Abstract
Extracellular acidification activates a family of proteins known as acid-sensing ion channels (ASICs). One ASIC subtype, ASIC type 1 (ASIC1), may play an important role in synaptic plasticity, memory, fear conditioning and ischemic brain injury. ASIC1 is found primarily in neurons, but one report showed its expression in isolated mouse cerebrovascular cells. In this study, we sought to determine if ASIC1 is present in intact rat and human major cerebral arteries. A potential physiological significance of such a finding is suggested by studies showing that nitric oxide (NO), which acts as a powerful vasodilator, may modulate proton-gated currents in cultured cells expressing ASIC1s. Because both constitutive NO synthesizing enzymes, neuronal nitric oxide synthase (nNOS) and endothelial NOS (eNOS), are expressed in cerebral arteries we also studied the anatomical relationship between ASIC1 and nNOS or eNOS in both rat and human cerebral arteries. Western blot analysis demonstrated ASIC1 in cerebral arteries from both species. Immunofluorescent histochemistry and confocal microscopy also showed that ASIC1-immunoreactivity (IR), colocalized with the smooth muscle marker alpha-smooth muscle actin (SMA), was present in the anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA) and basilar artery (BA) of rat and human. Expression of ASIC1 in cerebral arteries is consistent with a role for ASIC1 in modulating cerebrovascular tone both in rat and human. Potential interactions between smooth muscle ASIC1 and nNOS or eNOS were supported by the presence of nNOS-IR in the neighboring adventitial layer and the presence of nNOS-IR and eNOS-IR in the adjacent endothelial layer of the cerebral arteries.
Keywords: Acid-sensitive ion channels, nitric oxide synthase, cerebral artery, Western blot, immunofluorescent histochemistry, human
Introduction
Acid-sensitive ion channels (ASICs) are a class of proton-gated cation channels that are widely distributed in the central and peripheral nervous system (CNS and PNS, respectively) where they are activated in response to decreases in extracellular pH as may occur with inflammation, ischemic stroke, traumatic brain injury and epileptic seizure (Lingueglia, 2007; Waldmann, 2001). The ASIC channel consists of three homomeric or heteromeric subunits encoded by four genes (Chu and Xiong, 2013). There are seven types of ASIC subunits (1a, 1b1, 1b2, 2a, 2b, 3 and 4), and each type of ASIC subunit consists of two transmembrane domains (TM1 and TM2) and a large cysteine-rich loop (Chu and Xiong, 2013). In addition to their acting as chemosensors responding to extracellular acidosis, some ASIC channels are also implicated in mechano-transduction (Chung et al., 2010).
ASIC type 1 (ASIC1) is the most broadly expressed channel of the ASIC family in CNS areas including the olfactory bulb, cerebral cortex, hippocampus, basolateral amygdaloid nuclei, subthalamic nuclei and cerebellum (Alvarez et al., 2003). Although the function of ASIC1 in the CNS has not been established, studies have shown that homomeric ASIC1a plays an important role in normal brain functions such as synaptic plasticity, learning/memory, and fear conditioning (Ziemann et al., 2009). In addition, ASIC1 channels may contribute to development of a number of pathological conditions (Pignataro et al., 2011; Xiong et al., 2004). For example, loss of ASIC1a function after intracerebroventricular injection of an ASIC1a blocker or in ASIC1a gene knockout animals protects the brain from ischemic injury (Xiong et al., 2004). Also, pre- or post-conditioning modulates expression of ASIC1a during ischemia (Pignataro et al., 2011). Therefore, it is plausible that ASIC1 may play a role in the development of acidosis-mediated ishemic brain damage in stroke. ASIC1 is predominantly expressed in axons, axon terminals and cell bodies in the CNS, PNS and cultured neurons (Zha, 2013) but also may be found in vascular smooth muscle cells where it may regulate muscle cell migration and influence vascular tone (Drummond et al., 2008b; Grifoni et al., 2008).
A vascular and neuronal origin for ASIC is shared with nitric oxide (NO), a short-lived mediator that plays an important role in modulation of cerebral blood flow and may provide tonic vasodilatory influences on cerebral vessels (McCarron et al., 2006; Talman et al., 2007). Neuronal nitric oxide synthase (nNOS), the enzyme responsible for neuronal synthesis of NO, is found in nerve fibers that surround and innervate cerebral arteries in experimental animals and in humans (Nozaki et al., 1993; Taktakishvili et al., 2010). NO may modify the function of a wide variety of proteins, including ASICs (Wang et al., 2012), by two major pathways (Ahern et al., 2002). One pathway involves activation of soluble guanylate cyclase, which produces cGMP and through it activates protein kinase G (PKG) to affect other proteins (Potter, 2011). Another pathway directly modifies the tertiary structure of proteins by S-nitrosylation of the thiol side chains of neighboring cysteine (Marozkina and Gaston, 2012). One such S-nitrosylated product, S-nitroso-N-acetyl-penicillamine (SNAP), itself an NO donor, has been shown to modulate ASIC1 activity and to potentiate proton-gated currents in rat cultured dorsal root ganglion neurons and in Chinese hamster ovary cells expressing ASIC1a or ASIC1b (Cadiou et al., 2007).
Although ASIC1 (both ASC1a and ASIC1b) expression has been found in isolated mouse cerebral artery smooth muscle cells (Chung et al., 2010), there have been no studies to determine if ASIC1 is present in intact cerebral arteries from rats or humans and, if so, in what layers the ASIC1 may be found. We hypothesized that ASIC1 is present in cerebral arteries and that it is expressed in close proximity to nNOS expressing nerve fibers that are found in the adventitial layer of the arteries. In this study, utilizing an antibody that recognizes both ASIC1a and 1b, we performed Western blot analyses and immunofluorescent histochemistry with confocal microscopy in the human and rat anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA) and basilar artery (BA). To determine if there is an anatomical basis for an interaction between ASIC1 and nNOS in cerebral arteries, we performed multiple-labeling immunofluorescent histochemistry for ASIC1 and nNOS in cerebral arteries. Because endothelial NOS (eNOS) is a major source of NO in blood vessels, we also performed multiple-labeling immunofluorescent histochemistry for ASIC1 and eNOS in these cerebral arteries.
