Abstract
Despite increasing numbers of women availing themselves of assisted reproductive technology (ART), effects on cancer risk remain unresolved. Given hormonal exposures, breast cancer risk is of particular concern. The aim of this study is to investigate breast cancer risk amongst women giving birth following ART as compared to that amongst women who gave birth without ART.
Data on all women who gave birth in Norway with or without ART, between 1984 and 2010 was obtained from the Medical Birth Registry of Norway (MBRN). 808 834 women eligible for study were linked to the Cancer Registry of Norway. Cox proportional hazards model computed relative risk of breast cancer between the two groups, adjusting for age, parity, age at first birth, calendar period and region of residence.
A total of 8037 women were diagnosed with breast cancer during the study period, 138 ART women and 7899 unexposed. Total follow-up time was 12 401 121 person-years (median 16.0), median age at entry was 32.5 years (range18.6-49.9) for ART women and 26.3 (range 10.5-54.6) for women without ART.
Women exposed to ART had an elevated risk of breast cancer (adjusted HR 1.20, 95% CI 1.01-1.42). Subgroup analyses resulted in an HR of 1.30 (95% CI 1.07-1.57) for women treated with IVF and 1.35 (95 % CI 1.07-1.71) for women with follow-up >10 years, compared with controls.
Our findings of increased risk in the study population, warrant continued monitoring of women treated with ART as this population advances into more typical cancer age ranges.
Introduction
The 1980s and early 1990s experienced a significant increase in the demand and availability of assisted reproductive technology (ART). (1) Presently 2-5 % of children in Europe are born with the aid of ART.(2) Considering the cumulative success rates of ART of about 50-60%, an even larger percentage of infertile women are being exposed to ART.(3) In the early 1990s, studies were published showing an increased risk of ovarian cancer in women treated for infertility.(4-6) Much research has subsequently been conducted to assess the relationship between infertility, fertility treatment and cancer, including several cancer forms.(7-9) The results are inconsistent, and the question is whether a potential increase in risk is due to the hormone treatment in ART or to the infertility itself.
Breast cancer is of particular concern because established aetiological factors in its development include those of both hormonal and reproductive origin. First, reproductive factors such as nulliparity and older age at first birth are known to increase the risk of breast cancer, both of which apply more frequently to infertile patients. Second, duration of exposure to endogenous and exogenous hormones (menopausal hormone therapy and oral contraceptive use) is also a risk factor for developing breast cancer.(10, 11) A third issue of concern is that the risk of both breast cancer and infertility increases with age.(12, 13)
Although many studies have investigated the risk of breast cancer amongst infertile women, few have addressed the effects of hormones specifically used in ART (mainly gonadotropins and gonadotropin releasing hormone (GnRH) analogues). Most of these studies do not demonstrate increased breast cancer risk for infertile women after fertility treatment,(14-22) although some do demonstrate significant increases in risk, this is limited to subgroups of patients.(19, 20, 23-25) One large study has demonstrated a decreased risk of breast cancer in women exposed to ART,(26) which also was suggested in a recent meta-analysis.(7) A number of investigators have been burdened with methodological difficulties such as short follow-up periods, few cancer cases in each comparison group or inability to adjust for confounding factors important in the aetiology of breast cancer.(27)
In Norway there has been increasing use of ART since it became available as treatment for infertile women in 1983, and currently 3 % of Norwegian children are born with the aid of ART. The study aimed to determine the risk of breast cancer amongst women giving birth following ART (in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI)) compared to that of women giving birth without ART, using the entire population of Norwegian women who gave birth over a 27-year period.
Materials and Methods
The study population
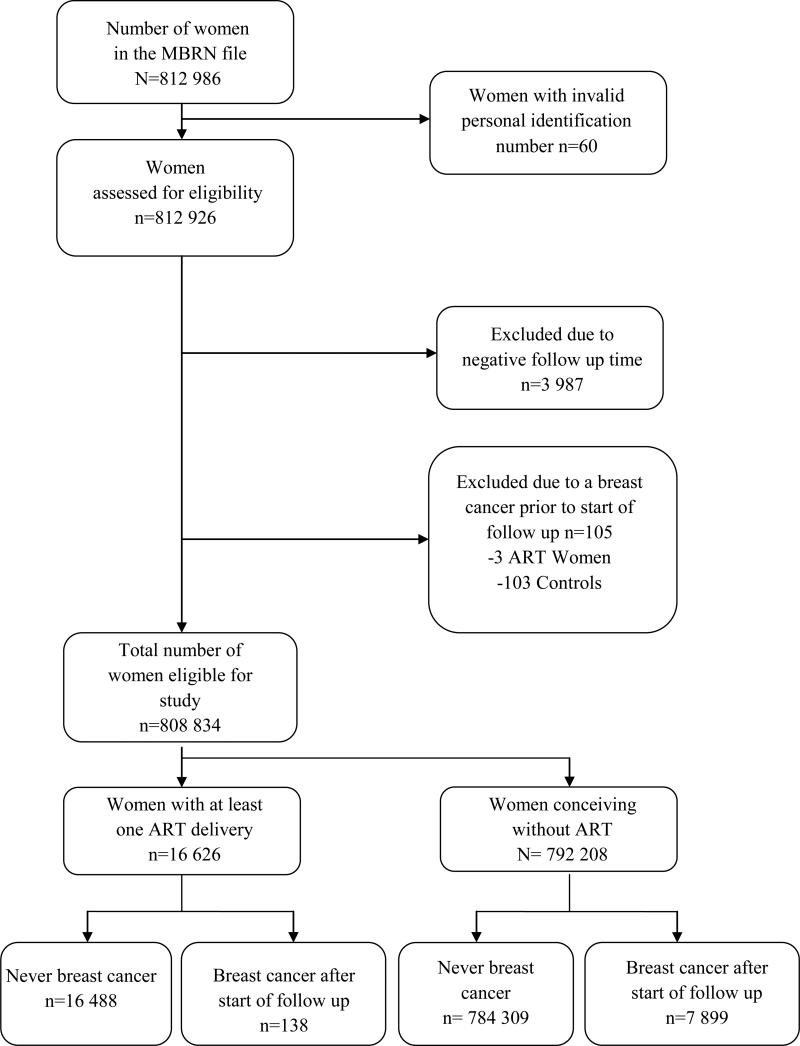
All women who were registered in the Medical Birth Registry of Norway (MBRN) as having given birth to a child (>22 weeks gestation) in Norway between 1 January, 1984 and 31 December, 2010 constituted the study cohort (n= 812 986). Of these, 4047 subjects were not eligible for follow up due to invalid personal identification numbers or negative follow up time. Women who were diagnosed with breast cancer before start of follow up were excluded from analysis (n= 105) (Figure 1). The final study population consequently comprised 808 834 subjects.
