Abstract
Recent advances in molecular technology have unraveled the complexity of leukemogenesis and provided the opportunity to design more personalized and pathophysiology-targeted therapeutic strategies. Despite the use of intensive chemotherapy, relapse remains the most common cause for therapeutic failure in acute myelogenous leukemia (AML). The interactions between leukemia stem cells (LSC) and marrow microenvironment appear to be critical in promoting therapeutic resistance through progressive acquisition of genetic and epigenetic changes within leukemia cells and immune evasion, resulting in leukemia cell survival. With advances in genomic sequencing efforts, epigenetic and phenotypic characterization, personalized therapeutic strategies aimed at critical leukemia survival mechanisms may be feasible in the near future. Here, we review select novel approaches to therapy of AML such as targeting LSC, altering leukemia/marrow microenvironment interactions, inhibiting DNA repair or cell cycle checkpoints, and augmenting immune-based anti-leukemia activity.
Background
Acute myelogenous leukemias (AML) are a heterogeneous group of disorders that differ in their genotypic, phenotypic, and epigenetic characteristics, and in their net responses to anti-leukemic interventions. Despite the achievement of complete remission (CR) in substantial proportions of AML subgroups, relapse occurs in the majority and remains the most common reason for treatment failure.
Contrary to what might be expected for such a diverse group of diseases, the AML genome on average contains only 13 gene mutations, and the vast majority of AML patients carry at least one pathogenic mutation affecting biologically relevant pathways, with unique patterns of mutual exclusivity and cooperation (1). Nonetheless, clonal complexity evolves from diagnosis through treatment and disease progression, at least in part due to selective pressure from chemotherapy (2, 3).
The ability to measure minimal residual disease (MRD) seems critical to determining optimal post-induction strategies that can eventually lead to disease eradication. Several AML subtypes have well-defined molecular aberrations and/or gene mutations, e.g., NPM-1 or FLT-3, that permit the use of high-sensitivity molecular detection of the leukemic burden by reverse transcriptase quantitative (qRT)-PCR (4–8). Alternatively, in AMLs lacking such specific molecular hallmarks, qRT-PCR for WT1, a zinc-finger transcription factor that is preferentially overexpressed in AML patients, may provide valuable information regarding MRD status. Several studies, including the recent European LeukemiaNet study, have found that the magnitude of WT1 log reduction following induction chemotherapy is an independent predictor of relapse (5, 9).
Flow cytometry provides an alternative method for detection of MRD based on the presence of aberrant cell surface marker expression. Detection of MRD by flow cytometry correlates with relapse (5). Additionally, flow cytometry holds the promise to track residual leukemia stem cells (LSCs). Although to date there is a limited consensus regarding LSC phenotypes, there are discrete markers reported to facilitate the isolation and identification of LSCs, including CD34, CD38, CD44, CD47, CD96, CD32, CD25, CD133, CD90, CD117, CD123, TIM3, CLL-1, and ALDH1 (10, 11). As a case in point, Gerber et al. (12), used flow cytometry to assess aldehyde dehydrogenase (ALDH) expression in CD34+ cells, and identified a population of CD34+CD38− cells with intermediate ALDH activity that was 89% leukemic by fluorescence in situ hybridization (FISH), reproducibly generated AML upon transplantation into mice, and was highly predictive of relapse.
If we are to combat AML more effectively, we must develop strategies that take into account the multiple factors contributing to leukemia pathogenesis and pathophysiology, including the LSC, its interaction with its surrounding bone marrow (BM) microenvironment, and the development of net drug resistance over time. In this review, we discuss selected approaches that address aspects of both the leukemic clone and its supportive milieu.
On the Horizon
Targeting leukemia stem cells and marrow microenvironment
Leukemia stem cell directed therapies
LSCs share many properties with normal hematopoietic stem cells (HSCs) such as self-renewal, quiescence, and resistance to traditional cell-cycle dependent chemotherapeutic agents (13). An ability to target LSCs offers a possibility of eradicating AML at its roots. Such eradication, however, requires the ability to exploit differences between LSCs and HSCs in terms of dependence on specific survival pathways, alterations in the genetic, epigenetic and metabolic landscapes, and immunophenotypes. As new drugs are developed to selectively target the abnormalities responsible for leukemia initiation and perpetuation, there may be an opportunity to eradicate LSC clones before acquisition of additional mutations renders them resistant to therapy (Table 1).
