Abstract
Intrauterine growth restriction (IUGR) programs neurodevelopmental impairment and long-term neurological morbidities. Neurological morbidities in IUGR infants are correlated with changes hippocampal volume. We previously demonstrated that IUGR alters hippocampal cellular composition in both neonatal and juvenile rat pups in association with altered hippocampal gene expression and epigenetic determinants. PPARγ signaling is important for neurodevelopment as well as epigenetic integrity in the brain via the PPARγ-Setd8-H4K20me1 axis and Wnt signaling. We hypothesized that IUGR would decrease expression of PPARγ, Setd8, and H4K20me1 in juvenile rat hippocampus. We further hypothesized that reduced PPARγ-Setd8-H4K20me1 would be associated with reduced Wnt signaling genes Wnt3a and β-catenin, and wnt target gene Axin2. To test our hypothesis we used a rat model of uteroplacental insufficiency-induced IUGR. We demonstrated that PPARγ localizes to oligodendrocytes, neurons and astrocytes within the juvenile rat hippocampus. We also demonstrated that IUGR reduces levels of PPARγ, Setd8 and H4K20me1 in male and female juvenile rat hippocampus in conjunction with reduced Wnt signaling components in only male rats. We speculate that reduced PPARγ and Wnt signaling may contribute to altered hippocampal cellular composition which, in turn, may contribute to impaired neurodevelopment and subsequent neurocognitive impairment in IUGR offspring.
Keywords: Intrauterine growth restriction, brain development, PPARγ, epigenetics
1. Introduction
Intrauterine growth restriction (IUGR) occurs when a fetus fails to reach optimal growth potential in utero. In developed countries, IUGR frequently occurs secondary to maternal hypertensive disorders and uteroplacental insufficiency. Insults such as IUGR, which occur during developmentally plastic periods, program neurodevelopmental impairment and long-term neurological morbidities (Geva et al. 2006, von Beckerath et al. 2013).
In IUGR infants, neurological impairments include both short and long-term cognitive disabilities evident as early as 2 years of age and persisting beyond school entry (Sung et al. 1993). Long-term neurological morbidities in IUGR infants are correlated with changes in brain connectivity and reduced volume of the hippocampus, a brain region key for the formation of certain types of memory (Olton et al. 1978, Morris et al. 1982, Zola-Morgan et al. 1986, Batalle et al. 2012, Padilla et al. 2014). However, mechanisms for the programming of neurodevelopmental impairment in IUGR are not well understood.
In order to understand the mechanisms driving the programming of neurodevelopmental impairment following IUGR, our group utilizes a rat model of uteroplacental insufficiency induced IUGR. In our model, IUGR results from multiple components including nutrient restriction and hypoxia. We previously demonstrated that IUGR rat pups have cognitive impairment as adults (D. Caprau 2012). We also demonstrated that IUGR alters hippocampal cellular composition in both neonatal and juvenile rat pups (Schober et al. 2009, Fung et al. 2012). Importantly, alterations in cellular composition are accompanied by changes in both hippocampal epigenetic determinants and changes in hippocampal gene expression (Ke et al. 2006, Ke et al. 2010, Ke et al. 2011). Our previous results suggest that IUGR disrupts pathways regulating hippocampal phenotype, epigenetics and gene expression in the rat.
An important pathway unexplored in the IUGR rat hippocampus is the peroxisome proliferator-activated receptor gamma (PPARγ) pathway. PPARγ is a transcription factor that belongs to the PPAR subfamily of nuclear receptors. The PPARγ gene is well conserved between the human and rat and gives rise to multiple mRNA variants, and two protein isoforms, PPARγ1 and PPARγ2 (Zhu et al. 1995, Fajas et al. 1997, Ershov and Bazan 2000). The PPARγ pathway is involved in hippocampal repair and plasticity (Zhao et al. 2009). PPARγ is expressed throughout the developing brain, however, hippocampal PPARγ variant expression patterns remain unknown (Cullingford et al. 1998, Moreno et al. 2004). PPARγ activation improves hippocampus-dependent cognitive function in neurodegenerative disorders in both human and animals (Watson et al. 2005, Pedersen et al. 2006, Risner et al. 2006, Escribano et al. 2009, Rodriguez-Rivera et al. 2011, Denner et al. 2012). In addition, neural PPARγ knockout mice show that postnatal reduction of PPARγ in neurons heightens sensitivity to ischemia (Zhao et al. 2009).
A novel means by which PPARγ may contribute to brain development is the transcriptional regulation of epigenetic modifying enzymes. Epigenetic modifying enzymes affect developmental processes by altering expression patterns of target genes. The genes coding for several chromatin modifying enzymes contain PPAR response elements (PPRE) and are demonstrated transcriptional targets of PPARγ (Wakabayashi et al. 2009). One of these PPARγ responsive genes is the set domain containing histone methyltransferase, Setd8, which puts a monomethyl (me1) group on lysine (K) 20 of Histone (H) 4.
Accumulating evidence shows that H4K20me1 can function as a transcriptional activator in canonical Wnt signaling (Li et al. 2011). Canonical Wnt signaling plays an important role in a wide range of biological and pathophysiological processes involving central nervous system development (Logan and Nusse 2004, Clevers 2006, Willert and Jones 2006). A transcriptional target of active Wnt signaling is Axin inhibition protein 2 (Axin2). Axin2 is an essential regulator of normal myelination and remyelination (Fancy et al. 2011).