Figure 1. Number of women included in the original cohort; of which 4092 were excluded from analysis.
ART women are those who had a child following assisted reproductive technology (ART) and controls are those who had a child following natural concetption only. Those with negative follow up time due to a registered delivery after emigration were excluded from analysis, as well as those with breast cancer prior to start of follow up.
Data sources and items
The data from the MBRN were linked to the Cancer Registry of Norway (CRN) for cancer data, using each woman's unique personal identification number. These two registries cover the total Norwegian population and completeness and validity is reported to be high, both in the CRN (28) and MBRN.(29, 30) The reporting of neoplasms and certain precancerous lesions has been compulsory by law since the CRN systematically started to collect notifications on cancer in 1953. Similarly, the reporting of all pregnancies and deliveries from gestational week 12 has been compulsory since the establishment of MBRN in 1967. Reporting data on ART pregnancies to MBRN was started in 1984, and became compulsory by law in 1988.
For each child born, a record with the following variables was extracted from the MBRN database: date of birth of mother and child, parity, present region of residence, exposure to ART, the specific method of ART (IVF, ICSI, a combination of the two or any other kind of treatment). Other treatments include frozen embryo transfer or ART received abroad, but does not include artificial insemination by husband or donor. Data on smoking was available, but not included in analysis as 68 % of the records had missing values.
The cancer diagnoses were categorised according to The International Classification of Diseases version 10, (ICD-10), and all information on cancer history (C00-96) was extracted from the CRN. At the time of data linkage (January 2013), the latest update of the CRN was 31 December, 2010.
Ascertainment of exposure
Assisted reproductive technology (ART) denotes “all procedures that include the in vitro handling of both human oocytes and sperm, or embryos, for the purpose of establishing a pregnancy”.(31) Women who had at least one pregnancy initiated by ART were classified as “ART women”, and women who had no registered ART pregnancies were classified as controls. Women who had one or several deliveries before the first ART pregnancy, were enrolled in the study at the time of the first ART pregnancy. Women who gave birth without ART after a prior ART delivery remained in the ART group. In this study we did not have information about the type of ART medication given, only that some women received IVF, others received ICSI, some received other forms of treatment (previously mentioned) and finally some had missing information about type of ART used. Although FSH (recombinant-FSH) became the preferred gonadotropin preparation for obtaining controlled ovarian hyper-stimulation during the mid-nineties (32, 33), during the first years of ART in Norway, the hormone used to obtain controlled ovarian stimulation was hMG and to a lesser extent clomiphene citrate.
Identification of cases
All women with at least one case of breast cancer (ICD10, C50) in the period 1 January, 1952 and 31 December, 2010 were identified through linkage with the CRN. For women who were diagnosed with breast cancer more than once, only the first case was counted. In calculations of cancer risk before inclusion to the study, only the first cancer was counted (ICD 10, C00-C96).
Follow up
In order to identify the number of cancers occurring after exposure to ART, the start of follow-up was set at the estimated time of fertility treatment, i.e. the start of the ART derived pregnancy. The date was calculated by subtracting the gestational length (in days) of the first pregnancy from ART during the observational period. To ensure suitable comparability between ART women and controls, start of follow up was set in the same manner for the latter. Gestational length was missing for 5% of the study subjects. In these women, 282 days (the mean length of a pregnancy), (34) was used as gestational length, and start of follow up was calculated accordingly. Consequently, the observational period spans from 8 April, 1983 through 31 December, 2010. Subjects were followed until the date of their first cancer diagnosis, the date of death or emigration, or to 31 January, 2010, whichever occurred first. Date of death or emigration was obtained from the central person's registry (www.ssb.no).
To evaluate the risk of cancer for ART women and controls before inclusion to the study, a separate analysis was performed. Here each woman's date of birth was used as the start of follow up, and the end of follow up was set at the first ART pregnancy or pregnancy in the period 1 January, 1984 to 31 December, 2010 for ART women and controls respectively.
Statistical analyses
Descriptive statistics are presented as median/interquartile range (IQR), and as frequency/percentage wherever appropriate. Ranges of values are also given where this is of interest.
Cox proportional hazards models were used to compare risk of breast cancer amongst ART women compared to controls. We examined the assumption of proportional hazards for each covariate in two ways. Firstly, using Schoenfeld residuals to test the null hypothesis of proportionality and secondly, plotting the cumulative hazard functions for each category.(35) To adjust for the age difference between ART women and controls (attained age) , we used the age of study subjects as the timescale in the Cox model.(36) In addition, adjustment was made for age at start of follow up (categorised: <30 yrs, 30-34 yrs and ≥35 yrs), calendar year at follow up (categorised as 1983-1992, 1993-2002, 2003-2010), and region of residence on 31 December, 2010 (South East, South West, West, Middle, North and the capital Oslo separately). Parity was included in the model as a time dependent covariate, and categorised into one, two, three or four and more children. Those who ended follow up between start of follow up and the delivery of their first child were classified as parity one.
Stratified analyses were performed by age groups (<30 yrs, 30-39 yrs, 40-49 yrs and ≥50 yrs), by calendar period (1983-1992, 1993-2002, 2003-2010), parity at inclusion (parous / nulliparous) parity at end of follow up (one, two, three or more), method of ART (1: IVF, 2: ICSI or 3:a combination of IVF/ICSI, other or unknown), and time from inclusion to diagnosis (<=1yr, >1-5yrs, >5-10yrs, >10yrs).
Two sensitivity analyses were performed: The first analysis excluded women with breast cancer within the first year of start of follow up. This was to allow for a minimum of time from exposure to failure and to remove the possibility that a pre-existing cancer was diagnosed after inclusion. The second analysis excluded women with any cancer before start of follow up.
