1423. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D. Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol. 2000;23:190–194. doi: 10.1097/00002826-200007000-00004. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Knapp PE, Hauser KF. CCL5/RANTES gene deletion attenuates opioid-induced increases in glial CCL2/MCP-1 immunoreactivity and activation in HIV-1 Tat-exposed mice. J Neuroimmune Pharmacol. 2008;3:275–285. doi: 10.1007/s11481-008-9127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Ensoli F, Fiorelli V, DeCristofaro M, Santini Muratori D, Novi A, Vannelli B, Thiele CJ, Luzi G, Aiuti F. Inflammatory cytokines and HIV-1-associated neurodegeneration: oncostatin-M produced by mononuclear cells from HIV-1-infected individuals induces apoptosis of primary neurons. J Immunol. 1999;162:6268–6277. [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E HNRC Group. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. Brain Pathology. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. The human immunodeficiency virus-1-associated protein, Tat(1–86), impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: A no-net-flux microdialysis study. Neuroscience. 2009;159:1292–1299. doi: 10.1016/j.neuroscience.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Differential long-term neurotoxicity of HIV-1 proteins in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2008a;18:135–147. doi: 10.1002/hipo.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Dose-dependent long-term effects of Tat in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2010a;20:469–480. doi: 10.1002/hipo.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006a;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006b;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal gp120 injection: an examination early in development. Neurotoxicology. 2007;28:101–107. doi: 10.1016/j.neuro.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal injection of the HIV-1 proteins gp120 and Tat: differential effects on behavior and the relationship to stereological hippocampal measures. Brain Res. 2008b;1232:139–154. doi: 10.1016/j.brainres.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010b;177(3):1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SB, Lu M, Glaze DG, Reuben JM, Harris LL, Cohen EN, et al. Associations of cytokines, sleep patterns, and neurocognitive function in youth with HIV infection. Clin Immunol. 2012;144:13–23. doi: 10.1016/j.clim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Biancalana S, Hudson D. Activity of synthetic peptides from the Tat protein of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:7397–7401. doi: 10.1073/pnas.86.19.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S, Liu Y. Insights into the role of immune activation in HIV neuropathogenesis. J Neurovol. 2002;8:69–75. doi: 10.1080/13550280290049525. [DOI] [PubMed] [Google Scholar]
- Gary DS, Milhavet O, Camandola S, Mattson MP. Essential role for integrin linked kinase in Akt-mediated integrin survival signaling in hippocampal neurons. J Neurochem. 2003;84:878–890. doi: 10.1046/j.1471-4159.2003.01579.x. [DOI] [PubMed] [Google Scholar]
- Gavin P, Yogev R. Central nervous system abnormalities in pediatric human immunodeficiency virus infection. Pediatr Neurosurg. 1999;31:115–123. doi: 10.1159/000028845. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 2010;5:92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Gendelman HE, Poluektova LY. Can humanized mice reflect the complex pathobiology of HIV-associated neurocognitive disorders? J Neuroimmune Pharmacol. 2012;7:352–362. doi: 10.1007/s11481-011-9335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi S, Dieni C, Frondaroli A, Pettorossi VE. Influence of visual experience on developmental shift from long-term depression to long-term potentiation in the rat medial vestibular nuclei. J Physiol. 2004;560:767–777. doi: 10.1113/jphysiol.2004.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Gupta S, Shah DM, Shah I. Neurological disorders in HIV-infected children in India. Ann Trop Paediatr. 2009;29:177–181. doi: 10.1179/027249309X12467994693734. [DOI] [PubMed] [Google Scholar]
- Heinen K, Bosman LW, Spijker S, van Pelt J, Smit AB, Voorn P, Baker RE, Brussaard AB. GABAA receptor maturation in relation to eye opening in the rat visual cortex. Neuroscience. 2004;124:161–171. doi: 10.1016/j.neuroscience.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samini A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice overexpressing interleukin 6 in the brain. Proc Natl Acad Sci USA. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Covin AC, Juncos JL, Justice JB., Jr Individual differences in basal and cocaine-stimulated extracellular dopamine in the nucleus accumbens using quantitative microdialysis. Brain Res. 1992;587:306–312. doi: 10.1016/0006-8993(92)91012-4. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Juncos JL, Justice JB, Jr, Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. J Neurosci. 1994;14:6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang KT. Human Retroviruses and AIDS: A Compilation and Analysis of Nucleic Acid and Amino Acid Sequences. Los Alamos National Laboratory; Los Alamos, NM: 1996. pp. III-3–III-18. [Google Scholar]