Experimental Procedures
1. Animals and tissue preparation
All procedures conformed to standards established in Guide for Care and Use of Laboratory Animals (National Academy Press, Washington, D.C. 2011). The Institutional Animal Care and Use Committees of the University of Iowa and Department of Veterans Affairs Medical Center, Iowa City reviewed and approved all protocols. Both institutions are accredited by AAALAC, International. All efforts were made to minimize the number of animals used and to avoid their experiencing pain or distress. As the studies on human tissues were performed on post mortem material obtained through the Autopsy Service at the University of Iowa Hospitals and Clinics, approval was not required from the Institutional Review Board (IRB).
For studies utilizing Western blot analysis of rat cerebral vessels to validate the ASIC antibody, we euthanized adult male Sprague-Dawley rats (280 – 330g) under deep pentobarbital (150 mg/kg) anesthesia as we have previously described (Lin et al., 2011). The brains were then removed and placed on ice. The ACA, MCA, PCA and BA from 6 rats were carefully dissected from surrounding tissue. Because the amount of vascular tissue from any single rat was insufficient for analysis, we divided the 6 rats into 2 groups of 3 rats per group, pooled together tissues from each group, and homogenized the pooled tissue for Western blot analysis (see below). Although the majority of the dissected tissue consisted of ACA, MCA, PCA and BA, it also contained part of the basal vein, which runs along part of the ACA and part of the PCA (Greene, 1970), and small veins that run along these cerebral arteries. A piece of parietal cortex (approximately 50 mg) was also removed from one rat in each group and was homogenized for Western blot analysis to provide comparison between ASIC1 in predominantly cortical tissue vs. predominantly vascular tissue.
For immunofluorescent staining of rat cerebral arteries, we euthanized and perfused adult male Sprague-Dawley rats (280 – 330 g, n = 5) under pentobarbital (50 mg/kg) anesthesia according to procedures described in our earlier publications (Lin et al., 2011; Lin et al., 2007; Lin and Talman, 2005a). The brain was then removed, post-fixed in 4% paraformaldehyde for 2 h and then cryo-protected for 2 days in 30% sucrose in phosphate buffered saline (PBS) at 4°C. Frozen 20 μm coronal sections were cut with a cryostat and mounted on Colorfrost Plus microscope slides (Fisher Scientific, PA, USA). Brain sections that contained the ACA, MCA, PCA and BA were processed for immunofluorescent staining as will be described later.
2. Preparation of human cerebral arteries
Cerebral arteries were collected from five patients at necropsy approximately 15–22 h after each patient’s death. One patient had died of lung cancer (age 74), three of septic shock in the setting of enterococcal bacteremia, coagulopathy, and pneumonia (age 75, 63 and 16, respectively) and one of heart failure associated with mitral valve prolapse (age 84). We obtained an un-fixed 1 cm segment from each of the ACA, MCA, PCA and BA. Tissue was fixed in 4% paraformaldehyde for 1 h at 4°C and then cryo-protected for 1 h in 30% sucrose at 4°C. Frozen 30 μm cross sections were cut with a cryostat and processed for immunofluorescent staining. We also obtained a 0.5 cm un-fixed segment from each of the ACA, MCA, PCA and BA from the same subjects and processed each piece of tissue from each subject for Western blot analysis (see below).
3. Western blot analysis of ASIC1
Procedures like those described in our previous publications (Lin et al., 2012; Lin and Talman, 2005b) were used for Western blot analysis of ASIC1. In brief, we homogenized tissue in homogenization buffer containing 2% sodium dodecyl sulphate (SDS), 1 mM phenylmethylsulfonyl flouride, 1 mM dithiothreitol and 1 mM EDTA in Tris buffered saline, pH 7.4, using disposable polypropylene pestles in 1.5 ml microcentrifuge tube (Kimble Chase, NY, USA). The volume of homogenization buffer was 120 μl for pooled rat cerebral arteries, 300 μl for 50 mg rat cortex, and 150 μl for each segment of 0.5 cm human cerebral arteries. After centrifugation, protein concentration of the supernate was determined by means of Bio-Rad DC Protein Assay (Bio-Rad Laboratories, CA, USA). Supernate containing 10 μg (rat) or 50 μg (human) protein was separated alongside Bio-Rad Precision Plus Proteins Standards (Bio-Rad Laboratories) by 7.5 % SDS-polyacrylamide gel electrophoresis (Ready Gel, Bio-Rad Laboratories) using the Mini Protein II System (Bio-Rad Laboratories) according to Laemmli (Laemmli U.K., 1970). The separated proteins were transferred to nitrocellulose membrane (Bio-Rad Laboratories) using the Mini Trans-Blot Cell (Bio-Rad Laboratories). The blot was blocked in 10% milk in PBS and then incubated with ASIC1 antibody (1:1000 dilution, Santa Cruz Biotechnology, TX, USA) at 4°C for 24 h. After thorough washes, the blot was incubated with horseradish peroxidase-conjugated anti-rabbit antibody (1:10,000 dilution, Jackson ImmunoResearch Lab., PA, USA) at 25°C for 4 hr. Protein bands were visualized with ECL Plus™ Western Blotting Reagents (GE Healthcare/Amersham Biosciences, South San Francisco, CA, USA) and exposed to X-ray films. Because ASICs are assembled from homomultimeric or heteromultimeric subunits and because the exact subunit combination of ASICs in native tissue is not clear, there are no accepted molecular weights of ASIC1a and 1b. Therefore, molecular weights of visualized bands were compared with known (Chung et al., 2010; Grifoni et al., 2008; Jahr et al., 2005) molecular weights of ASIC1 protein.