Childhood cancer survivors are less likely to become parents, (37, 38) and have been shown to have a higher risk of being treated with ART.(38) In analyses of cancer risk before inclusion, cox regression model was used to compare risk of cancer (at all sites) prior to follow up in ART women compared to controls. Age was also here used as the timescale; adjustment was made for calendar period (10-year categories from 1960 to the calendar year at first birth) and parity at end (yes/no).
The distribution by calendar period at inclusion was unbalanced; more than 50% of ART women were included in the last ten-year period, and almost 50% of controls were included in the first ten-year period. To explore if this was a major selection bias of our study, we drew a sub cohort of controls who were matched by period of inclusion with the ART women, so that the distribution of controls in each category (1983-1992, 1993-2002, and 2003-2010) was equal to the distribution seen in the ART women. As many matched controls as possible were drawn. This gave a study population of 365 923, and the analyses were performed as for the total cohort.
Significance levels were set to p<0.05. Analyses were conducted using the software package STATA.(39)
Ethical Approval
The study was approved by the Ethical committee for the South Eastern Health region of Norway.
Results
Of the total study population of 808 834 women, 16 626 gave birth to a child following ART. A total of 141 ART women were registered with a breast cancer, out of which 138 (97.9%) cancers were diagnosed after study entry. Amongst the controls (n= 792 208), of the total of 8001 breast cancers, 7899 (98.7%) were diagnosed after study entry. A total of 2427 women had a cancer at any site (other than breast cancer) before start of follow up, of which 116 were ART women and 2311 were controls (data not shown). Of the 116 ART women who had cancer before start of follow up, the most prevalent sites were melanoma of the skin (C43 n=28), thyroid cancer (C73 n=16) and cervical cancer (C53 n=13). Amongst the 2311 controls with previous cancer, the most prevalent sites were melanoma of the skin (C43 n=576), thyroid cancer (C73 n=239) and central nervous system (C71 n=199) (counting first cancers only)(data not shown). Tables 1 and 2 show the characteristics of the study population. Compared with the controls, the ART women were older at entry, had shorter follow up times (as a larger proportion were recruited from a later time period), and were slightly younger at their first cancer diagnosis.
Table 1.
Characteristics of the study population (n = 808 834)
| ART women, n= 16 626 | Controls, n= 792 208 | Total Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Breast Cancer | All | Breast Cancer | All | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Age at entry | ||||||||||
| < 25 | 495 | 3 | 1 | 1 | 316 408 | 40 | 1 445 | 18 | 316 903 | 39 |
| 25-29 | 4 022 | 24 | 22 | 16 | 282 199 | 36 | 2 843 | 36 | 286 221 | 35 |
| 30-34 | 7 550 | 45 | 59 | 43 | 141 139 | 18 | 2 304 | 29 | 148 689 | 18 |
| 35-39 | 4 208 | 25 | 54 | 38 | 45 146 | 6 | 1 092 | 14 | 49 354 | 6 |
| 40-44 | 341 | 2 | 2 | 1 | 7 049 | 1 | 208 | 3 | 7 390 | 1 |
| ≥45 | 10 | 0 | 0 | 0 | 267 | 0 | 7 | 0 | 277 | 0 |
| Total | 16 626 | 100 | 138 | 100 | 792 208 | 100 | 7 899 | 100 | 808 834 | 100 |
| Parity at entry | ||||||||||
| P0 | 12 538 | 75 | 96 | 70 | 630 967 | 80 | 4 570 | 58 | 643 505 | 80 |
| P1 | 3 333 | 20 | 35 | 25 | 95 211 | 12 | 1 912 | 24 | 98 544 | 12 |
| P2+ | 755 | 5 | 7 | 5 | 66 030 | 8 | 1 417 | 18 | 66 785 | 8 |
| Total | 16 626 | 100 | 138 | 100 | 792 208 | 100 | 7 899 | 100 | 808 834 | 100 |
| Parity at exit* | ||||||||||
| 1 | 7 612 | 46 | 59 | 43 | 178 980 | 23 | 1 240 | 16 | 186 592 | 23 |
| 2 | 6 970 | 42 | 62 | 45 | 343 918 | 43 | 3 549 | 45 | 350 888 | 43 |
| 3 | 1 696 | 10 | 13 | 9 | 197 833 | 25 | 2 315 | 29 | 199 529 | 25 |
| ≥4 | 348 | 2 | 4 | 3 | 71 477 | 9 | 795 | 10 | 71 825 | 9 |
| Total | 16 626 | 100 | 138 | 100 | 792 208 | 100 | 7 899 | 100 | 808 834 | 100 |
| Calendar year at entry | ||||||||||
| 1983-1992 | 1 383 | 9 | 40 | 29 | 363 350 | 46 | 6 471 | 82 | 364 773 | 45 |
| 1993-2002 | 6 233 | 38 | 89 | 64 | 244 461 | 31 | 1 316 | 17 | 250 783 | 31 |
| 2003-2010 | 8 872 | 53 | 9 | 7 | 184 397 | 23 | 112 | 1 | 193 278 | 24 |
| Total | 16 626 | 100 | 138 | 100 | 792 208 | 100 | 7 899 | 100 | 808 834 | 100 |
| Method of ART | ||||||||||
| IVF | 10 112 | 61 | 111 | 80 | - | - | - | - | 10 112 | 61 |
| ICSI | 4 968 | 30 | 11 | 8 | - | - | - | - | 4 968 | 30 |
| Combination/Missing/Unknown | 1 546 | 9 | 16 | 12 | - | - | - | - | 1 546 | 9 |
| Total | 16 626 | 100 | 138 | 100 | - | - | - | - | 16 626 | 100 |
| Age at first diagnosis | ||||||||||
| < 30 | - | - | 0 | 0 | - | - | 129 | 2 | - | - |
| 30 - 39 | - | - | 36 | 26 | - | - | 1 694 | 21 | - | - |
| 40 - 49 | - | - | 80 | 58 | - | - | 4 010 | 51 | - | - |
| 50 - 59 | - | - | 21 | 15 | - | - | 1 934 | 24 | - | - |
| ≥ 60 | - | - | 1 | 1 | - | - | 132 | 2 | - | - |
| Total | - | - | 138 | 100 | - | - | 7 899 | 100 | - | - |
| Region of present residence | ||||||||||
| South East | 5 265 | 32 | 40 | 29 | 266 553 | 34 | 2 746 | 35 | 271 818 | 34 |
| Oslo | 2 514 | 15 | 25 | 18 | 123 231 | 16 | 1 229 | 16 | 125 745 | 16 |
| South | 3 018 | 18 | 24 | 17 | 114 651 | 14 | 1 088 | 14 | 117 669 | 15 |
| West | 2 702 | 16 | 20 | 14 | 135 481 | 17 | 1 358 | 17 | 138 183 | 17 |
| Middle | 1 780 | 11 | 16 | 12 | 68 834 | 9 | 634 | 8 | 70 614 | 9 |
| North | 1 344 | 8 | 13 | 9 | 83 086 | 10 | 840 | 11 | 84 430 | 10 |
| Missing or unknown | 3 | 0 | 0 | 0 | 372 | 0 | 4 | 0 | 375 | 0 |
| Total | 16 626 | 100 | 138 | 100 | 792 208 | 100 | 7 899 | 100 | 808 834 | 100 |
Number of births per woman at the end of follow up
Table 2.