Despite the importance of PPARγ and Wnt signaling in hippocampal outcomes in IUGR, the effects of IUGR on PPARγ-Setd8-H4K20me1 and wnt signaling components is unknown. We hypothesized that IUGR would decrease expression of PPARγ and its target gene Setd8, as well as subsequent levels of H4K20me1 in juvenile rat hippocampus. We further hypothesized that reduced PPARγ-Setd8-H4K20me1 would be associated with reduced expression of Wnt signaling genes Wnt3a and β-catenin, as well as wnt target gene Axin2. To test our hypothesis we used a well characterized rat model of IUGR.
2. Methods
2.1 Rat Model of IUGR
All animal procedures were approved by the University of Utah Animal Research Committee and are in accordance with the American Physiological Society’s guiding principles (Society 2002). Surgical methods have been described previously (Ke et al. 2005, Ke et al. 2006, Joss-Moore et al. 2010) and are briefly described below. IUGR was induced in rat pups by ligation of both uterine arteries on day 19 of gestation, following anesthesia. Dams for control pups received identical anesthesia. IUGR and control rat dams delivered spontaneously and litters were culled to six in order to normalize postnatal nutrition. At postnatal day 21 (d21), IUGR and control rats were separated from their dams for 4 hours, anesthetized, and killed. We chose to assess the effects of IUGR on the PPARγ and Wnt signaling pathways at postnatal d21 (juvenile) because this time point follows key maturational processes such as myelination and synaptogenesis in the rat brain (Rice and Barone 2000). Brains were quickly removed, and hippocampi dissected and flash frozen in liquid nitrogen and stored in −80°C.
Whole brain from one male and one female rat pup from each litter were individually fixed via intra-cardiac perfusion as previously described (Fung et al. 2012). Dissected whole brains were postfixed at 4 °C overnight, cryoprotected with 15% and 30% sucrose at 4 °C overnight, and embedded in 2% gelatin, 0.9% NaCl, 0.05% NaN3 (Sigma-Aldrich) under freezing conditions. Brains were sectioned coronally at 12 μm per section (Microm HM550; Microm International, Walldorf, Germany). Sections containing dorsal hippocampus were collected sequentially on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA).
All experiments used 6 non-sibling rat pups per group (male control, male IUGR, female control, female IUGR).
2.2 Immunofluorescent staining for PPARγ localization
Immunofluorescent (IF) double labeling was used to co-localize PPARγ in neurons, oligodendrocytes and astrocytes in the hippocampus as previously described (Fung et al. 2012). 49,6-diamidino-2-phenylindole (DAPI) was used for nuclei counter staining. The following primary antibodies were used: anti-PPARγ (#2435, Cell Signaling) labeled in red, anti-neuron marker NeuN antibody (MAB377; Millipore, Billerica, MA); anti- oligodendrocyte marker APC antibody (ab16794, abcam) and anti-astrocyte marker GFAP antibody (IF30L-100ug; Millipore, Billerica, MA). Negative control sections underwent similar staining procedures with the omission of primary antibody.
Sections were imaged with the Nikon’s A1 Nikon’s A1 multiphoton laser scanning confocal microscope (Nikon Instruments Inc./Americas) and taken at ×20 and ×60 magnification to encompass all subregions of the hippocampus (CA1, CA3, and DG).
2.3 Real-Time RT-PCR
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was used to evaluate mRNA abundance of hippocampal PPARγ variants, Setd8, Wnt3a, β-catenin and Axin2 as previously described (Ke et al. 2006, Joss-Moore et al. 2010). The following Assay-on-demand primer/probe sets were used: PPAR γ1 – Rn01492275_m1, PPARγ 2 – Rn00440940_m1, Setd8 – Rn01477383_g1, Wnt3a-RN01470643_m1, β-catenin-00584431_g1, Axin2-Rn00577441_m1 (Applied Biosystems, Foster, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. GAPDH primer and probe sequences; Forward: CAAGATGGTGAAGGTCGGTGT; Reverse: CAAGAGAAGGCAGCCCTGGT; Probe: GCGTCCGATACGGCCAAATCCG.
2.4 Immunoblot
Hippocampal levels of PPARγ, Setd8, Wnt3a and γ-catenin protein were quantified using immunoblot as previously described (Joss-Moore et al. 2010, Ke et al. 2010). GAPDH was used as a loading control. The following primary antibodies were used: PPARγ (H-100, sc-7196, Santa Cruz Biotechnology), Setd8 (#2996, Cell Signaling Technology), Wnt3a (ab28472, abcam), β-catenin (H102, sc-7199, Santa Cruz Biotechnology) and GAPDH (rabbit polyclonal, Cell Signaling)
Global hippocampal H4K20me1 was quantified by immunoblot with acid-extracted histones, prepared as previously described (Ke et al. 2006, Joss-Moore et al. 2010). Levels of histone H4 monomethyl Lys20 (H4K20Me pAb, #39727, Active Motif) was quantified relative to total H4 (#61299, Active Motif).
2.5 Statistics
All data presented are expressed as mean ± SD. Effects of IUGR on PPARγ-Setd8-H4K20me1 and wnt signaling in male and female rats were analyzed by Mann Whitney U test. Statistical significance was accepted as p<0.05.
3. Results
3.1 Weights of IUGR Rat Pups
IUGR rat pups weighed significantly less than age- and sex-matched control pups at day of life 1 and at day of life 21 (Table 1).
Table 1.