4. Immunofluorescent histochemistry
Procedures similar to those described in our previous publications (Lin et al., 2007; Lin and Talman, 2005a; Lin and Talman, 2006) were used for immunofluorescent staining of rat and human tissue sections. Immunofluorescent histochemistry for ASIC1 was performed with or without biotin-streptavidin amplification. Sections were incubated in rabbit anti-ASIC1 antibody (1:100 dilution with biotin-streptavidin amplification, 1:10 dilution without biotin-streptavidin amplification, Santa Cruz Biotechnology, catalogue number SC-28756) in 10% donkey normal serum for 24 h in a humid chamber at 25°C. An epitope corresponding to amino acids 505–574 mapping at the C-terminus of ASIC1 of human origin was used as immunogen for the antibody. This antibody recognizes isoforms a and b of mouse and rat origin as a single band of predicted molecular weight of ASIC1 by Western blotting (information provided by Santa Cruz Biotechnology, also see Results for our confirming the specificity of this antibody). For immunofluorescent staining of ASIC1 with biotin-streptavidin amplification, sections were washed with PBS after being incubated with primary antibody. They were then incubated with biotin-conjugated donkey anti-rabbit IgG (1:200 dilution, Jackson ImmunoResearch Labs.) in PBS for 20–24 h at 4°C. They were washed again and incubated with DyLight 488-conjugated streptavidin (1:200 dilution, Jackson ImmunoResearch Labs.) in PBS for 20–24 h at 4°C. For immunofluorescent staining of ASIC1 without biotin-streptavidin amplification, sections were incubated with rhodamine red X (RRX)-conjugated donkey anti-rabbit IgG (1:200 dilution, Jackson ImmunoResearch Labs.) in PBS for 20–24 h at 4°C after they have been incubated with primary ASIC1 antibody and washed. Stained sections were cover-slipped with Prolong Gold Anti-fade Reagents (Invitrogen-Molecular Probes) after the final washes with PBS and then were examined with a confocal microscope (see below). Negative controls consisted of tissue processed in the absence of primary antibodies.
As we have previously described (Lin et al., 2011; Lin et al., 2008) procedures were used for multiple-label immunofluorescent staining of ASIC1 and alpha-smooth muscle actin (SMA); ASIC1 (both with and without biotin-streptavidin amplification), nNOS and eNOS; nNOS and rat endothelial cell antigen (RECA-1). Sections were incubated in 10% donkey serum with a mixture of primary antibodies that were made in different species. The sheep nNOS antibody was a gift from Dr. Piers C. Emson. The specificity of anti-nNOS, which we have also used in previous published studies, has been demonstrated (Herbison et al., 1996; Lin et al., 2004; Lin and Talman, 2006; Simonian and Herbison, 1996). The mouse SMA antibody (1:100 dilution of ascites fluid, Sigma, MO, USA) was generated by using an NH2 terminal synthetic decapeptide of SMA coupled to keyhole limpet hemocyanin. The antibody has been shown to react specifically with alpha-smooth muscle actin in immunoblotting analysis (Skalli et al., 1986). The specificity of mouse antibody for eNOS (1:10 dilution, BD Transduction Labs., CA, USA) has also been shown by its recognition of a single band corresponding to the molecular weight of eNOS (Cao et al., 2001). The antibody to endothelial cell marker, RECA-1, made in mouse (1:2000 dilution, AbD Serotec, NC, USA), has also been shown to be specific (Duijvestijn et al., 1992). After washing, sections were incubated in a mixture of donkey fluorophore-conjugated secondary antibodies (DyLight 488, RRX or DyLight 649, all in 1:200 dilution, Jackson ImmunoResearch Labs., against species from which the primary antibodies were made) for multiple-labeling, washed and cover-slipped as described above.
5. Confocal laser scanning microscopy
As previously described (Lin et al., 2007; Lin and Talman, 2006) we analyzed stained sections with a Zeiss LSM 710 confocal laser scanning microscope. Multiple-labeled sections were scanned sequentially in different channels to separate labels. Images from different channels were each assigned a pseudo-color and then were superimposed. Confocal images were obtained and processed with software provided with the Zeiss LSM 710. Adobe Photoshop image editing software (Adobe Photoshop CS2) was used as we switched between channels on the monitor to determine if a structure was labeled by one or more antibodies. Another image editing program, Microsoft PowerPoint (2010), was used to create montages. Images were not otherwise manipulated.
Results
1. Specificity of ASIC1 antibody
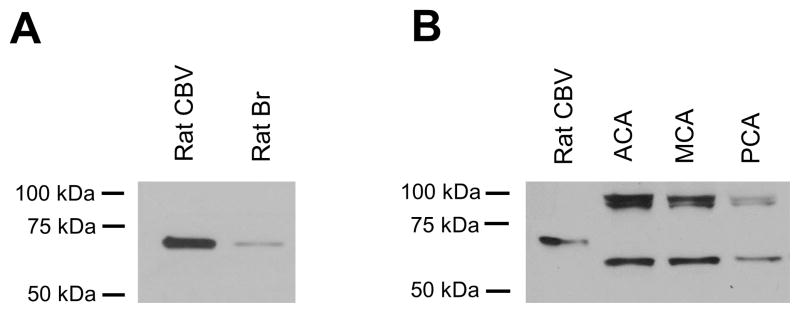
A single clear band of predicted molecular weight (about 70 kDa) was seen in pooled rat cerebral arteries by Western blot analysis and a band of the same molecular weight was found in the homogenate of rat cortex (Fig. 1A). In that a band of similar molecular weight was reported in an earlier study that used a different antibody to analyze tissue from different areas of rat brain (Alvarez et al., 2003) the current data support specific labeling of ASIC1 by the antibody used in this study.
Fig. 1.
Western blots of ASIC1 in rat and human cerebral blood vessels. (A) A major band is seen in rat cerebral blood vessels (lane: Rat CBV) and rat cortex (lane: Rat Br) homogenate. (B) ASIC1 is seen as two major bands in human cerebral blood vessels (lanes: ACA, MCA and PCA). Rat cerebral blood vessels (lane: Rat CBV) are included in this blot for size comparison. Molecular weight markers are shown on the left side of each blot.
2. Expression of ASIC1 in rat and human cerebral blood vessels detected by western blot analysis
We included rat cerebral blood vessels in a different lane in the same Western blot for human cerebral blood vessels for size comparison. Similar to the blot obtained above (Fig. 1A), a single band of the same molecular weight (70 kDa) was noted for rat cerebral blood artery homogenates (Fig. 1B). In contrast, two major bands of different molecular weights were observed in homogenates of human ACA, MCA, PCA (Fig. 1B), and BA (not shown because western blot analysis of BA, though showing the same result, was run separate from the other 3 vessel types). Again validating the specificity of the ASIC antibody used in this study the two bands corresponded to the 55 kDa and 90 kDa signal of ASIC1 previously found in COS-7 cells transfected with human ASIC1 (Leonard et al., 2003).