Age at inclusion and diaqnosis, and follow up time of ART women (n = 16 626) and controls (n=792 208)
| ART Women | Controls | Total Cohort | ||||
|---|---|---|---|---|---|---|
| Total | Women with breast cancer | Total | Women with breast cancer | Total | Women with breast cancer | |
| Age at start of follow up | ||||||
| Median | 32.5 | 34.0 | 26.3 | 29.4 | 26.4 | 29.5 |
| IQR | 29.7-35.3 | 31.1-36.6 | 22.9-29.9 | 26.1-33.2 | 23.0-30.1 | 26.2-33.4 |
| Range | 18.6-49.9 | 24.7-41.7 | 10.5-54.6 | 16.2-50.9 | 10.5-54.6 | 16.2-50.9 |
| Follow up time | ||||||
| Median | 7.3 | 10.3 | 16.3 | 16.1 | 16.0 | 16.0 |
| IQR | 3.7-12.3 | 6.9-13.9 | 8.1-23.1 | 10.9-20.6 | 7.9-22.9 | 10.8-20.5 |
| Range | 0.4-27.2 | 0.6-21.8 | 0.0-27.9 | 0.1-27.6 | 0.0-27.9 | 0.1-27.6 |
| Age at end of follow up | ||||||
| Median | 40.5 | 43.9 | 42.1 | 45.5 | 42.1 | 45.5 |
| IQR | 36.1-45.5 | 39.9-48.1 | 35.2-49.1 | 40.5-50.2 | 35.2-49.0 | 40.5-50.2 |
| Range | 19.5-65.9 | 32.3-61.3 | 14.5-81.2 | 21.2-68.1 | 14.5-81.2 | 21.2-68.1 |
| Age at diagnosis | ||||||
| Median | - | 43.9 | - | 45.5 | - | 45.5 |
| IQR | - | 39.9-48.1 | - | 40.5-50.2 | - | 40.5-50.2 |
| Range | - | 32.3-61.3 | - | 21.2-68.1 | - | 21.2-68.1 |
| Time to diagnosis | ||||||
| Median | - | 10.6 | - | 16.5 | - | 16.4 |
| IQR | - | 7.1-14.3 | - | 11.2-21.1 | - | 11.0-21.0 |
| Range | - | 0.6-22.3 | - | 0.1-28.3 | - | 0.1-28.3 |
All numeric values in years. IQR = Interquartile range.
The median age at study entry was 32.5 years for ART women (IQR 29.7 – 35.3) and 26.3 years for controls (IQR 22.9-29.9). Median age at end of follow up was 40.5 years for ART women (IQR 36.1-45.5) and 42.1 years for the controls (IQR 35.2-49.1)(table 2). The total follow-up time for the whole cohort was 12 401 121 person-years (median 16.0, IQR 10.8-20.5) (table 2). Of those diagnosed with breast cancer, the average time from inclusion to diagnosis was 10.6 years (range 0.6-22.4) for ART women and 16.5 years (range 0.1-28.3) for controls (table 2).
Amongst ART women, 10 112 had received IVF only, 4968 had received ICSI only, whilst 330 had received either a combination of IVF and ICSI or some other form of treatment. Information on mode of treatment was missing for 1216 records. For region of present residence, 375 (0.05 %) values were missing or unknown; they were classified as a separate category. No other variables had any missing values.
For the variables IVF, region and period the assumption of proportionality was valid (large and insignificant p-values, and parallel cumulative hazard functions). For the variables parity and age at start follow-up the results were ambiguous. We therefore analysed the data both using standard Cox-regressions and Cox-regressions where we allowed parity and age at start follow-up to interact with time. The results were unaltered and we therefore applied the simplest model assuming proportionality throughout.
In analyses of the total cohort, the crude hazard ratio for ART women compared to controls was 1.35 (95 % CI 1.14-1.60). Including adjustment for calendar period, region of present residence, parity and age at start of follow up gave a risk estimate of 1.20 (95% CI 1.01-1.42)(table 3). Adjustment for parity at the end of follow up and age at start of follow up had the most pronounced effect on the crude estimate.
Table 3.