IUGR Decreased Body Weight (gm) in Rat Pups at day of life 1 and 21 (mean ± SD)
| Postnatal | Male |
Female |
||
|---|---|---|---|---|
| Age (days) |
Control | IUGR | Control | IUGR |
| DOL1 | 7.1 ± 0.4 | 5.1 ± 0.5* | 6.7 ± 0.3 | 5.4 ± 0.3* |
| DOL21 | 68.3 ± 3.7 | 55.3 ± 7.7* | 63.4 ± 1.9 | 54.6 ± 4.2* |
Different from age- and sex-matched control group, p<0.05.
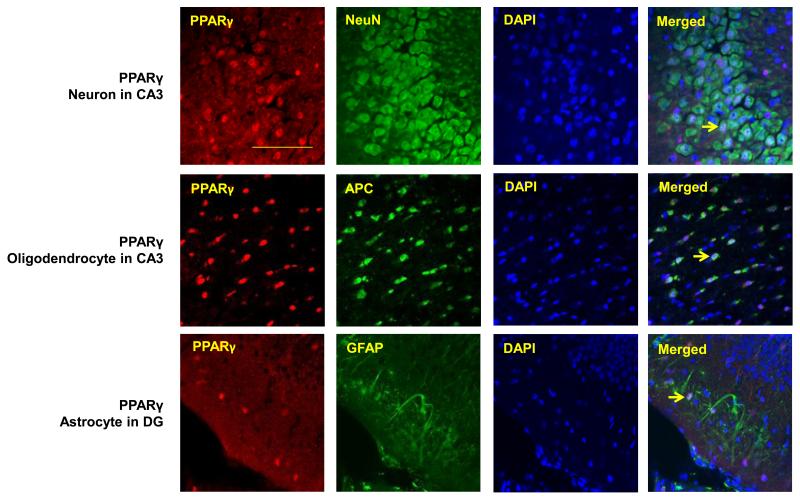
3.2 PPARγ co-localizes to oligodendrocytes, neurons and astrocytes in d21 rat hippocampus
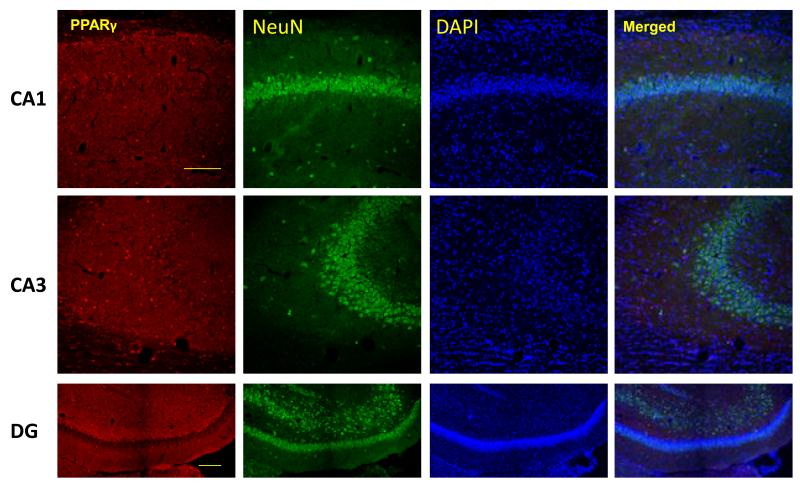
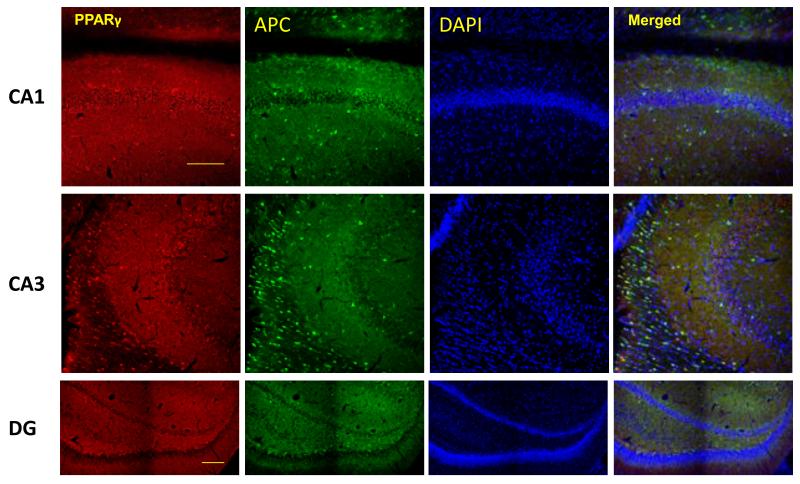
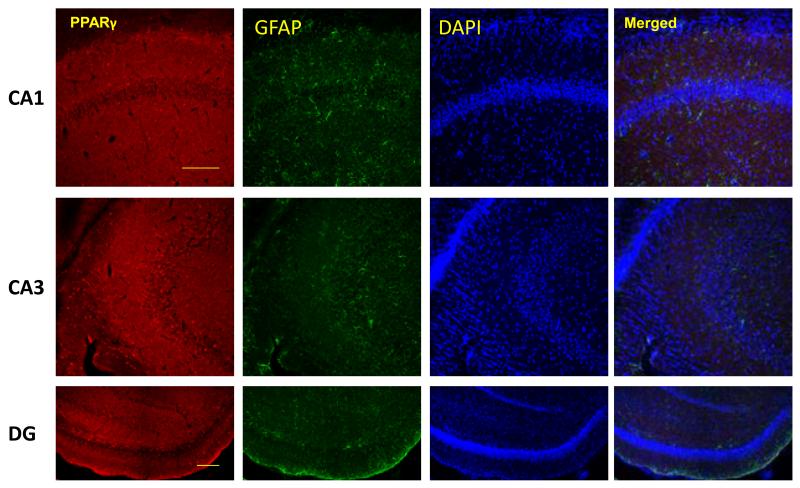
To determine the cellular distribution of PPARγ in the d21 rat hippocampus, PPARγ co-immunofluorescence was used with markers for oligodendrocytes, neurons and astrocytes. All 3 cell types stained positive for PPARγ (Figure 1A-D). Oligodendrocytes qualitatively displayed greater PPARγ expression than neurons and astrocytes. Neurons expressing PPARγ were primarily localized to the CA3 region of the hippocampus.