3. Presence of ASIC1 in rat and human cerebral blood vessels by immunofluorescent histochemistry
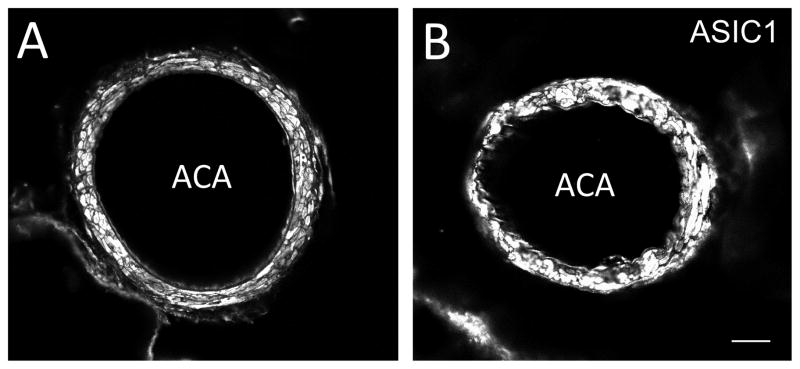
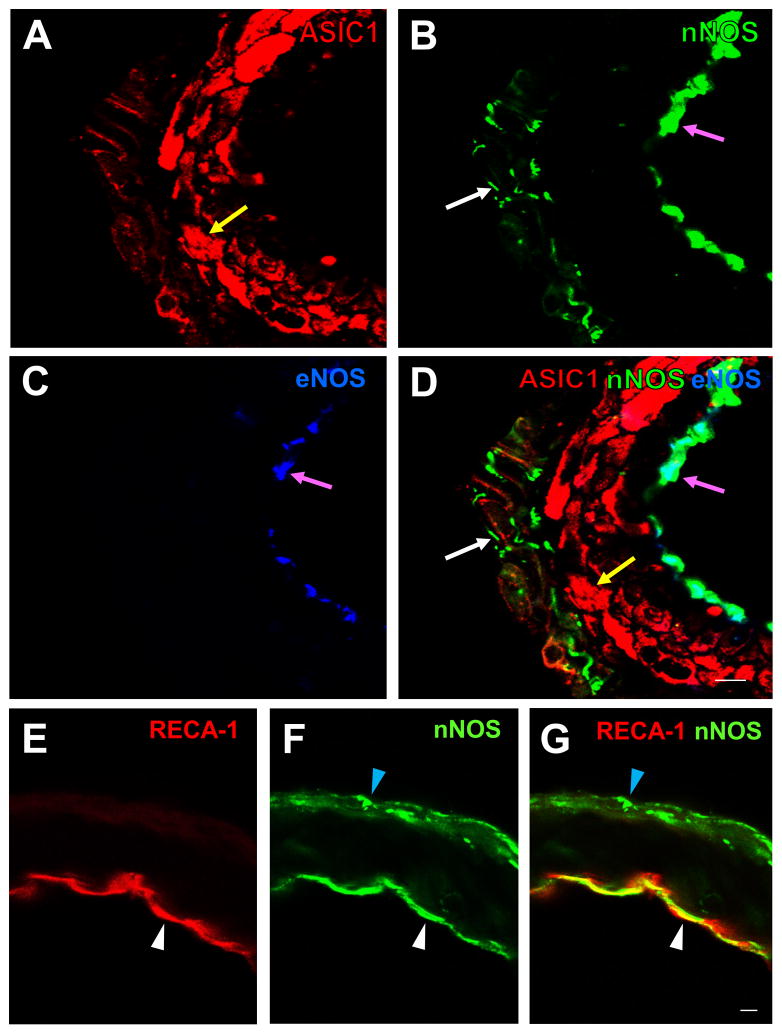
In the negative controls (omission of ASIC1 antibody), there was no ASIC1 immunoreactivity (IR) (Fig. 2A). In contrast, ASIC1-IR was observed in rat ACA, MCA, PCA and BA incubated with ASIC1 antibody (Fig. 2B–E). Similar staining patterns of ASIC1 in these arteries were observed when immunofluorescent staining was performed with (Fig. 3A) or without (Fig. 3B) biotin-streptavidin amplification. Double-labeling immunofluorescent staining showed that the majority of ASIC1-IR colocalized with SMA-IR (Fig. 2F–H). Thus, ASIC1-IR was present in the smooth muscle layer of rat cerebral blood vessels. When the arteries were processed for triple-labeling for ASIC1, nNOS and eNOS, we observed ASIC1-IR in proximity to nNOS-IR, which was present in nerve fibers in the adventitia layer and in endothelial cells in the endothelial layer of the rat cerebral blood vessel (Fig. 4A–D). Localization of nNOS-IR (Fig. 4B) to the endothelium was confirmed by presence of eNOS-IR (Fig. 4C–D) and the endothelial cell marker, RECA-1 (Fig. 4 E–G) in the tissue. Note that ASIC1-IR is also in proximity of eNOS-IR (Fig. 4A–D).
Fig. 2.
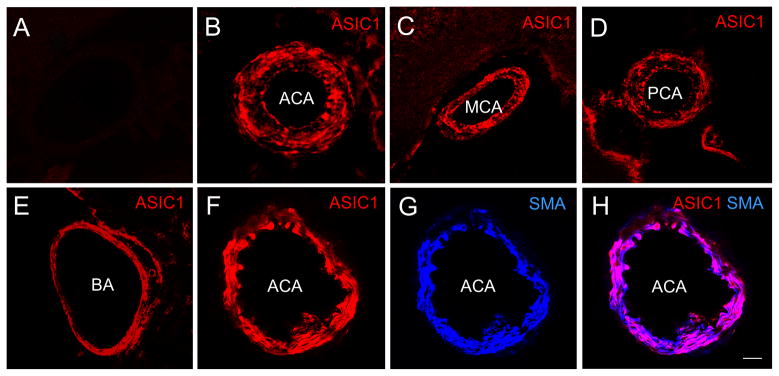
Confocal images showing the presence of ASIC1-IR in rat cerebral arteries. No ASIC1-IR was noted in the negative control (A). In contrast, ASIC1-IR was observed in rat ACA (panel B, RRX) when ASIC1 antibody was present. Similar to what was observed in the ACA, ASIC1-IR was present in the MCA (panel C), PCA (panel D) and BA (panel E). Double-labeling fluorescent immunostaining of ASIC1 (panel F, RRX, red) and alpha-smooth muscle actin (SMA, panel G, DyLight 649, blue), a marker for smooth muscle, shows that ASIC1-IR and SMA-IR colocalizes and appears as purple in the merged image in H. That colocalization confirms that ASIC1-IR is located in the smooth muscle layer of the ACA. Colocalization of ASIC1-IR and SMA-IR was also seen in the MCA, PCA and BA (not shown). Scale bar = 50 μm.