Hazard ratio with 95% confidence intervals (CI), of breast cancer in ART women (n = 16 629) versus controls (n=792 208)
| ART women | Controls | HR | (95 % CI) | |||
|---|---|---|---|---|---|---|
| n | Person years | n | Person years | |||
| Breast cancer after start of follow up (crude)a | 138 | 141 629 | 7899 | 12 259 492 | 1.35 | (1.14-1.60) |
| Breast cancer after start of follow upb | 138 | 141 629 | 7899 | 12 259 492 | 1.20 | (1.01-1.42) |
| Excluding those with cancer first year | 137 | 141 628 | 7868 | 12 259 476 | 1.20 | (1.01-1.43) |
| Excluding those with any cancer before inclusion | 134 | 140 855 | 7852 | 12 228 308 | 1.17 | (0.98-1.40) |
| Periodb | ||||||
| 1883-1992 | 0 | 3809 | 368 | 1 975 710 | NA | |
| 1993-2002 | 27 | 40 972 | 2304 | 4 801 834 | 1.05 | (0.72-1.55) |
| 2003-2010 | 111 | 96 848 | 5227 | 5 481 949 | 1.23 | (1.01-1.49) |
| Parity at inclusionb | ||||||
| P0 | 96 | 107 056 | 4570 | 8 878 894 | 1.12 | (0.91-1.38) |
| P1 + | 42 | 34 573 | 3329 | 3 380 599 | 1.39 | (1.01-1.90) |
| Parity at end of follow upb,c | ||||||
| 1 | 59 | 59 058 | 1 240 | 1 647 599 | 1.01 | (0.78-1.33) |
| 2 | 62 | 63 102 | 3 549 | 5 362 014 | 1.24 | (0.96-1.60) |
| ≥3 | 17 | 19 468 | 3 110 | 5 249 879 | 1.52 | (0.94-2.46) |
| Age at follow up, yearsb | ||||||
| <40 | 36 | 87 683 | 1 850 | 8 296 713 | 1.17 | (0.84-1.64) |
| 40-50 | 80 | 47 659 | 3 983 | 3 122 918 | 1.12 | (0.89-1.40) |
| =>50 | 22 | 6 286 | 2 066 | 839 862 | 1.20 | (0.78-1.84) |
| Mode of fertility treatmentb | ||||||
| IVF | 111 | 97 368 | 7899 | 12 259 492 | 1.30 | (1.07-1.57) |
| ICSI | 11 | 28 962 | 7899 | 12 259 492 | 0.70 | (0.39-1.27) |
| IVF/ICSI in combination/other/unknown | 16 | 15 299 | 7899 | 12 259 492 | 1.13 | (0.69-1.84) |
| Duration of follow-up, yearsb | ||||||
| < 1 | <5 | 16 582 | 32 | 790 615 | 0.53 | (0.07-4.00) |
| 1 - 5 | 13 | 53 751 | 485 | 2 904 719 | 0.61 | (0.35-1.06) |
| 5 -10 | 50 | 41 079 | 1 197 | 3 046 830 | 1.33 | (0.99-1.78) |
| > 10 | 74 | 30 216 | 6 185 | 5 517 329 | 1.35 | (1.07-1.71) |
In the crude estimate, no adjustments for confounding have been made.
Adjusted HR: adjusted for attained age, calendar period of follow up, region of residence, parity and age at start of follow up. Each covariate is omitted from the model when it is used for stratification.
In multiple gestations, each birth is counted once.
Stratified analyses by ART method showed that women subjected to only IVF had a significantly increased risk of breast cancer, HR 1.30 (95% CI 1.07-1.57), compared to controls, whereas no significant difference was found for women who had received ICSI or other methods (table 3). Women subjected to ICSI had shorter follow up time (median 5.0) and were younger at the end of follow up (median 38.0 years); compared with IVF women (median follow up time 8.8 years and median age at end of follow up 41.9) (data not shown).
Amongst those with follow up time exceeding five and ten years, ART women had significantly increased risk of breast cancer compared to controls, HR 1.35 (95% CI 1.01-1.80) and HR 1.34 (95% CI 1.07-1.71), respectively. Restricting these analyses to those only treated with IVF produced estimates for those with >5 years follow up of 1.54 (95% CI 1.12-2.14) and for more than 10 years 1.41 (95% CI 1.09-1.81) (data not shown). Stratification by parity, period and age group are demonstrated in table 3.
The sensitivity analyses excluding women with breast cancer during the first year of follow up (1 ART woman and 31 controls) computed an HR of 1.20 (95% CI 1.01-1.43), whereas analyses restricted to patients without previous cancer gave an HR of 1.17 (95% CI 0.98-1.40) (table 3). Sensitivity analyses restricted to patients without previous cancer for those followed for ten years or more and who were exposed only to IVF, gave similar risk estimates; HR 1.29 (95% CI 1.01-1.63) and HR 1.26 (95% CI 1.04-1.53) respectively(data not shown).
The adjusted HR of any cancer before inclusion to the study for ART women compared to controls was 1.15 (95 % CI 0.96-1.39)(data not shown).
The analysis performed on the sub cohort (n=366 538) resulted in an adjusted HR of 1.28, 95% CI 1.08-1.62) in ART women compared to controls. Results in the stratified analyses were similar, for those treated with IVF treatment specifically the HR was 1.38 (95% CI 1.13-1.69) and for those with follow up of more than 10 years HR was 1.42 (95% CI 1.10-1.83)(data not shown).
Discussion
The results indicate an elevated risk of breast cancer amongst women giving birth following ART compared to that in women giving birth without ART. Stratified analyses showed a significantly increased risk for women who were followed for longer than ten years and those specifically subjected to IVF. The results did not change appreciably when the analysis excluded previous cancer diagnoses or cancers that were diagnosed within one year following fertility treatments.
The study includes all women who gave birth in Norway through 27 years. The legislation of reporting to the national health registries (MBRN and CRN) is important for sustaining high data quality, and the completeness and validity of both the CRN (28) and MBRN (29, 40) are reported to be high. The 11-digit personal identification number prevents potential duplication of registered cases, and concurrently provides a unique possibility for merging data as well as obtaining complete follow up with information on essential events like births and cancer occurrences for the total Norwegian female population. The registry based design ensured that the data on exposure is unaffected by the outcome variable, i.e. no recall bias, and allows for adjustment of important confounders like age and parity.
The Norwegian health care system has since the advent of ART provided fully state financed fertility treatment with IVF/ICSI for three cycles for all women, regardless of social class or area of residence. We believe that this makes our study population heterogenous (in that no specific socioeconomic groups are represented in the ART group) and that the results are externally valid. Dos Santos Silva and colleagues took into account socioeconomic status, but this did not alter the estimates.(41) On the other hand, Yli Kuha found that adjustment for socioeconomic status did attenuate risks.(16)
A limitation of this study is that we lacked information on some important aetiological factors in the development of breast cancer, such as family history of cancer, age at menarche and breastfeeding history. Obesity is another factor suggested to influence the risk of breast cancer, and it is also a known risk factor for infertility. Unfortunately, information on body weight and height was not available for the study population. Furthermore, we lacked information on prior exposure to other fertility hormones (such as clomiphene citrate) in ART women. An unknown number of women in the MBRN receive ART in countries other than Norway, (42) and fertility treatment of these women is not systematically registered in the MBRN. This might bias the result, underestimating the risks for ART women. Furthermore, a very young population is examined in this study, (mean age at end of follow up was 45 years) below the average age of first diagnosis of breast cancer, which also may limit statistical power. Our study did not include data on infertility diagnoses, and it has been shown that different infertility diagnoses possess inherent differences with regard to risk of cancer development.(15, 43) Neither did we have information on use of exogenous hormones and screening history; important, considering that mammography in Norway was introduced in a stepwise manner by different health counties, (44) resulting in a possible influence on regional cancer rates through the last three decades. However, most women in the study population were below the age of 50 years and thus have not yet been included in the national screening programme.