Figure 1.
PPARγ is expressed in neurons, oligodendocytes and astrocytes of the rat hippocampus. Representative images of PPARγ (red) co-immunoflorescent staining with A) NeuN for neurons(green), B) APC for oligodendrocytes (green), and GFAP for astrocytes(green) in CA1, CA3 and dentate gyrus (DG) subregions of the rat hippocampus. Nuclei were counterstained with DAPI (blue). Three colors equally merged shows in white. D) Arrows show representative PPARγ positive cells in each case. Scale bar=100μm.
3.3 IUGR decreases Rat Hippocampal PPARγ-Setd8-H4K20me1
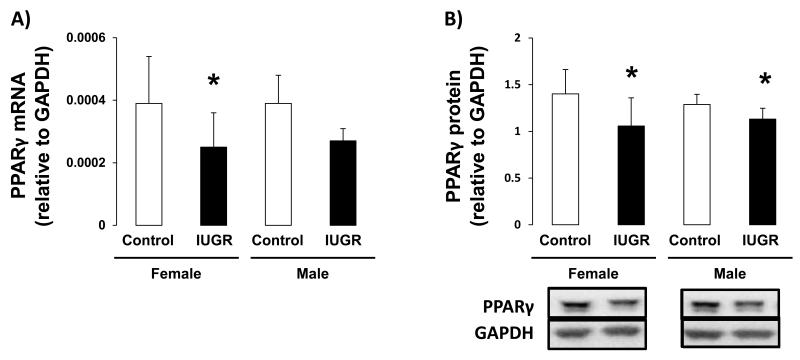
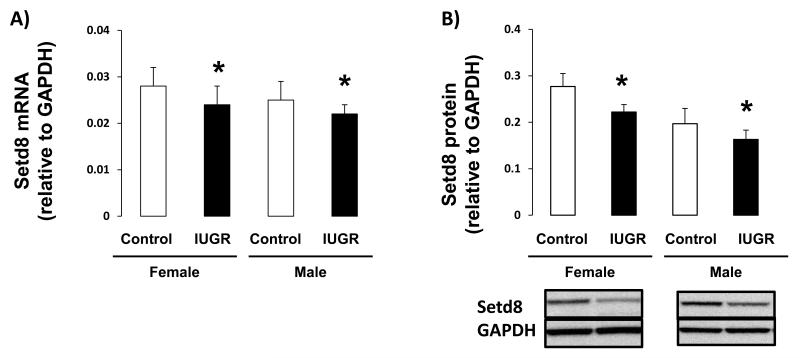
The effect of IUGR on hippocampal expression of PPARγ, and downstream target Setd8, was assessed in female and male d21 rats. IUGR decreased PPARγ1 mRNA and protein levels, relative to sex-matched controls, in both female (mRNA p=0.026, protein p=0.015) and male (mRNA p=0.009, protein p=0.026) rat hippocampus (Figure 2A-B). The PPARγ2 variant was undetectable in d21 male or female rat hippocampus. Similarly, IUGR decreased Setd8 mRNA and protein abundance in both female (mRNA p=0.026, protein p=0.004) and male (mRNA p=0.002, protein p=0.015) hippocampus (Figure 3A-B).
Figure 2.
PPARγ mRNA and protein levels in d21 hippocampus. IUGR significantly decreased PPARγ mRNA (A) and protein levels (B) in both female and male hippocampus n=6. *p<0.05
Figure 3.
Setd8 mRNA and protein levels in d21 hippocampus. IUGR significantly decreased Setd8 mRNA levels (A) and protein levels (B) in both female and male hippocampus n=6. *p<0.05
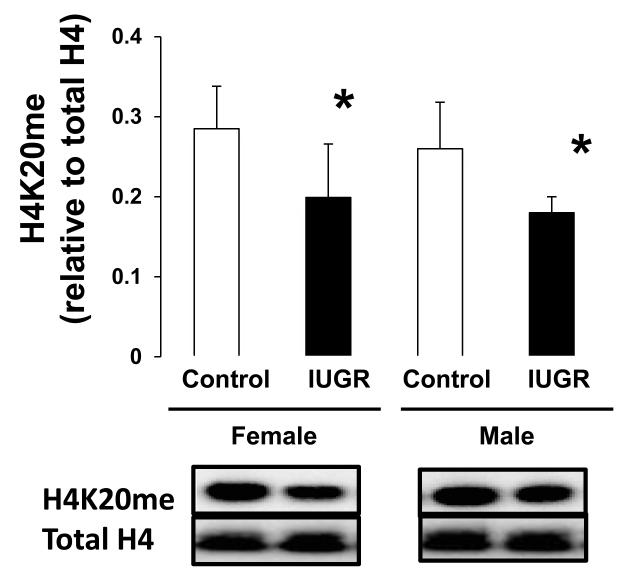
Because Setd8 places the H4K20me1 mark, we measured global levels of H4K20me1 in d21 rat hippocampus. IUGR reduced global H4K20me1 both female (p=0.026) and male (p=0.041) hippocampus (Figure 4).