Table 1.
Select agents targeting leukemia stem cell and microenvironment
| Molecular Target | Targeting Agent | Study in AML |
|---|---|---|
| Leukemia Stem Cell | ||
| Survival pathways | ||
| NF-ĸB | Parthenolide, bortezomib | Preclinical, phase I-III |
| PI3K/Akt/mTOR | BKM120, CAL-101, MK-2206, perifosine, GSK21110183, sirolimus, temsirolimus, deferolimus, everolimus, BEZ235, OSI-207 |
Preclinical, phase I-II |
| Self-renewal pathways | ||
| Wnt/β-catenin | CWP232291, PRI-724 | Phase I-II |
| Hedgehog | PF-04449913, LDE225 | Phase I-II |
| Mitochondrial targets | ||
| Bcl-2 | Oblimersen sodium, obatoclax, ABT-737, ABT-199 | Preclinical, Phase I-III |
| Mitochondrial translation | Tigecycline | Preclinical, Phase I |
| Cell surface antigen | ||
| CD123 | CSL362, SL-401 (IL-3-diphteria toxin), *CAR T cells, MGD006 (*DART CD123, CD3) |
Preclinical, Phase I-II |
| CLL-1 | mAB, nanoparticle-daunorubicin | Preclinical |
| CD25 | Basiliximab, Daclizumab, denileukin diftitox immunotoxin |
Preclinical, Phase I-II |
| CD47 | mAb, SIRPa Fc fusion protein | Preclinical |
| CD33 | Gemtuzumab ozogamicin, SGN-CD33A, 225-AcLintuzumab, AMG-330 (CD3, CD33 *BITE Ab), *CAR T cells |
Preclinical, Phase I-III |
| Bone marrow microenvironment | ||
| Adhesion, homing | ||
| CXCR4/SDF-1 | BMS-936564, BL-8040, plerixafor; NOX-A12 (SDF-1) | Preclinical, phase I-II |
| VLA-4 | natalizumab | Preclinical |
| CD44 | mAb | Preclinical |
| MUC1-C | peptide inhibitor GO-203 | Preclinical |
| Hypoxia | ||
| HIF-1a | ehinomycin | Preclinical |
| VEGF | bevacizumab, lenalidomide, sunitinib, sorafenib | Preclinical, Phase I-III |
| Hypoxia-activated prodrugs | PR-104, TH-302 | Preclinical, Phase I |
CAR (Chimeric Antigen Receptor) T cells; BITE Ab (Bispecific T cell Engager Ab); DART (Dual Affinity Retargeting Molecule)
Several pathways appear to promote LSC survival preferentially. NF-kB, a transcription factor that promotes cell growth and inhibits apoptosis, is constitutively activated in LSCs (14). Parthenolide induces LSC apoptosis via NF-kB inhibition and increase in reactive oxygen species (ROS), and decreases engraftment of LSCs but not HSCs in mice (15). The proteasome inhibitor Bortezomib produces an anti-NF-kB effect by inhibiting the degradation of IkB. Bortezomib given with traditional induction chemotherapy produced encouraging results (CR 65%; DFS and OS of 7.4 and 17.5 months) in newly diagnosed elderly AML patients (16). Whether or not this is a consequence of more effective LSC eradication will require further studies. The PI3K/AKT/mTOR pathway is frequently up-regulated in AML and plays a central role in multiple key survival processes within the cells such as in regulation of formation of reactive oxygen species (ROS), modulation of Bcl-2 family proteins, up-regulation of NF-kB, and self-renewal of LSCs through Wnt/B-catenin pathway (17). PI3K/mTOR inhibitors were found to augment the effects of parthenolide on LSCs possibly through down-regulation of NrF2, a transcription factor involved in activating the expression of antioxidant enzymes such as heme oxygenase (HMOX-1) (18). Thus, inhibition of PI3K/AKT/mTOR may sensitize LSCs to agents that induce oxidative stress by increasing that stress and thereby reducing LSC self-renewal. However, when given as single agents or in combination with chemotherapy, mTOR analogues or perifosine demonstrated only modest clinical activity in AML, (19–21) likely due to mTORC1 but not mTORC2 inhibition. New PI3K/mTOR inhibitors are in clinical trials in AML (NCT01756118, NCT01396499).