To our knowledge only Swedish researchers have used a whole population of parous women to examine breast cancer risk in women treated for infertility problems. Kristiansson et al, demonstrated a non-significant decreased risk of breast cancer following IVF treatment leading to a pregnancy (21) and four years later Källén demonstrated a decreased risk in cancer for parous women treated with IVF.(26) Our study was not in agreement with the suggestions of a preventive effect of ART treatment on breast cancer risk.
The present study focuses on the effects of fertility drugs specific to ART, important because these are a whole different set of medications than the “older” fertility drugs that have been most frequently assessed in relation to cancer risk. Research that has been published assessing the risk of breast cancer after treatment with these older fertility medications, either separately or combined, (14, 17, 18, 19-23, 25, 26, 45) has in some cases demonstrated increased risk in subgroups of the study populations.(20, 23, 25, 45) Others, however, did not detect any increased risk of breast cancer (14, 17, 18, 19, 22, 26). Amongst the few that have reported on cancer risk after use of ART solely, Stewart and colleagues (17) found no overall increase in risk of breast cancer, although in subgroup analyses they discovered an excess risk in women commencing treatment at younger ages. In a case control study from 2008, Katz and colleagues demonstrated an increased risk of breast cancer in those treated with IVF, and that the mean age at diagnosis was lower in the IVF group.(24) This is concordant with our findings of a slightly lower age at diagnosis for ART women.
Stratified analysis on mode of fertility treatment demonstrated elevated risk of breast cancer in women only treated with IVF. Women treated only with ICSI, a procedure specifically used for couples diagnosed with male infertility, did not have higher risk than controls, although this group was small. The hormone regimens used for ICSI and IVF are comparable. Unfortunately, we could not stratify our analyses by different causes of infertility. The present finding, however, of an association between breast cancer and IVF, infers that the underlying female infertility and not the hormone treatment may lead to an increased risk of breast cancer. It is thought that within a heterogeneous group of infertile women, the risk profiles are different amongst those with different infertility diagnoses.(15) Orgeas and colleagues found in a cohort study an increased risk of breast cancer in women referred for non-ovulatory infertility, compared to those referred for ovulatory dysfunction.(42) Our results also show that those treated with ICSI had a significantly lower age at end of follow up and shorter follow up time, which might partly explain the difference in risk we observed between IVF and ICSI treatment. We may also be lacking statistical power for proper analysis of ICSI women due to their lower age and shorter follow up time.
In our material, we found no significant risk increase of cancer at any site for ART women before inclusion to the study. This is contrary to Källén and colleagues; who observed a higher odds ratio for cancer before treatment in women receiving IVF compared with controls. Childhood cancer survivors have been shown to have a higher risk of being treated with ART.(38) In this study, however, excluding women with cancer before the start of follow up did in fact alter the main result somewhat, but not analyses of subgroups of women with longer follow up, nor for women subjected to IVF in particular.
Of those with more than 10 years of follow up, risk of breast cancer was significantly increased. Brinton and colleagues (45) also found a slightly increased risk after more than 20 years follow up after use of fertility drugs (clomiphene citrate and gonadotropins). Some have argued that the risk of late onset breast cancer is more related to hormone exposure than early onset breast cancer.(46) The study population in the cohort is relatively young, and hence cancer rates in the cohort will increase with the passing of time. The increased risk difference we observed after 10 years of follow up may be a consequence of that only after 10 years or more of follow up, are study subjects old enough to detect any significant risk difference between ART women and controls. The delayed effect may also be explained by latency of the hormone exposure, causing risk increase only to appear after the passing of time.
In conclusion, this population-based study of all parous Norwegian women over a 27-year period showed an increased risk of breast cancer in women who received ART compared to women who did not. Subgroup analyses showed a significantly increased risk for ART women subjected to IVF, and to women followed up for at least ten years. Although the absolute risk increase was small, it is important to stress that a large portion of the study population is young, and follow up time is relatively short. The results confirm the importance of continued monitoring of cancer risks in ART women, as the population of infertile women exposed to fertility treatment advances into more typical cancer age ranges.
Examining risks in all nulliparous women who have received ART compared to risks in nulliparous women who have not been exposed to ART will be an important addition to this material.
Summary.
The study uses high quality data from two Norwegian registries, the Cancer Registry and the Medical Birth Registry to assess the risk of breast cancer in parous women following assisted reproductive technology (ART). It uses data from the whole Norwegian population through 27 years, no related studies document longer follow-up time for their study cohorts. Many studies to date assess exposure to other forms of fertility treatment, and not only ART as this one does.
Acknowledgements
The authors are grateful to the Medical Birth Registry of Norway and the Cancer Registry of Norway for supplying the data.
Abbreviations
- ART
Assisted reproductive technology
- IVF
In vitro fertilisation
- ICSI
Intracytoplasmic sperm injection
- MBRN
The Medical Birth Registry of Norway
- CRN
The Cancer Registry of Norway
Footnotes
Disclaimer
This study has used data from the Medical Birth Registry of Norway. The interpretation and reporting of these data is the sole responsibility of the authors, and no endorsement by the Medical Birth Registry of Norway is intended nor should be inferred
Contributor Information
Marte Myhre Reigstad, Norwegian Resource Centre for Women's Health, Oslo University Hospital, Rikshospitalet, Oslo, Norway.
Inger Kristin Larsen, Cancer Registry of Norway, Institute of Population-based Cancer Research, Norway.
Tor Åge Myklebust, Cancer Registry of Norway, Institute of Population-based Cancer Research, Norway.