Figure 4.
H4K20me protein levels in d21 hippocampus. IUGR significantly reduced H4K20me protein abundance in both female and male hippocampus. n=6. *p<0.05
3.4 IUGR Decreases Hippocampal Wnt3a and β-catenin expression, and Axin2 mRNA
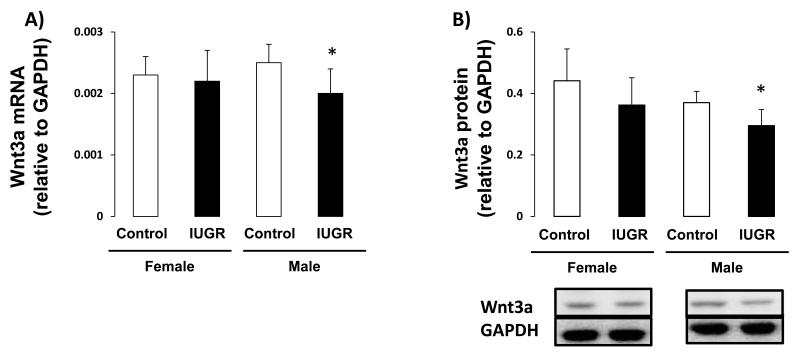
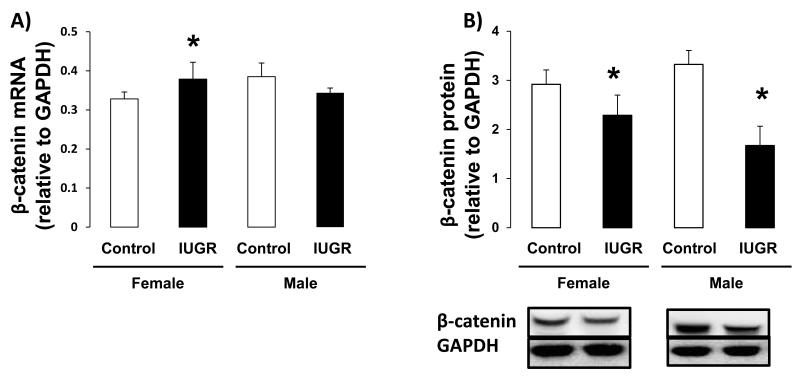
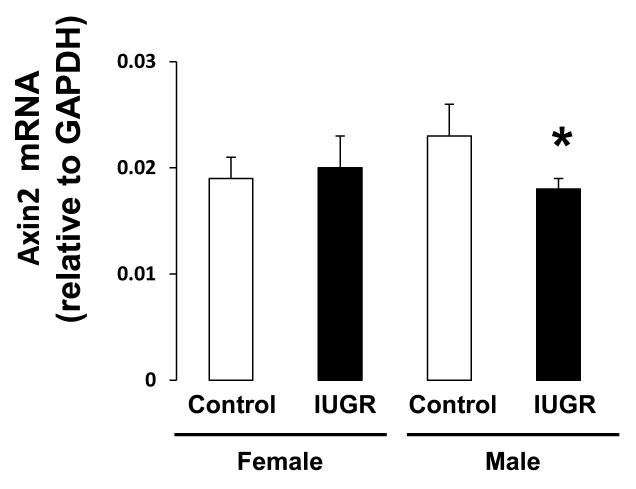
Because the regulation of Wnt signaling is a downstream effect of H4K20me1, we measured the effect of IUGR on expression of Wnt signaling components, Wnt3a and β-catenin, as well the Wnt signaling target, Axin2. In female hippocampus, IUGR did not affect Wnt3a mRNA or protein abundance (Figure 5A-B). However, IUGR did decrease β-catenin protein abundance (p=0.015) relative to female control (Figure 6B). Wnt target gene, Axin2 mRNA was not altered by IUGR in female rat hippocampus (Figure 7). In contrast, in male hippocampus, IUGR decreased mRNA and protein levels of Wnt3a (mRNA p=0.026, protein p=0.015), β-catenin protein levels (p=0.015) as well as mRNA levels of Axin2 (p=0.041) (Figure 5,6,7).
Figure 5.
Wnt3a mRNA and protein levels in d21 hippocampus. A-B: IUGR significantly decreased Wnt3a mRNA and protein abundance in male rat hippocampus but not in female rat hippocampus. n=6. *p<0.05
Figure 6.
β-catenin mRNA and protein levels in d21 hippocampus. A-B: IUGR significantly decreased β-catenin protein abundance in male rat hippocampus. IUGR also decreased β-catenin protein levels in female rat hippocampus. n=6. *p<0.05
Figure 7.
Axin 2 mRNA levels in d21 hippocampus.: IUGR significantly decreased Axin2 mRNA levels in male rat hippocampus without affecting female rat hippocampus. n=6. *p<0.05
4. Discussion
The results of our study demonstrate that IUGR disrupts the PPARγ-Setd8-H4K20me1 axis and Wnt signaling in the juvenile rat hippocampus. Our novel data show that PPARγ is expressed in oligodendrocytes, neurons, and astrocytes in juvenile rat hippocampus. While overall decreases in the PPARγ-Setd8-H4K20me1 axis were observed in both male and female IUGR rat hippocampus, effects on Wnt signaling components and Axin2 were predominantly seen in male rat hippocampus. Given the importance of PPARγ and Wnt signaling in appropriate neural development, decreased PPARγ-Setd8-H4K20me1 and Wnt signaling may contribute to the IUGR-induced alterations in hippocampal phenotype previously observed in the model.