- Jeang KT, Xial H, Rich EA. Multifaceted activities of the HIV-1 transactivator of transcription. Tat J Biol Chem. 1999;274:28837–28840. doi: 10.1074/jbc.274.41.28837. [DOI] [PubMed] [Google Scholar]
- Jeremy RJ, Kim S, Nozyce M, Nachman S, McIntosh K, Pelton SI, et al. 377 Study Teams. Neuropsychological functioning and viral load in stable antiretroviral therapy-experienced HIV-infected children. Pediatrics. 2005;115:380–387. doi: 10.1542/peds.2004-1108. [DOI] [PubMed] [Google Scholar]
- Jin CZ, Zhao Y, Zhang FJ, Yao HP, Wu LJ, Zhao HX, Wei HS, Wu NP. Different plasma levels of interleukins and chemokines: comparison between children and adults with AIDS in China. Chin Med J (Engl) 2009;122:530–535. [PubMed] [Google Scholar]
- Jones MV, Bell JE, Nath A. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. AIDS. 2000;14:2709–2713. doi: 10.1097/00002030-200012010-00010. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Digicaylioglu M, Russo R, Kaul M, Achim CL, Fletcher L, Masliah E, Lipton SA. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Ann Neurol. 2010;68:342–352. doi: 10.1002/ana.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Keller MA, Venkatraman TN, Thomas A, Deveikis A, LoPresti C, Hayes J, et al. Altered neurometabolite development in HIV-infected children: correlation with neuropsychological tests. Neurology. 2004;62:1810–1817. doi: 10.1212/01.wnl.0000125492.57419.25. [DOI] [PubMed] [Google Scholar]
- Ketlinskii SA, Kalinina NM, Tsvetkova SN, Rakhmanova AG, Maslov VN, Khaldeeva NA. Tumor necrosis factor-alpha and interleukin-1beta in the blood plasma of patients with HIV infection. Vestn Ross Akad Med Nauk. 1992:36–41. [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Le Doare K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130:1326–1344. doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Brenneman DE, Silverstein FS, Masliah E, Mucke L. gp120 and neurotoxicity in vivo. Trends Pharmacol Sci. 1995;16:122. doi: 10.1016/s0165-6147(00)88998-1. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Midde NM, Huang X, Gomez AM, Booze RM, Zhan CG, Zhu J. Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions. J Neuroimmune Pharmacol. 2013;8:975–987. doi: 10.1007/s11481-013-9464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz M. Clinical comparison of adult and pediatric NeuroAIDS. Adv Neuroimmunol. 1994;4:207–221. doi: 10.1016/s0960-5428(06)80259-7. [DOI] [PubMed] [Google Scholar]
- Miura Y, Misawa N, Kawano Y, Okada H, Inagaki Y, Yamamoto N, et al. Tumor necrosis factor-related apoptosis-inducing ligand induces neuronal death in a murine model of HIV central nervous system infection. Proc Natl Acad Sci U S A. 2003;100(5):2777–2782. doi: 10.1073/pnas.2628048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Motz BA, Alberts JR. The validity and utility of geotaxis in young rodents. Neurotoxicol Teratol. 2005;27:529–533. doi: 10.1016/j.ntt.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Murase S, Owens DF, McKay RD. In the newborn hippocampus, neurotrophin-dependent survival requires spontaneous activity and integrin signaling. J Neurosci. 2011;31:7791–7800. doi: 10.1523/JNEUROSCI.0202-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Paramesparan Y, Garvey LJ, Ashby J, Foster CJ, Fidler S, Winston A. High rates of asymptomatic neurocognitive impairment in vertically acquired HIV-1-infected adolescents surviving to adulthood. J Acquir Immune Defic Syndr. 2010;55:134–136. doi: 10.1097/QAI.0b013e3181d90e8c. [DOI] [PubMed] [Google Scholar]
- Parisi T, Ison JR. Development of the acoustic startle response in the rat: ontogenetic changes in the magnitude of inhibition by prepulse stimulation. Dev Psychobiol. 1979;12:219–230. doi: 10.1002/dev.420120305. [DOI] [PubMed] [Google Scholar]
- Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Dyke RB, Seage GR, 3rd Pediatric AIDS Clinical Trials Group 219/219C Study Team. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis. 2008;46:1751–1760. doi: 10.1086/587900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Ming X, Williams PL, Robertson KR, Oleske JM, Seage GR., 3rd Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009;23:1893–1901. doi: 10.1097/QAD.0b013e32832dc041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrella O, Carrieri PB, Guarnaccia D, Soscia M. Cerebrospinal fluid cytokines in AIDS dementia complex. J Neurol. 1992;239:387–388. doi: 10.1007/BF00812156. [DOI] [PubMed] [Google Scholar]