Trude Eid Robsahm, Cancer Registry of Norway, Institute of Population-based Cancer Research, Norway.
Nan Birgitte Oldereid, Section of Reproductive Medicine, Oslo University Hospital, Rikshospitalet, Oslo, Norway.
Anne Katerine Omland, Section of Reproductive Medicine, Oslo University Hospital, Rikshospitalet, Oslo, Norway.
Siri Vangen, Norwegian Resource Centre for Women's Health, Oslo University Hospital, Rikshospitalet, Oslo, Norway.
Louise Annette Brinton, National Cancer Institute, Division of Cancer Epidemiology & Genetics, Hormonal and Reproductive Epidemiology Branch, Bethesda, Maryland, U.S.A..
Ritsa Storeng, Norwegian Resource Centre for Women's Health, Oslo University Hospital, Rikshospitalet, Oslo, Norway.
References
- 1.Stephen EH, Chandra A. Updated projections of infertility in the United States: 1995-2025. Fertil Steril. 1998;70:30–34. doi: 10.1016/s0015-0282(98)00103-4. [DOI] [PubMed] [Google Scholar]
- 2.Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, de Mouzon J, et al. Assisted reproductive technology in Europe, 2009: results generated from European registers by ESHRE. Hum Reprod. 2013;28:2318–2331. doi: 10.1093/humrep/det278. [DOI] [PubMed] [Google Scholar]
- 3.Group ECW. Failures (with some successes) of assisted reproduction and gamete donation programs. Hum Reprod Update. 2013;19:354–365. doi: 10.1093/humupd/dmt007. [DOI] [PubMed] [Google Scholar]
- 4.Whittemore AS. The risk of ovarian cancer after treatment for infertility. N Engl J Med. 1994;331:805–806. doi: 10.1056/NEJM199409223311211. [DOI] [PubMed] [Google Scholar]
- 5.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136:1184–1203. doi: 10.1093/oxfordjournals.aje.a116427. [DOI] [PubMed] [Google Scholar]
- 6.Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331:771–776. doi: 10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- 7.Li LL, Zhou J, Qian XJ, Chen YD. Meta-analysis on the possible association between in vitro fertilization and cancer risk. Int J Gynecol Cancer. 2013;23:16–24. doi: 10.1097/IGC.0b013e318277608b. [DOI] [PubMed] [Google Scholar]
- 8.Brinton LA, Sahasrabuddhe VV, Scoccia B. Fertility drugs and the risk of breast and gynecologic cancers. Semin Reprod Med. 2012;30:131–145. doi: 10.1055/s-0032-1307421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo Russo G, Spinelli GP, Tomao S, Rossi B, Frati L, et al. Breast cancer risk after exposure to fertility drugs. Expert Rev Anticancer Ther. 2013;13:149–157. doi: 10.1586/era.12.181. [DOI] [PubMed] [Google Scholar]
- 10.Nelson HD, Zakher B, Cantor A, Fu R, Griffin J, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann Intern Med. 2012;156:635–648. doi: 10.1059/0003-4819-156-9-201205010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumitrescu RG, Cotarla I. Understanding breast cancer risk -- where do we stand in 2005? J Cell Mol Med. 2005;9:208–221. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer in Norway 2012 Cancer incidence, mortality, survival and prevalence in Norway. Oslo. 2014 [Google Scholar]
- 13.Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17:1399–1403. doi: 10.1093/humrep/17.5.1399. [DOI] [PubMed] [Google Scholar]
- 14.Brinton L, Trabert B, Shalev V, Lunenfeld E, Sella T, et al. In vitro fertilization and risk of breast and gynecologic cancers: A retrospective cohort study within the Israeli Maccabi Healthcare Services. Fertility and Sterility. 2013;99:1189–1196. doi: 10.1016/j.fertnstert.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerner-Geva L, Rabinovici J, Olmer L, Blumstein T, Mashiach S, et al. Are infertility treatments a potential risk factor for cancer development? Perspective of 30 years of follow-up. Gynecol Endocrinol. 2012;28:809–814. doi: 10.3109/09513590.2012.671391. [DOI] [PubMed] [Google Scholar]
- 16.Yli-Kuha AN, Gissler M, Klemetti R, Luoto R, Hemminki E. Cancer morbidity in a cohort of 9175 Finnish women treated for infertility. Human Reproduction. 2012;27:1149–1155. doi: 10.1093/humrep/des031. [DOI] [PubMed] [Google Scholar]
- 17.Stewart LM, Holman CD, Hart R, Bulsara MK, Preen DB, et al. In vitro fertilization and breast cancer: is there cause for concern? Fertil Steril. 2012;98:334–340. doi: 10.1016/j.fertnstert.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Dor J, Lerner-Geva L, Rabinovici J, Chetrit A, Levran D, et al. Cancer incidence in a cohort of infertile women who underwent in vitro fertilization. Fertil Steril. 2002;77:324–327. doi: 10.1016/s0015-0282(01)02986-7. [DOI] [PubMed] [Google Scholar]
- 19.Jensen A, Sharif H, Svare EI, Frederiksen K, Kjaer SK. Risk of breast cancer after exposure to fertility drugs: results from a large Danish cohort study. Cancer Epidemiol Biomarkers Prev. 2007;16:1400–1407. doi: 10.1158/1055-9965.EPI-07-0075. [DOI] [PubMed] [Google Scholar]
- 20.Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with invitro fertilisation. Lancet. 1999;354:1586–1590. doi: 10.1016/S0140-6736(99)05203-4. [DOI] [PubMed] [Google Scholar]
- 21.Kristiansson P, Bjor O, Wramsby H. Tumour incidence in Swedish women who gave birth following IVF treatment. Hum Reprod. 2007;22:421–426. doi: 10.1093/humrep/del411. [DOI] [PubMed] [Google Scholar]
- 22.Lerner-Geva L, Geva E, Lessing JB, Chetrit A, Modan B, et al. The possible association between in vitro fertilization treatments and cancer development. Int J Gynecol Cancer. 2003;13:23–27. doi: 10.1136/ijgc-00009577-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Brinton LA, Scoccia B, Moghissi KS, Westhoff CL, Althuis MD, et al. Breast cancer risk associated with ovulation-stimulating drugs. Hum Reprod. 2004;19:2005–2013. doi: 10.1093/humrep/deh371. [DOI] [PubMed] [Google Scholar]