Fig. 3.
Confocal images showing similar patterns of ASIC1-IR in rat ACA either with (A) or without (B) biotin-streptavidin amplification in immunofluorescent staining procedures. A higher dilution (1:100) of the ASIC1 antibody was used when the procedures included biotin-streptavidin amplification. A lower dilution (1:10) of the antibody was used without biotin-streptavidin amplification. Scale bar = 50 μm.
Fig. 4.
(A–D): High magnification confocal images of rat ACA showing triple-labeling fluorescent immunostaining for ASIC1 (A, red, RRX), nNOS (B, green, DyLight 488), and eNOS (C, blue, DyLight 649). ASIC1-IR (yellow arrow in A and D) is present in the smooth muscle layer, nNOS-IR in the adventitial layer (white arrows in B and D) and the endothelial layer (pink arrows in B and D), and eNOS-IR in the endothelial layer (pink arrows in C and D). Similar localization of ASIC1-IR, nNOS-IR and eNOS was also seen in the MCA, PCA and BA (not shown). A merged image (D) shows that nNOS-IR colocalizes with eNOS-IR in the endothelial layer (pink arrows in B, C and D) but not in the adventitial layer. (E–G): Confocal images of rat ACA showing double-labeling fluorescent immunostaining for the endothelial cell marker RECA-1 (E, red, RRX) and nNOS (F, green, DyLight 488). A merged image (G) shows that nNOS-IR colocalizes with IR of RECA-1 in the endothelial layer (white arrow heads in E–G), but not in the adventitial layer where RECA-1 was not found (blue arrow heads in F and G). Scale bar = 10 μm.
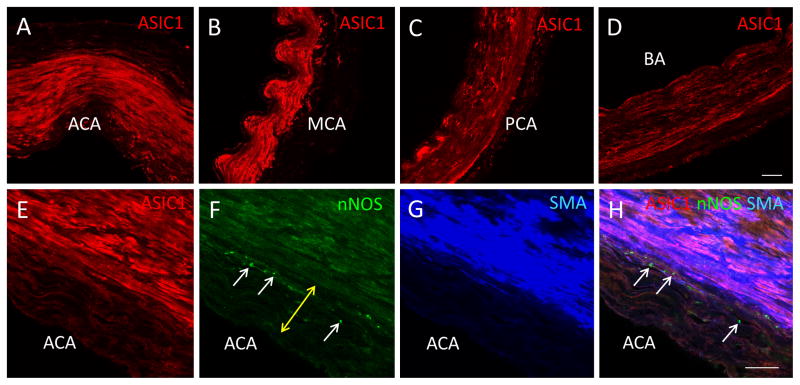
ASIC1-IR was also noted in the human ACA, MCA, PCA and BA (Fig. 5A–D) even though these cerebral arteries were collected 15–22 h after each patient’s death and postmortem degradation might have occurred. In addition, staining intensity of ASIC1-IR in cerebral arteries was not consistent among patients. This lack of consistency could have resulted from the different causes of death. We did not notice any difference between the staining intensity of ASIC1-IR in cerebral arteries from the only young patient and those from older patients. Multiple-labeling immunofluorescent staining showed that ASIC1-IR also colocalized with SMA-IR in human cerebral blood arteries (Fig. 5E–H) as in rat cerebral blood arteries. We observed similar colocalization of ASIC1-IR and SAM-IR in cerebral blood arteries from different patients. ASIC1-IR in human cerebral arteries was located in proximity to nerve fibers that were positive for nNOS-IR in the adventitia layer (Fig. 5E–H) and in the nNOS-IR and eNOS-IR positive endothelial cells (not shown).
Fig. 5.
Presence of ASIC1-IR in human cerebral arteries. We observed ASIC1-IR in human ACA (panel A, red, RRX), MCA (panel B, red, RRX), PCA (panel C, red, RRX) and BA (panel D, red, RRX). Triple-labeling immunofluorescent staining of human ACA shows that ASIC1-IR (panel E, red, RRX) is in close proximity to nNOS-IR (panel F, green, DyLight 488) positive nerve fibers (white arrows in F and H) in the adventitia layer (indicated by the yellow double-headed arrow in F) and endothelial layer (not shown). Colocalization of ASIC1-IR and SMA-IR (G, blue, DyLight 649) is demonstrated in a merged image in H. Thus, as in the rat, ASIC1-IR is present in the smooth muscle layer of human ACA. A similar distribution of ASIC1-IR, nNOS-IR and SMA-IR was also noted in human MCA, PCA and BA (not shown). Scale bar = 100 μm.
Discussion
We have demonstrated by both Western blot analysis and immunofluorescent histochemistry that ASIC1-IR was expressed in the ACA, MCA, PCA and BA of rat and human. We also showed that ASIC1-IR, located in the smooth muscle layer, nNOS-IR, located in the adventitia layer and endothelial layer, and eNOS-IR, located in the endothelial layer, were in proximity in these cerebral arteries.
Our observation that ASIC1-IR was present in the smooth muscle layer of rat ACA, MCA, PCA and BA is consistent with an earlier observation that ASIC1 was detected in mouse isolated cerebral vascular smooth muscle cells (Chung et al., 2010). Most importantly, we also demonstrated that ASIC1-IR was found in human ACA, MCA, PCA and BA as it was also in rat. In Western blot analysis we observed one major band of correct molecular weight in rat cerebral vessels, but we observed two bands in human samples. Different sizes of ASIC have been reported previously and were thought to have been due to glycosylation, multimer assembly or species differences in ASIC subunits (Chung et al., 2010; Grifoni et al., 2008; Jahr et al., 2005). We detected a stronger signal for ASIC1 in rat cerebral blood vessels and a weaker one in rat cerebral cortex. However, that result does not necessarily mean that rat cerebral blood vessels contain a higher level of ASIC1 than rat cerebral cortex. An alternative explanation would be that the antibody we used recognizes a form of ASIC1 that is enriched in cerebral blood vessels in contrast to cortex. This conjecture is in line with suggestions that signal intensities in Western blot may vary due to differences in antibody sources and that conclusions regarding qualities and quantities of antigen should be drawn with caution (Jahr et al., 2005). Such variances may also explain why we detect a stronger ASIC1 signal in rat cerebral arteries than in rat brain, although an earlier paper (Chung et al., 2010) detected a stronger ASIC1 signal in mouse brain than isolated mouse cerebral vessels. Of note, however, the antibodies against ASIC1a and ASIC1b used in that paper were different from the one we used. In addition, we acknowledge that some of the human samples came from patients who died of conditions that could affect blood pH and oxygenation. As a result, ASIC1 expression in these cerebral arteries might have been altered and may not reflect normal ASIC1 qualities and quantities in health human subjects.