- Philippon V, Vellutini C, Gambarelli D, Harkiss G, Arbuthnott G, Metzger D, Roubin R, Filippi P. The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: involvement of cytokines. Virology. 1994;205:519–529. doi: 10.1006/viro.1994.1673. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Sultana R, Mohmmad-Abdul H, Nath A, Butterfield DA. HIV-dementia, Tat-induced oxidative stress, and antioxidant therapeutic considerations. Brain Res Brain Res Rev. 2005;50:14–26. doi: 10.1016/j.brainresrev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Romani B, Engelbrecht S, Glashoff RH. Functions of Tat: the versatile protein of human immunodeficiency virus type 1. J Gen Virol. 2010;91:1–12. doi: 10.1099/vir.0.016303-0. [DOI] [PubMed] [Google Scholar]
- Roscoe RF, Mactutus CF, Booze RM. HIV-1 transgenic female rat: Synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol. 2014 doi: 10.1007/s11481-014-9555-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol. 2004;107:97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 tat protein: Involvement of D1 dopamine receptor. Neurotoxicology. 2007;28:1184–1190. doi: 10.1016/j.neuro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Pentlicky S, Klase Z, Singh M, Neuveut C, Lu CY, Reitz MS, Jr, Yarchoan R, Marx PA, Jeang KT. An in vivo replication-important function in the second coding exon of Tat is constrained against mutation despite cytotoxic T lymphocyte selection. J Biol Chem. 2003;278:44816–44825. doi: 10.1074/jbc.M307546200. [DOI] [PubMed] [Google Scholar]
- Suksomboon N, Poolsup N, Ket-Aim S. Systematic review of the efficacy of antiretroviral therapies for reducing the risk of mother-to-child transmission of HIV infection. J Clin Pharm Ther. 2007;32:293–311. doi: 10.1111/j.1365-2710.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–93. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tohmi M, Tsuda N, Watanabe Y, Kakita A, Nawa H. Perinatal inflammatory cytokine challenge results in distinct neurobehavioral alterations in rats: implication in psychiatric disorders of developmental origin. Neurosci Res. 2004;50:67–75. doi: 10.1016/j.neures.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Torre D, Ferrario G, Speranza F, Martegani R, Zeroli C. Increased levels of nitrite in the sera of children infected with human immunodeficiency virus type 1. Clin Infect Dis. 1996;22:650–653. doi: 10.1093/clinids/22.4.650. [DOI] [PubMed] [Google Scholar]
- UNAIDS. [Last accessed May 30 2014];2013 UNAIDS Report on the global AIDS epidemic. 2013 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- Vallat AV, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, et al. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- Volmink J, Siegfried NL, van der Merwe L, Brocklehurst P. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2007:CD003510. doi: 10.1002/14651858.CD003510.pub2. [DOI] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120-and tat(1–72)-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Walton KD, Harding S, Anschel D, Harris YT, Llinas R. The effects of microgravity on the development of surface righting in rats. J Physiol. 2005;565:593–608. doi: 10.1113/jphysiol.2004.074385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Watson K, Edwards RJ. HIV-1 trans-Activating (Tat) Protein. Biochem Pharmacol. 1999;58:1521–1528. doi: 10.1016/s0006-2952(99)00209-9. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, Dailey P, Achim CL. Distribution of brain HIV load in AIDS. Brain Pathol. 1998;8:277–284. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willen EJ. Neurocognitive outcomes in pediatric HIV. Ment Retard Dev Disabil Res Rev. 2006;12:223–228. doi: 10.1002/mrdd.20112. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. 2. McGraw-Hill; New York: 1971. [Google Scholar]
- Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4:430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. Recombinant human immunodeficiency virus-1 transactivator of transcription(1–86) allosterically modulates dopamine transporter activity. Synapse. 2011;65:1251–1254. doi: 10.1002/syn.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Bruntz RC, Stairs DJ, Dwoskin LP. Individual differences in response to novelty predict prefrontal cortex dopamine transporter function and cell surface expression. Eur J Neurosci. 2007;26:717–728. doi: 10.1111/j.1460-9568.2007.05690.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat Protein-induced rapid and reversible decrease in [H-3]dopamine uptake: dissociation of [H-3]dopamine uptake and [H-3]2 beta-carbomethoxy-3-beta -(4-fluorophenyl) tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]