In our study, we identified PPARγ expression in different cell types within the hippocampus. We observed PPARγ expression in hippocampal neurons, consistent with previous studies (Inestrosa et al. 2005, Di et al. 2009). Interestingly, we also observed that PPARγ is primarily localized to neurons in CA3 region of the hippocampus. The hippocampal CA3 subregion plays a significant role in spatial memory formation (Handelmann and Olton 1981, Sutherland et al. 1983, Stubley-Weatherly et al. 1996). Our study also showed significant PPARγ expression in hippocampal oligodendrocytes. In oligodendrocytes, PPARγ is involved in lipid metabolism and differentiation in vitro (Roth et al. 2003). Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that enable formation of myelin and saltatory nerve conduction, and provide a supporting role for neurons in the CNS. We speculate that decreased PPARy expression and its downstream signaling may account for reduced oligodendrocyte density previously reported in IUGR male rats (Schober et al. 2009).
In the past, PPARγ has been investigated for its action in ameliorating the development and progression of a number of CNS diseases. PPARγ activation has been shown to increase neuron survival and decrease lesion sizes in animal models of Parkinson’s disease, central inflammation, intracerebral hemorrhage, and cerebral ischemia (Heneka et al. 2000, Breidert et al. 2002, Dehmer et al. 2004, Ou et al. 2006, Victor et al. 2006, Zhao et al. 2006). PPARγ agonists have been shown to improve impaired hippocampus-dependent cognitive function in neurodegenerative disorders in both human and animals (Watson et al. 2005, Pedersen et al. 2006, Risner et al. 2006, Escribano et al. 2009, Rodriguez-Rivera et al. 2011, Denner et al. 2012). Our finding of IUGR-induced reductions in hippocampal PPARγ expression suggests that PPARγ contribute to impaired hippocampal development and impaired learning and memory function seen in IUGR rats. Further investigations into the causative nature of PPARγ levels on the IUGR phenotype are warranted.
Specific roles of PPARγ in hippocampal development and function are currently unknown. The discovery that PPARγ regulates the expression of chromatin modifying enzymes (Wakabayashi et al. 2009) provides a novel mechanism by which PPARγ may impact hippocampal development. Epigenetic regulation provides a way to selectively express information contained within the genome. This is particularly important in the context of developmentally specific gene expression. One mode of epigenetic regulation is methylation of histones by methyltransferase enzymes. The PPARγ target gene, Setd8 is a histone lysine methyltransferase, which places the H4K20me1 mark.
Roles of H4K20me1 are varied and include transcriptional repand transcriptional activation repression (Nishioka et al. 2002, Talasz et al. 2005, Vakoc et al. 2006). One pathway that is activated by H4k20me1 is canonical Wnt/β-catenin signaling (Li et al. 2011). Setd8 mediates Wnt/β-catenin signaling and Wnt signaling stimulates H4K20me1 enrichment at target gene promoters (Li et al. 2011). One of the Wnt signaling genes Wnt3a is crucial for normal growth of the hippocampus. Wnt3a acts locally to regulate the expansion of the caudomedial cortex, from which the hippocampus develops (Lee et al. 2000). Moreover, the Wnt signaling target gene Axin2 is essential for myelination and remyelination in brain development (Fancy et al. 2011). In this study, we found that IUGR-induced decreased hippocampal PPARγ expression is associated with decreased Setd8 expression and H4K20me1 abundance, as well as reductions of Wnt3a, β-catenin and Axin2, in male rat hippocampus.
Our finding of IUGR-induced decreased Wnt3a and Axin2 expression in male rats, but not in female rats, suggests a gender-specific response to brain Wnt signaling. This is important in the context of IUGR because gender-specific molecular changes are often observed (Joss-Moore et al. 2010, Ke et al. 2010, Ke et al. 2011, Fung et al. 2012). Mechanisms driving the gender-specific molecular responses are not fully elucidated. However, cross-talk between Wnt and estrogen signaling pathways is known to occur via a functional interaction between β-catenin and estrogen receptor alpha (ERα) (Kouzmenko et al. 2004). Furthermore, we previously demonstrated that IUGR reduces expression of the estrogen synthase, aromatase and ERα mRNA levels in male newborn rat hippocampus (O’Grady et al. 2010, Numpang et al. 2013). Collectively, we speculate that reduction of aromatase and ERα expression may play a role in decreased Wnt3a and subsequent axin2 levels in male IUGR rat hippocampus.
Our study is not without limitations. Our molecular findings are limited to global hippocampus and are not specific to any hippocampal subregion or specific cell type. In our study, we only examined the juvenile IUGR brain. In order to separate prenatal effects (IUGR) from postnatal effects (growth rate) on PPARγ-Setd8-H4K20me1 and wnt signaling, newborn rats will need to be studied. Our study is also descriptive in nature. Future studies examining the effect of PPARγ activators and antagonists on downstream targets will be an important next step in establishing cause and effect relationships.
In conclusion, IUGR decreases hippocampal PPARγ expression with an associated decreased in PPARγ downstream target Setd8 and H4K20me1 abundance. Reduction in H4K20me1 is further associated with decreased expression of Wnt signaling genes Wnt3a and β-catenin, as well as axin2 in male pups. We speculate that reduced PPARγ and Wnt signaling may contribute to altered hippocampal cellular composition which, in turn, may contribute to impaired neurodevelopment and subsequent neurocognitive impairment seen in IUGR offspring.
Highlights.
Intrauterine growth restriction programs neurodevelopmental impairment and long-term neurological morbidities.