- 24.Katz D, Paltiel O, Peretz T, Revel A, Sharon N, et al. Beginning IVF Treatments After Age 30 Increases the Risk of Breast Cancer: Results of a Case-Control Study. Breast Journal. 2008;14:517–522. doi: 10.1111/j.1524-4741.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 25.Pappo I, Lerner-Geva L, Halevy A, Olmer L, Friedler S, et al. The possible association between IVF and breast cancer incidence. Ann Surg Oncol. 2008;15:1048–1055. doi: 10.1245/s10434-007-9800-2. [DOI] [PubMed] [Google Scholar]
- 26.Kallen B, Finnstrom O, Lindam A, Nilsson E, Nygren KG, et al. Malignancies among women who gave birth after in vitro fertilization. Hum Reprod. 2011;26:253–258. doi: 10.1093/humrep/deq307. [DOI] [PubMed] [Google Scholar]
- 27.Zreik TG, Mazloom A, Chen Y, Vannucci M, Pinnix CC, et al. Fertility drugs and the risk of breast cancer: a meta-analysis and review. Breast Cancer Res Treat. 2010;124:13–26. doi: 10.1007/s10549-010-1140-4. [DOI] [PubMed] [Google Scholar]
- 28.Larsen I, Smastuen M, Johannesen T, Langmark F, Parkin D, et al. Data quality at the Cancer Registry of Norway: An overview of comparability, completeness, validity and timeliness. European Journal of Cancer. 2009;45:1218–1231. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Vikanes A, Magnus P, Vangen S, Lomsdal S, Grjibovski AM. Hyperemesis gravidarum in the Medical Birth Registry of Norway - a validity study. BMC Pregnancy Childbirth. 2012;12:115. doi: 10.1186/1471-2393-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engeland A, Bjorge T, Daltveit AK, Vollset SE, Furu K. Validation of disease registration in pregnant women in the Medical Birth Registry of Norway. Acta Obstet Gynecol Scand. 2009;88:1083–1089. doi: 10.1080/00016340903128454. [DOI] [PubMed] [Google Scholar]
- 31.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, et al. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod. 2009;24:2683–2687. doi: 10.1093/humrep/dep343. [DOI] [PubMed] [Google Scholar]
- 32.Out HJ, Mannaerts BM, Driessen SG, Bennink HJ. A prospective, randomized, assessor-blind, multicentre study comparing recombinant and urinary follicle stimulating hormone (Puregon versus Metrodin) in in-vitro fertilization. Hum Reprod. 1995;10:2534–2540. doi: 10.1093/oxfordjournals.humrep.a135740. [DOI] [PubMed] [Google Scholar]
- 33.Recombinant Human FSH Study Group Clinical assessment of recombinant human follicle-stimulating hormone in stimulating ovarian follicular development before in vitro fertilization. Recombinant Human FSH Study Group. Fertil Steril. 1995;63:77–86. [PubMed] [Google Scholar]
- 34.Hansmann M. Ultraschallbiometrie im II. und III. Trimester des Schwangerschaft. Gynäkologe. 1976;9:133–155. [Google Scholar]
- 35.Schoenfeld D. Partial Residuals for the Proportional Hazards Regression-Model. Biometrika. 1982;69:239–241. [Google Scholar]
- 36.Commenges D, Letenneur L, Joly P, Alioum A, Dartigues JF. Modelling age-specific risk: application to dementia. Stat Med. 1998;17:1973–1988. doi: 10.1002/(sici)1097-0258(19980915)17:17<1973::aid-sim892>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Syse A, Kravdal O, Tretli S. Parenthood after cancer - a population-based study. Psychooncology. 2007;16:920–927. doi: 10.1002/pon.1154. [DOI] [PubMed] [Google Scholar]
- 38.Stensheim H, Cvancarova M, Moller B, Fossa SD. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer. 2011;129:1225–1236. doi: 10.1002/ijc.26045. [DOI] [PubMed] [Google Scholar]
- 39.StataCorp . Stata Statistical Software: Release 13. StataCorp LP; College Station, TX: 2013. [Google Scholar]
- 40.Engeland A, Bjorge T, Daltveit AK, Vollset SE, Furu K. Validation of disease registration in pregnant women in the Medical Birth Registry of Norway. Acta Obstetricia Et Gynecologica Scandinavica. 2009;88:1083–1089. doi: 10.1080/00016340903128454. [DOI] [PubMed] [Google Scholar]
- 41.Hudson N, Culley L, Blyth E, Norton W, Rapport F, et al. Cross-border reproductive care: a review of the literature. Reproductive Biomedicine Online. 2011;22:673–685. doi: 10.1016/j.rbmo.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Orgeas CC, Sanner K, Hall P, Conner P, Holte J, et al. Breast cancer incidence after hormonal infertility treatment in Sweden: a cohort study. Am J Obstet Gynecol. 2009;20072:e71–77. doi: 10.1016/j.ajog.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 43.Hofvind S, Geller B, Vacek PM, Thoresen S, Skaane P. Using the European guidelines to evaluate the Norwegian Breast Cancer Screening Program. European Journal of Epidemiology. 2007;22:447–455. doi: 10.1007/s10654-007-9137-y. [DOI] [PubMed] [Google Scholar]
- 44.Gauthier E, Paoletti X, Clavel-Chapelon F. Breast cancer risk associated with being treated for infertility: results from the French E3N cohort study. Hum Reprod. 2004;19:2216–2221. doi: 10.1093/humrep/deh422. [DOI] [PubMed] [Google Scholar]
- 45.Louise A, Brinton BS, Kamran S, Moghissi, Carolyn L, Westhoff, Michelle D, Althuis, Jerome E, Mabie, Emmet J, Lamb Breast cancer risk associated with ovulationstimulating drugs. Human Reproduction. 2004;19:2005–2013. doi: 10.1093/humrep/deh371. [DOI] [PubMed] [Google Scholar]
- 46.Benz CC. Impact of aging on the biology of breast cancer. Critical Reviews in Oncology Hematology. 2008;66:65–74. doi: 10.1016/j.critrevonc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]