LSCs reside in a hypoxic environment in the BM and depend heavily on oxidative phosphorylation, which is a pivotal function of mitochondrial proteins. This oxidative phosphorylation is critical to LSC maintenance and survival. As a case in point, LSCs upregulate specific Bcl-2 family members to evade apoptosis and promote survival and chemoresistance. Bcl-2 and Bcl-XL are highly expressed in LSC while Mcl-1 has a key role in maintenance of HSCs (22). This difference provides a therapeutic opportunity to sensitize LSC to chemotherapy via Bcl-2 inhibition (23). For instance, anti-Bcl2/BH3 mimetic ABT-737 preferentially inhibited LSC survival in preclinical studies; however, compensatory increases in Mcl-1 may limit its net activity (24). Dual inhibition of Bcl-2 and Mcl-1 promoted anti-leukemia activity (25, 26), but the clinical applicability of this approach could be challenged by potential hematologic toxicity.
Several phenotypic markers preferentially expressed on LSC may serve as therapeutic targets. Targeting surface antigens with monoclonal antibodies (mAb), immunotoxins, chimeric antigen receptor modified T cells (CAR T-cells) are in diverse stages of clinical testing. For instance, CD123 is preferentially expressed in CD34+CD38− AML cell population (27) and pre-treatment of NOD/SCID with anti-CD123 mAb decreases AML cell engraftment (28). Two biologic agents targeting anti-CD123 are in clinical testing: mAb CSL362 (NCT01632852) and DT388IL3 (NCT00397579)(29). The LSC express CD47 that binds to signal-regulatory-protein-alpha (SIRPα) on macrophages and inhibits phagocytosis. The disruption of CD47-SIRPa interaction using anti-CD47 Abs or SIRPα-Fc fusion protein decreases LSC engraftment in xenograft models and promotes macrophage-mediated phagocytosis of AML cells (30, 31).
Microenvironment: the LSC niche as a therapeutic target
Two distinct BM microenvironmental niches, osteoblastic and vascular, are required for maintenance of HSCs but also provide a sanctuary for leukemic cells to evade chemotherapy-induced death. In principal, both LSC and HSC are dependent on signals from their microenvironment, including stromally-produced cytokines, chemokines, and intracellular signals initiated by cellular adhesion; however, LSC are able to outcompete HSCs, hijacking the BM environment and creating their foster home through reversible changes in BM stromal cell function (32).
Interactions between CXCL12 (stromal cell derived factor alpha-1) and its receptor CXCR4 on leukemia cells contribute to LSC homing to the microenvironment. CXCR4 expression is increased on AML cells, particularly in FLT3-mutated AML, and is associated with poor outcome (33). Anti-CXCR4 mAb given to NOD/SCID mice engrafted by human AML decreased the numbers of AML cells in blood, BM, and spleen, but did not affect homing of HSCs (34). CXCR4 inhibitors are currently in clinical studies in AML (NCT01120457, NCT01352650, NCT01160354). Plerixafor, a small molecule antagonist of CXCR4, mobilizes AML cells into peripheral blood where they are more sensitive to chemotherapy. Plerixafor in combination with chemotherapy produced complete remission in 46% of relapsed/refractory AML patients, and correlative studies demonstrated two-fold increased mobilization of leukemia cells into peripheral blood (35). Adhesion of LSCs to the BM microenvironment promotes survival, self-renewal and resistance to therapy. Interaction between VLA-4 on the surface of leukemia cells and fibronectin on stromal cells activates pro-survival pathways and contributes to MRD persistence (36). Another adhesion molecule, CD44, promotes LSC homing to microenvironmental niches by mediating cell-cell and cell-extracellular matrix interactions through binding to hyalouronan (37).