We use a rat model to test the effects of IUGR on two developmentally important pathways; PPARγ-Setd8-H4K20me1 and Wnt signaling.
IUGR reduces mRNA and protein components of both pathways as well as mRNA of final target gene Axin2.
IUGR programming of neurodevelopmental impairment likely involves disruption to the PPARγ-Setd8-H4K20me1 and Wnt signaling pathways.
Acknowledgements
We would like to acknowledge the Division of Neonatology for support. This study was supported by the NIH (K01-DK080558 (LJM) and R03-DK095970 (LJM)).
Footnotes
Conflict of Interest Statement
None of the authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batalle D, Eixarch E, Figueras F, Munoz-Moreno E, Bargallo N, Illa M, Acosta-Rojas R, Amat-Roldan I, Gratacos E. Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage. 2012;60(2):1352–1366. doi: 10.1016/j.neuroimage.2012.01.059. [DOI] [PubMed] [Google Scholar]
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem. 2002;82(3):615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem. 1998;70(4):1366–1375. doi: 10.1046/j.1471-4159.1998.70041366.x. [DOI] [PubMed] [Google Scholar]
- Caprau D, S ME, Bass K, O’Grady S, Ke X, Block B, Callaway CW, Hale M, Yu X, McKnight RA, Kesner RP, Lane RH. Altered expression and chromatin structure of the hippocampal IGF1r gene is associated with impaired hippocampal function in the adult IUGR male rat. Journal of Developmental Origins of Health and Disease. 2012;3(02):83–91. doi: 10.1017/S2040174411000791. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88(2):494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- Denner LA, Rodriguez-Rivera J, Haidacher SJ, Jahrling JB, Carmical JR, Hernandez CM, Zhao Y, Sadygov RG, Starkey JM, Spratt H, Luxon BA, Wood TG, Dineley KT. Cognitive enhancement with rosiglitazone links the hippocampal PPARgamma and ERK MAPK signaling pathways. J Neurosci. 2012;32(47):16725–16735a. doi: 10.1523/JNEUROSCI.2153-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Z, Tian Y, Ma H, Du F, Lei H, Zhang G, Liang H. [Expression of peroxisome proliferator-activated receptor gamma in hippocampus neurons in rats after oxygen deprivation/oxygen supply in vitro] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34(12):1238–1242. [PubMed] [Google Scholar]
- Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPARgamma expression in retinal pigment epithelial cells. J Neurosci Res. 200060(3):328–337. doi: 10.1002/(SICI)1097-4547(20000501)60:3<328::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Escribano L, Simon AM, Perez-Mediavilla A, Salazar-Colocho P, Del Rio J, Frechilla D. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer’s disease mouse model. Biochem Biophys Res Commun. 2009;379(2):406–410. doi: 10.1016/j.bbrc.2008.12.071. [DOI] [PubMed] [Google Scholar]
- Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272(30):18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14(8):1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C, Ke X, Brown AS, Yu X, McKnight RA, Lane RH. Uteroplacental insufficiency alters rat hippocampal cellular phenotype in conjunction with ErbB receptor expression. Pediatr Res. 2012;72(1):2–9. doi: 10.1038/pr.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva R, Eshel R, Leitner Y, Valevski AF, Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006;118(1):91–100. doi: 10.1542/peds.2005-2343. [DOI] [PubMed] [Google Scholar]
- Handelmann GE, Olton DS. Recovery of function after neurotoxic damage to the hippocampal CA3 region: importance of postoperative recovery interval and task experience. Behav Neural Biol. 1981;33(4):453–464. doi: 10.1016/s0163-1047(81)91815-x. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J Neurosci. 2000;20(18):6862–6867. doi: 10.1523/JNEUROSCI.20-18-06862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M. Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Exp Cell Res. 2005;304(1):91–104. doi: 10.1016/j.yexcr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Joss-Moore LA, Wang Y, Baack ML, Yao J, Norris AW, Yu X, Callaway CW, McKnight RA, Albertine KH, Lane RH. IUGR decreases PPARgamma and SETD8 Expression in neonatal rat lung and these effects are ameliorated by maternal DHA supplementation. Early Hum Dev. 2010;86(12):785–791. doi: 10.1016/j.earlhumdev.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, Yu X, Wang L, Callaway CW, Gill G, Chan GM, Albertine KH, McKnight RA, Lane RH. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics. 2006;5(1):16–28. doi: 10.1152/physiolgenomics.00093.2005. [DOI] [PubMed] [Google Scholar]
- Ke X, McKnight RA, Caprau D, O’Grady S, Fu Q, Yu X, Callaway CW, Albertine KH, Lane RH. Intrauterine growth restriction affects hippocampal dual specificity phosphatase 5 gene expression and epigenetic characteristics. Physiol Genomics. 2011;43(20):1160–1169. doi: 10.1152/physiolgenomics.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X, McKnight RA, Wang ZM, Yu X, Wang L, Callaway CW, Albertine KH, Lane RH. Nonresponsiveness of cerebral p53-MDM2 functional circuit in newborn rat pups rendered IUGR via uteroplacental insufficiency. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R1038–1045. doi: 10.1152/ajpregu.00701.2004. [DOI] [PubMed] [Google Scholar]
- Ke X, Schober ME, McKnight RA, O’Grady S, Caprau D, Yu X, Callaway CW, Lane RH. Intrauterine growth retardation affects expression and epigenetic characteristics of the rat hippocampal glucocorticoid receptor gene. Physiol Genomics. 2010;42(2):177–189. doi: 10.1152/physiolgenomics.00201.2009. [DOI] [PubMed] [Google Scholar]