As mentioned in the Methods section, the antibody we used recognizes both isoforms a and b of ASIC1. Therefore, our data do not indicate which type of ASIC1 represented the majority of ASIC1-IR found in rat and human cerebral arteries. However, because an earlier report has shown a strong ASIC1b immunoreactivity and very weak ASIC1a immunoreactivity in isolated cerebral vascular smooth muscles cells (Chung et al., 2010), it is likely that the ASIC1-IR we observed in the cerebral arteries was mostly represented by ASIC1b. This suggestion was supported by our observation that ASIC1-IR was observed in cerebral arteries of ASIC1a knockout mouse (a generous gift from Dr. John A. Wemmie) using the ASIC1 antibody we used in this study (unpublished observation). Unfortunately, neither an ASIC1b knockout animal nor a combined ASIC1a and b knockout animal is available. Therefore, it is not possible to conclude unequivocally that ASIC1b is the major form of ASIC1 in cerebral arteries. Similarly, with regard to the specificity of the antibody we used, although our Western blot analysis does support the specificity of the antibody, a complete validation of this antibody awaits availability of a combined ASIC1a and ASIC1b knockout animal (Baek et al., 2013).
Our double-labeling immunofluorescent staining of ASIC1 and SMA provides evidence that ASIC1 is located in the smooth muscle layer of rat and human ACA, MCA, PCA and BA. The precise role of ASIC1 in the vascular smooth muscle is not yet clear. However, an earlier study showed that the specific ASIC1 inhibitor psalmotoxin 1, a toxin isolated from tarantula venom, inhibited vasoconstriction in pulmonary arteries (Jernigan et al., 2009). Supporting a role of ASIC1 in modulating vasoconstriction, treatment of pulmonary arteries with ASIC1 siRNA also resulted in inhibition of vasoconstriction (Jernigan et al., 2009). Our finding that ASIC1-IR was present in cerebral arteries would support the suggestion that ASIC1 may play a similar role in maintaining vascular tone in cerebral blood vessels as it does in pulmonary arteries. With ASIC1 being found in vascular smooth muscle it could be well positioned to influence smooth muscle contractility, which is regulated by changes in intracellular calcium concentration (Marchand et al., 2012). Indeed, psalmotoxin 1 and ASIC1 siRNA have been found to inhibit release of calcium from intracellular stores and thus to inhibit calcium entry in pulmonary arteries (Jernigan et al., 2009). In isolated cerebral artery smooth muscle cells, psalmotoxin 1 has been found to abolish acidosis-induced currents (Chung et al., 2010). These findings suggest that ASIC1 could modulate vascular tone by influencing calcium trafficking in cerebral blood vessels, but future studies are required to delineate the role of ASIC1 in cerebral blood vessels.
Given that nNOS-IR has been found in nerve fibers in the adventitia of cerebral arteries, it was not surprising that nNOS-IR was in proximity to ASIC1-IR, which was found in the adjacent smooth muscle layer. Currently, it is not clear how NO may affect the function of ASIC1 in cerebral vasculature, but the proximity we show now provides an anatomical basis for such an interaction given the need for that proximity due to limited diffusion of NO from its source (Garthwaite, 1995; Vanderkooi et al., 1994). As mentioned in the Introduction, the NO donor SNAP potentiates proton-gated currents in neonatal rat cultured dorsal root ganglion neurons and proton-gated currents in Chinese hamster ovary cells expressing ASIC1 (Cadiou et al., 2007). Another NO donor, sodium nitroprusside, and the NO precursor, L-arginine, also potentiated ASICs in a neural crest-derived cell line N2A (Jetti et al., 2010). This potentiation can be blocked completely by nNOS inhibitor 7-nitroindazole (Jetti et al., 2010). Taken together, these studies suggested that NO may influence ASIC1 activity. It has been suggested that S-nitrosylation of cysteine residues in the extracellular loop and not activation of guanylate cyclase may be the mechanism through which NO may affect ASIC1 (Cadiou et al., 2007). The physiological significance of the effect of NO on ASIC1 in blood vessels is unknown. However, because activation of ASIC channels leads to membrane depolarization and vasoconstriction, it is unlikely that ASIC channels function as mediators of acid-mediated dilation (Chung et al., 2010). It has been postulated that ASIC channels may serve as a counter-force to acid-induced dilation and prevent excessive dilation (Chung et al., 2010). Similarly vasoconstriction associated with ASIC1 may provide feedback control in blood vessels after NO-induced dilation and thus prevent uncontrollable vasodilation. These conjectures about ASIC/NO integration await physiological studies to test the hypotheses.
A possible interaction between ASIC and NO is also supported by the close proximity not only of nNOS and ASIC but also eNOS and ASIC. In general, eNOS is considered the main source of NO in the vasculature (Moncada and Higgs, 2006). However, consistent with our observations, several studies have demonstrated that nNOS is also found in endothelial cells (Bachetti et al., 2004; Daneshtalab and Smeda, 2010; Lekontseva et al., 2011). Although the functional significance of nNOS in the endothelium is not known, its role in vasorelaxation has been demonstrated (Capettini et al., 2011). Therefore, it is possible that NO produced either in the endothelium by eNOS or nNOS on in the adventitia by nNOS may interact with ASIC1 in cerebral blood vessels to contribute to as yet unidentified interactions in vasomotor control. Given that ASIC have been shown to participate in mechanotransduction (Chen and Wong, 2013; Drummond et al., 2008a), it is appealing to conjecture that the ASIC1/NOS proximity could provide an opportunity for a vasoconstrictor (ASIC)/vasodilator (NOS) balance as is seen with autoregulation of cerebral blood flow. In that condition we have shown that loss of nNOS function leads to persistent autoregulatory vasoconstriction even during excessive elevations of arterial blood pressure (Talman and Dragon, 1995). Loss of nNOS then could lead to unchecked ASIC-induced vasoconstriction in response to increasing stretch of cerebral arteries as pressure increases. This intriguing conjecture will, of course, also require further study to establish or refute it.