Leukemia progression may promote hypoxia in the BM niche, leading to overexpression of HIF-1a which promotes leukemia cell quiescence as well as recruitment and retention of leukemia cells through activation of CXCL12-CXCR4 signaling (38). Targeting HIF-1a transcription factor and its downstream target CIAX is now amenable to pharmacologic inhibition. The hypoxia-activated pro-drug PR-104 demonstrated clinical activity in AML patients but produced prolonged myelosuppression at higher doses (39). Nonetheless, since HSCs and LSCs use overlapping pathways of microenvironmental protection, it will be important to examine the safety and selectivity of these novel agents.
Novel treatments based on improved understanding of AML biology
Epigenetic therapies
Global epigenetic modulators such as DNA methyltransferase (DNMT) or histone deacetylase (HDAC) inhibitors are associated with reduction but not eradication of LSCs (40). The identification of recurring unique mutations in epigenetic mediators and overall epigenetic profiles opens the possibility of developing more effective inhibitors. Mutations such as IDH1/IDH2 or DNMT3 appear to be leukemia-initiating and may contribute to initial LSC expansion (41, 42). IDH mutations lead to production of 2-hydroxyglutarate which inhibits histone and DNA methyltransferases, resulting in increased DNA methylation and blocked cellular differentiation (43). Small molecule inhibitors of IDH2 (AG-6780, AG-221) reduce 2-HG levels, increase differentiation, and produce survival benefit in xenograft models (44, 45). AG-221 is currently in phase I testing (NCT01915498). The presence of DNMT3 mutations may be associated with an increased response to hypomethylating agents, as reported in a small number of AML patients treated with decitabine (46). DNMT3 mutations occur early in leukemogenesis (41), raising the possibility that DNMT3 inhibitors could be used selectively, not only in initial therapy, but also as maintenance or to target MRD following chemotherapy in DNMT3-mutated leukemias.
Increasing the DNA-damaging efficacy of chemotherapy
Cytarabine remains the single most effective drug for therapy of AML and there are ongoing efforts to identify strategies to enhance its activity. CPX-351 is a liposomal encapsulation of cytarabine and daunorubicin at a 5:1 molar concentration ratio that provides optimal synergistic activity (45). A randomized Phase II study of CPX-351 versus 7+3 in older adults with newly diagnosed AML resulted in improved CR rates (66.7% versus 51.2%) with survival advantage noted in patients with secondary AML (12 .1 vs 6.1 months), leading to a Phase III study in this population (NCT01696084)(47).