- Kouzmenko AP, Takeyama K, Ito S, Furutani T, Sawatsubashi S, Maki A, Suzuki E, Kawasaki Y, Akiyama T, Tabata T, Kato S. Wnt/beta-catenin and estrogen signaling converge in vivo. J Biol Chem. 2004;279(39):40255–40258. doi: 10.1074/jbc.C400331200. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127(3):457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Li Z, Nie F, Wang S, Li L. Histone H4 Lys 20 monomethylation by histone methylase SET8 mediates Wnt target gene activation. Proc Natl Acad Sci U S A. 2011;108(8):3116–3123. doi: 10.1073/pnas.1009353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123(1):131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nishioka K, Rice JC, Sarma K, Erdjument-Bromage H, Werner J, Wang Y, Chuikov S, Valenzuela P, Tempst P, Steward R, Lis JT, Allis CD, Reinberg D. PR-Set7 is a nucleosome-specific methyltransferase that modifies lysine 20 of histone H4 and is associated with silent chromatin. Mol Cell. 2002;9(6):1201–1213. doi: 10.1016/s1097-2765(02)00548-8. [DOI] [PubMed] [Google Scholar]
- Numpang B, Ke X, Yu X, Callaway C, McKnight R, Joss-Moore L, Lane R. Fetal growth restriction alters hippocampal 17-beta estradiol and estrogen receptor alpha levels in the newborn male rat. Syst Biol Reprod Med. 2013;59(4):184–190. doi: 10.3109/19396368.2013.786767. [DOI] [PubMed] [Google Scholar]
- O’Grady SP, Caprau D, Ke XR, Contreras Y, Haley S, Ermini F, Penn A, Moyer-Mileur L, McKnight R, Lane R. Intrauterine growth restriction alters hippocampal expression and chromatin structure of Cyp19a1 variants. Syst Biol Reprod Med. 2010;56(4):292–302. doi: 10.3109/19396368.2010.490871. [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978;139(2):295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Ou Z, Zhao X, Labiche LA, Strong R, Grotta JC, Herrmann O, Aronowski J. Neuronal expression of peroxisome proliferator-activated receptor-gamma (PPARgamma) and 15d-prostaglandin J2--mediated protection of brain after experimental cerebral ischemia in rat. Brain Res. 2006;1096(1):196–203. doi: 10.1016/j.brainres.2006.04.062. [DOI] [PubMed] [Google Scholar]
- Padilla N, Junque C, Figueras F, Sanz-Cortes M, Bargallo N, Arranz A, Donaire A, Figueras J, Gratacos E. Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res. 2014;1545:1–11. doi: 10.1016/j.brainres.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199(2):265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD, G. Rosiglitazone in Alzheimer’s Disease Study Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6(4):246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rivera J, Denner L, Dineley KT. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav Brain Res. 2011;216(1):255–261. doi: 10.1016/j.bbr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth AD, Leisewitz AV, Jung JE, Cassina P, Barbeito L, Inestrosa NC, Bronfman M. PPAR gamma activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J Neurosci Res. 2003;72(4):425–435. doi: 10.1002/jnr.10596. [DOI] [PubMed] [Google Scholar]
- Schober ME, McKnight RA, Yu X, Callaway CW, Ke X, Lane RH. Intrauterine growth restriction due to uteroplacental insufficiency decreased white matter and altered NMDAR subunit composition in juvenile rat hippocampi. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R681–692. doi: 10.1152/ajpregu.90396.2008. [DOI] [PubMed] [Google Scholar]
- Society AP. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;282(6):3. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- Stubley-Weatherly L, Harding JW, Wright JW. Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res. 1996;716(1-2):29–38. doi: 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- Sung IK, Vohr B, Oh W. Growth and neurodevelopmental outcome of very low birth weight infants with intrauterine growth retardation: comparison with control subjects matched by birth weight and gestational age. J Pediatr. 1993;123(4):618–624. doi: 10.1016/s0022-3476(05)80965-5. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic, kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7(2):133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- Talasz H, Lindner HH, Sarg B, Helliger W. Histone H4-lysine 20 monomethylation is increased in promoter and coding regions of active genes and correlates with hyperacetylation. J Biol Chem. 2005;280(46):38814–38822. doi: 10.1074/jbc.M505563200. [DOI] [PubMed] [Google Scholar]
- Vakoc CR, Sachdeva MM, Wang H, Blobel GA. Profile of histone lysine methylation across transcribed mammalian chromatin. Mol Cell Biol. 2006;26(24):9185–9195. doi: 10.1128/MCB.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24(6):1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- von Beckerath AK, Kollmann M, Rotky-Fast C, Karpf E, Lang U, Klaritsch P. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol. 2013;208(2):130 e131–136. doi: 10.1016/j.ajog.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Okamura M, Tsutsumi S, Nishikawa NS, Tanaka T, Sakakibara I, Kitakami J, Ihara S, Hashimoto Y, Hamakubo T, Kodama T, Aburatani H, Sakai J. The peroxisome proliferator-activated receptor gamma/retinoid X receptor alpha heterodimer targets the histone modification enzyme PR-Set7/Setd8 gene and regulates adipogenesis through a positive feedback loop. Mol Cell Biol. 2009;29(13):3544–3555. doi: 10.1128/MCB.01856-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13(11) doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20(11):1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, Grotta JC, Aronowski J. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J Neurosci. 2009;29(19):6186–6195. doi: 10.1523/JNEUROSCI.5857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d-Prostaglandin J2 activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2006;26(6):811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci U S A. 1995;92(17):7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]