Conclusions
ASIC1 is expressed in the ACA, MCA, PCA and BA of rat and human. ASIC1 is located in the smooth muscle layer of these cerebral arteries and in proximity of nNOS and eNOS. Our findings are consistent with a role of ASIC1 in modulating cerebrovascular tone and they provide an anatomical basis for potential interactions between ASIC1 and NO in cerebral arteries in rat and human.
Research Highlights.
Major cerebral arteries from rat and human were studied
Western blot analysis demonstrated ASIC1 in these cerebral arteries
Confocal microscopy showed ASIC1 colocalized with SMA in these arteries
ASIC1 and nNOS were present in adjacent layers in these cerebral arteries
Acknowledgments
This work was funded in part by NIH RO1 HL 59593 (to W. T. Talman), NIH RO1 HL 088090 (to L. H. Lin and W. T. Talman), and in part by a Merit Review from the Department of Veterans Affairs.
Abbreviations
- ACA
anterior cerebral artery
- ASIC
acid-sensitive ion channel
- ASIC1
acid-sensitive ion channel type1
- BA
basilar artery
- CNS
central nervous system
- eNOS
endothelial nitric oxide synthase
- IR
immunoreactivity
- MCA
middle cerebral artery
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
- PCA
posterior cerebral artery
- PBS
phosphate buffered saline
- PGP9.5
protein gene product 9.5
- PNS
peripheral nervous system
- RECA-1
rat endothelial cell antigen
- RRX
rhodamine red X
- SDS
sodium dodecyl sulphate
- SMA
alpha-smooth muscle actin
- SNAP
S-nitroso-N-acetyl-penicillamine
Footnotes
Author contribution
L. H. L. and W. T. T. designed experiments; L. H. L. and J. J. performed experiments; L. H. L and W. T. T. analyzed data; L. H. L., J. J., M. B. N, and W. T. T. wrote the article. All authors have approved the final article.
Conflict of Interest
None of the authors has any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25:510–517. doi: 10.1016/s0166-2236(02)02254-3. [DOI] [PubMed] [Google Scholar]
- Alvarez dlR, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol. 2003;546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachetti T, Comini L, Curello S, Bastianon D, Palmieri M, Bresciani G, Callea F, Ferrari R. Co-expression and modulation of neuronal and endothelial nitric oxide synthase in human endothelial cells. J Mol Cell Cardiol. 2004;37:939–945. doi: 10.1016/j.yjmcc.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Baek JH, Darlington CL, Smith PF, Ashton JC. Antibody testing for brain immunohistochemistry: brain immunolabeling for the cannabinoid CB(2) receptor. J Neurosci Methods. 2013;216:87–95. doi: 10.1016/j.jneumeth.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Cadiou H, Studer M, Jones NG, Smith ES, Ballard A, McMahon SB, McNaughton PA. Modulation of acid-sensing ion channel activity by nitric oxide. J Neurosci. 2007;27:13251–13260. doi: 10.1523/JNEUROSCI.2135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC, Shah V. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem. 2001;276:14249–14256. doi: 10.1074/jbc.M006258200. [DOI] [PubMed] [Google Scholar]
- Capettini LS, Cortes SF, Silva JF, Alvarez-Leite JI, Lemos VS. Decreased production of neuronal NOS-derived hydrogen peroxide contributes to endothelial dysfunction in atherosclerosis. Br J Pharmacol. 2011;164:1738–1748. doi: 10.1111/j.1476-5381.2011.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wong CW. Neurosensory mechanotransduction through acid-sensing ion channels. J Cell Mol Med. 2013;17:337–349. doi: 10.1111/jcmm.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Xiong ZG. Acid-sensing ion channels in pathological conditions. Adv Exp Med Biol. 2013;961:419–431. doi: 10.1007/978-1-4614-4756-6_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Farley JM, Swenson A, Barnard JM, Hamilton G, Chiposi R, Drummond HA. Extracellular acidosis activates ASIC-like channels in freshly isolated cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2010;298:C1198–C1208. doi: 10.1152/ajpcell.00511.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshtalab N, Smeda JS. Alterations in the modulation of cerebrovascular tone and blood flow by nitric oxide synthases in SHRsp with stroke. Cardiovasc Res. 2010;86:160–168. doi: 10.1093/cvr/cvp395. [DOI] [PubMed] [Google Scholar]
- Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda ) 2008a;23:23–31. doi: 10.1152/physiol.00034.2007. [DOI] [PubMed] [Google Scholar]
- Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension. 2008b;51:1265–1271. doi: 10.1161/HYPERTENSIONAHA.107.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn AM, van GH, Klatter F, Majoor GD, van BE, van Breda Vriesman PJ. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992;66:459–466. [PubMed] [Google Scholar]
- Garthwaite J. Neural nitric oxide signalling. Trends in Neurosciences. 1995;18:51–52. [PubMed] [Google Scholar]
- Greene EC. The Anatomy of The Rat. New York: Hafner Press; 1970. Circulatory System; pp. 177–336. [Google Scholar]
- Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res. 2008;75:202–210. doi: 10.1016/j.mvr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Endocrinol. 1996;8:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Jahr H, van DM, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun. 2005;337:349–354. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Jernigan NL, Paffett ML, Walker BR, Resta TC. ASIC1 contributes to pulmonary vascular smooth muscle store-operated Ca(2+) entry. Am J Physiol Lung Cell Mol Physiol. 2009;297:L271–L285. doi: 10.1152/ajplung.00020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetti SK, Swain SM, Majumder S, Chatterjee S, Poornima V, Bera AK. Evaluation of the role of nitric oxide in acid sensing ion channel mediated cell death. Nitric Oxide. 2010;22:213–219. doi: 10.1016/j.niox.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural protein during the assemble of the head of bacterophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lekontseva O, Chakrabarti S, Jiang Y, Cheung CC, Davidge ST. Role of neuronal nitric-oxide synthase in estrogen-induced relaxation in rat resistance arteries. J Pharmacol Exp Ther. 2011;339:367–375. doi: 10.1124/jpet.111.183798. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Yermolaieva O, Hruska-Hageman A, Askwith CC, Price MP, Wemmie JA, Welsh MJ. cAMP-dependent protein kinase phosphorylation of the acid-sensing ion channel-1 regulates its binding to the protein interacting with C-kinase-1. Proc Natl Acad Sci U S A. 2003;100:2029–2034. doi: 10.1073/pnas.252782799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Dragon DN, Jin J, Talman WT. Targeting neurons of rat nucleus tractus solitarii with the gene transfer vector adeno-associated virus type 2 to up-regulate neuronal nitric oxide synthase. Cell Mol Neurobiol. 2011;31:847–859. doi: 10.1007/s10571-011-9674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Edwards RH, Fremeau RT, Fujiyama F, Kaneda K, Talman WT. Localization of vesicular glutamate transporters colocalizes with and neuronal nitric oxide synthase in rat nucleus tractus solitarii. Neurosci. 2004;123:247–255. doi: 10.1016/j.neuroscience.2003.08.063. [DOI] [PubMed] [Google Scholar]
- Lin LH, Nitschke DD, Jin J, Tian X, Chu Y, Sigmund C, Talman WT. Decreased expression of neuronal nitric oxide synthase in the nucleus tractus solitarii inhibits sympathetically mediated baroreflex responses in rat. J Physiol. 2012;590:3545–3559. doi: 10.1113/jphysiol.2012.237966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Taktakishvili O, Talman WT. Identification and localization of cell types that express endothelial and neuronal nitric oxide synthase in the rat nucleus tractus solitarii. Brain Res. 2007;1171:42–51. doi: 10.1016/j.brainres.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Taktakishvili OM, Talman WT. Colocalization of neurokinin-1, N-methyl-d-aspartate, and AMPA receptors on neurons of the rat nucleus tractus solitarii. Neurosci. 2008;154:690–700. doi: 10.1016/j.neuroscience.2008.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Talman WT. Nitroxidergic neurons in rat nucleus tractus solitarii express vesicular glutamate transporter 3. J Chem Neuroanat. 2005a;29:179–191. doi: 10.1016/j.jchemneu.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Lin LH, Talman WT. Soluble guanylate cyclase and neuronal nitric oxide synthase colocalize in rat nucleus tractus solitarii. J Chem Neuroanat. 2005b;29:127–136. doi: 10.1016/j.jchemneu.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Lin LH, Talman WT. Vesicular glutamate transporters and neuronal nitric oxide synthase colocalize in aortic depressor afferent neurons. J Chem Neuroanat. 2006;32:54–64. doi: 10.1016/j.jchemneu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- Marchand A, Abi-Gerges A, Saliba Y, Merlet E, Lompre AM. Calcium signaling in vascular smooth muscle cells: from physiology to pathology. Adv Exp Med Biol. 2012;740:795–810. doi: 10.1007/978-94-007-2888-2_35. [DOI] [PubMed] [Google Scholar]
- Marozkina NV, Gaston B. S-Nitrosylation signaling regulates cellular protein interactions. Biochim Biophys Acta. 2012;1820:722–729. doi: 10.1016/j.bbagen.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron RM, Chen Y, Tomori T, Strasser A, Mechoulam R, Shohami E, Spatz M. Endothelial-mediated regulation of cerebral microcirculation. J Physiol Pharmacol. 2006;57(Suppl 11):133–144. [PubMed] [Google Scholar]
- Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Moskowitz MA, Maynard KI, Koketsu N, Dawson TM, Bredt DS, Snyder SH. Possible origins and distribution of immunoreactive nitric oxide synthase-containing nerve fibers in cerebral arteries. J Cereb Blood Flow Metab. 1993;13:70–79. doi: 10.1038/jcbfm.1993.9. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Cuomo O, Esposito E, Sirabella R, Di RG, Annunziato L. ASIC1a contributes to neuroprotection elicited by ischemic preconditioning and postconditioning. Int J Physiol Pathophysiol Pharmacol. 2011;3:1–8. [PMC free article] [PubMed] [Google Scholar]
- Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011;23:1921–1926. doi: 10.1016/j.cellsig.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonian SX, Herbison AE. Localization of neuronal nitric oxide synthase-immunoreactivity within sub-populations of noradrenergic A1 and A2 neurons in the rats. Brain Res. 1996;732:247–252. doi: 10.1016/0006-8993(96)00687-7. [DOI] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taktakishvili OM, Lin LH, Vanderheyden AD, Nashelsky MB, Talman WT. Nitroxidergic innervation of human cerebral arteries. Auton Neurosci. 2010;156:152–153. doi: 10.1016/j.autneu.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman WT, Corr J, Nitschke DD, Wang D. Parasympathetic stimulation elicits cerebral vasodilatation in rat. Auton Neurosci. 2007;133:153–157. doi: 10.1016/j.autneu.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman WT, Dragon DN. Inhibition of nitric oxide synthesis extends cerebrovascular autoregulation during hypertension. Brain Res. 1995;672:48–54. doi: 10.1016/0006-8993(94)01381-q. [DOI] [PubMed] [Google Scholar]
- Vanderkooi JM, Wright WW, Erecinska M. Nitric oxide diffusion coefficients in solutions, proteins and membranes determined by phosphorescence. Biochim Biophys Acta. 1994;1207:249–254. doi: 10.1016/0167-4838(94)00073-5. [DOI] [PubMed] [Google Scholar]
- Waldmann R. Proton-gated cation channels--neuronal acid sensors in the central and peripheral nervous system. Adv Exp Med Biol. 2001;502:293–304. doi: 10.1007/978-1-4757-3401-0_19. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Chu XP, Guo ML, Jin DZ, Xue B, Berry TJ, Fibuch EE, Mao LM. Modulation of ionotropic glutamate receptors and Acid-sensing ion channels by nitric oxide. Front Physiol. 2012;3:164. doi: 10.3389/fphys.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Zha XM. Acid-sensing ion channels: trafficking and synaptic function. Mol Brain. 2013;6:1. doi: 10.1186/1756-6606-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, III, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]