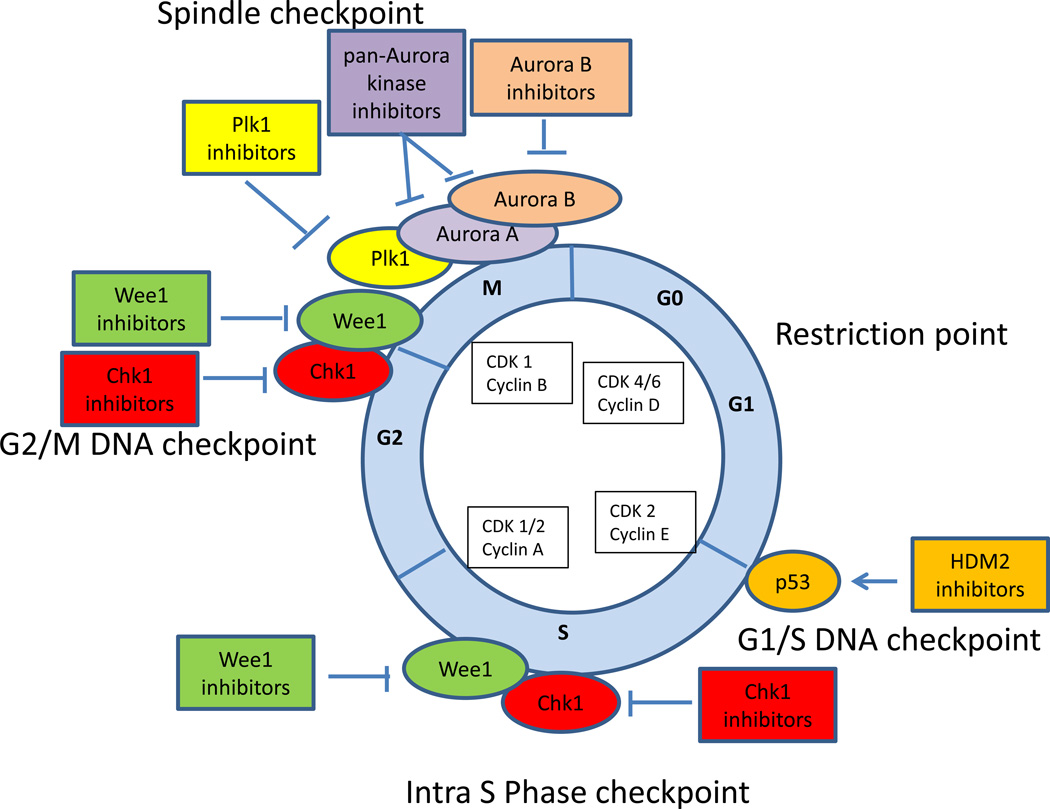
Incorporation of cytarabine into DNA activates Chk1, a serine/threonine kinase which stabilizes stalled replication fork, induces S phase arrest, and diminishes cytarabine toxicity. Depletion of Chk1 either by siRNA (48) or selective Chk1 inhibitor SCH900776 (MK8776) (49) can overcome S-phase checkpoint activation and enhance cytarabine cytotoxicity in AML. In a phase I study, SCH900776 given with timed sequential cytarabine produced CR in 33% of patients with relapsed/refractory AML, and increased H2Ax phosphorylation in marrow blasts consistent with unrepaired DNA damage (50). A randomized phase II study comparing cytarabine plus MK8776 to cytarabine alone is ongoing in patients with relapsed or refractory AML (NCT01870596). Several other cell cycle checkpoint inhibitors have shown preclinical or clinical activity in AML (Fig. 1). WEE-1 kinase is an essential G2/M as well as G1/S checkpoint kinase that phosphorylates CDK1, thereby delaying cell cycle progression, providing time to repair DNA damage and complete cell division. Inhibition of WEE-1 kinase by siRNA or pharmacologically with WEE-1 inhibitor MK1775 resulted in potentiation of ara-C cytotoxicity in AML cells (48). A study of WEE-1 kinase inhibitor in combination with cytarabine in AML is planned. Aurora kinases and Polo-like kinases (Plk) also play important roles in cell cycle progression. Aurora B kinase has a predominant role in mitosis and its inhibition by AZD1152 produced 25% response rate in poor-risk AML (51) and 45% response rate in older AML patients when given with low dose cytarabine (LDAC) (52). Plk inhibition leads to a disruption in spindle assembly causing a mitotic arrest and subsequent apoptosis. A randomized phase II study of volasertib, a Plk1 inhibitor, plus LDAC versus LDAC alone in older AML patients showed improvement in response rate and survival with the combination versus LDAC alone (31% vs 13%; OS 8 vs 5.2 months) (53), leading to Breakthrough Therapy designation and initiation of a phase III randomized study of the same regimens in AML patients older than 65 (NCT01721876).
Figure 1.
Cell cycle checkpoint inhibitors in AML. Cell cycle is controlled by successive activation of cyclin-cyclin dependent kinases (CDKs). Cell cycle checkpoints serve to stop progression of the cell cycle in response to DNA damage, to allow time for DNA repair and to preserve genomic integrity. The p53-dependent G1/S checkpoint blocks initiation of DNA replication. p53 is frequently altered (mutations or losses) in patients with complex karyotype AML. Inhibitors of HDM2 (RG7112, RO5503781 and MK-8242) are in clinical studies in AML alone or in combination with cytarabine and have a potential to be effective in leukemia cells that retain wild-type p53. Tumors that are defective in p53 function rely on an intact S phase and G2 checkpoint. Depending on the type of genotoxic stress, either ataxia-telangectasia mutated (ATM) protein kinase or ataxia-telangiectasia-related (ATR) protein kinase are activated leading to activation of Chk1 and Wee1 kinases that inactivate Cdk1 resulting in intra S phase and G2/M arrest, allowing time for DNA repair. Inhibition of Chk1 (MK8776) and Wee1 (MK1775) sensitizes leukemia cells to cytarabine cytotoxicity in preclinical studies and the combination of Chk1 inhibitor and cytarabine is in a phase II testing in AML. Synergistic activity has been described for Chk1 and Wee1 inhibition in leukemia cells. PLK1 and Aurora kinases are critical for centrosome maturation and proper formation of the mitotic spindle, and also play a role in chromosome segregation and cytokinesis. Plk1 inhibitor (volasertib) and Aurora B kinase inhibitor (AZD1152) have shown promising clinical activity in AML patients when given with low dose cytarabine. In addition, several pan CDK inhibitors (flavopiridol, dinaciclib) have been explored as a therapeutic strategy in AML. Flavopiridol in combination with cytarabine and mitoxantrone (FLAM) is in a phase III testing for newly diagnosed AML.
Aberrant or impaired repair of DNA double-strand breaks (DSB) is common feature of AML and MDS (54, 55). Inhibition of poly(ADP)ribose polymerases (PARP), a family of enzymes involved in base excision repair and other nuclear processes, may lead to an increase in single strand breaks (SSBs) which form DSBs upon encountering a replication fork which cannot be repaired in cells with a defective DSB repair background (defective BRCA, ATM, or Fanconi Anemia proteins). PARP inhibitors demonstrate single agent anti-leukemia activity in preclinical studies (56, 57) and potentiate the cytotoxic effects of diverse classes of DNA damaging agents in multiple tumor cell types, including AML (58, 59). Clinical trials are exploring activity of veliparib (ABT-888) with temozolomide (NCT01139970) or topotecan plus carboplatin (NCT00588991) in refractory AML.
Immunomodulation
Multiple mechanisms contribute to the dysfunction of effector T cells in AML, including 1) wide tissue expression of leukemia antigens; 2) the ability of AML cells to lose antigen/MHC expression limiting effective T cell response; 3) expression of negative co-stimulatory ligands by tumor cells; 4) expansion of regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSC); and 5) deletional peripheral T cell tolerance (60–62).
Recent attention has focused on blocking negative regulatory signals in order to activate T cell-mediated anti-tumor immunity (Table 2). CTLA-4 and PD-1, members of CD28 family, are the key inhibitory receptors that limit T cell activation. CTLA-4 is expressed on activated T cells and Tregs and binds CD80/CD86 ligands on antigen presenting cells (APCs). On effector T cells, CTLA-4 is able to outcompete CD28 for access to the immune synapse, thereby limiting co-stimulation (61, 63). The anti-CTLA-4 inhibitory mAb Ipilimumab was approved by FDA after clinical studies in advanced melanoma demonstrated remarkable activity (64). CTLA-4 blockade by mAb enhances AML specific T cell responses in vitro (65), and CTLA-4 polymorphism has been associated with AML relapse (66). Ipilimumab is now being evaluated in patients with relapsed MDS/AML (NCT01757639) or following allogeneic stem cell transplantation (NCT01822509).
Table 2.
Select Immunotherapeutic Strategies
| Target | Agent | Mechanisms/Studies in AML |
|---|---|---|
| Inhibitory pathways | ||
| CD28/B7 family receptors | ||
|
CTLA-4 (Cytotoxic T lymphocyte antigen-4) |
Ipilimumab* | CTLA-4 blockade enhances AML-specific T cell responses; CTLA-4 polymorphism associated with relapse (65, 66). *Clinical study in relapsed AML and after alloHSCT. |
|
PD-1 / PD-L1 (Programmed death-1) |
Nivolumab, MK-3475*, CT-011*, MEDI0680 BMS-936559, MEDI4736, MPDL3280A |
PD-L1 is expressed on AML blasts ; PD-1 expression increased on circulating T cells in leukemia patients; leukemia -specific T cell immunity and survival upon AML challenge increased in PD-1 knockout mice or upon PD-L1 blockade (61, 69, 70). *Clinical study of CT-011 and vaccine; and MK-3475 in MDS. |
| non-CD28/B7 family receptors | ||
|
LAG-3 (Lymphocyte activation gene-3) |
BMS-986016, IMP321 | No studies in AML. |
|
TIM-3/ galectin-9 (T cell immunoglobulin domain and mucin domain 3) |
mAb, TIM-3 fusion protein | Galectin-9 is expressed on AML cells; co- expression of TIM-3 and PD-1 identifies exhausted T cells in mice with advanced AML and increases during AML progression; combined blockade of TIM- 3/ PD-L1 had an additive effect in improving survival of AML-bearing mice(77). |
| Inhibitory enzymes | ||
|
IDO (Indoleamine 2,3-dioxygenase) |
INCB024360*, indoximod, NLG919 |
IDO overexpressed in AML cells, predicts poor prognosis, depletes tryptophan, thus, limiting T cell proliferation and stimulating Tregs accumulation. (78–80) *Clinical study in MDS. |
| Targeting T regs |
CD25 (Basiliximab*, Daclizumab, denileukindiftitoximmunotoxin); Metronomic cyclophosphamide; Immunomodulatory drugs (Pomalidomide* Lenalidomide*, Thalidomide); Fludarabine |
*Several clinical studies of anti-CD25 plus vaccine and chemotherapy plus immunomodulatory drugs ongoing in AML. |
| Targeting NK cells | ||
|
KIR(Killer-cell immunoglobulin-like receptors) CD200 / CD200R |
*Anti-KIR Ab (IPH2101, lirilumab) mAb (anti-CD200) |
*Phase I, II clinical studies-maintenance in older AML patients (81). CD200 is overexpressed in AML cells, correlates with poor prognosis, increases BM Tregs and directly inhibits the cytotoxic activity of NK cells (82, 83). |
| CD123/CD33/CD16 | Triplebody (SPM2) | Increased patient's NK cell cytolytic activity against AML cells ex vivo (84). |
| CD16 X CD33 | CD16xCD33 bispecific killer cell engager (BiKE) |
Reversed MDSC (myeloid-derived suppressor cell) immunosuppression of NK cells and induced CD33+ MDS and MDSC target cell lysis ex vivo (85). |
Denotes therapeutics in clinical studies in AML and MDS.
PD-1 is expressed on the surface of activated T cells, B cells, NK cells and monocytes in response to inflammation and binds two ligands: PD-L1 and PD-L2. PD-L1 is expressed on hematopoietic and non-hematopoietic cells and is over-expressed on multiple tumors, including AML blasts, while PD-L2 is mainly restricted to APCs (61, 67, 68). Leukemia-specific T cell immunity and survival upon AML challenge was increased in PD-1 knockout mice or in wild type mice upon PD-L1 blockade using mAb (69). PD-1 expression is increased on peripheral blood T cells in leukemia patients compared to healthy donors (70). PD-1/PD-L1 pathway blockade has shown promising activity in solid tumors (71, 72) and is now investigated in clinical trials in AML in combination with a dendritic cell based vaccine (NCT01096602). Additional non-CD28/B7 family T cell inhibitory receptors such as LAG-3 and TIM3 have also been identified as potential therapeutic targets.
Immunosuppressive Tregs are defined by their expression of FoxP3 transcription factor and have been implicated as major contributors to the defective immune response in AML. AML patients have a greater Treg frequency at diagnosis relative to normals, their Tregs more potently suppress the effector T cells, and those with greater numbers of Tregs appear to have a relatively poor clinical outcome (62, 73, 74). In mice, Tregs accumulate at leukemia sites and impede the activity of cytotoxic lymphocytes (CTL) whereas their removal, alone or in combination with PD-L1 blockade, results in increased frequency of CTL at tumor sites and improves the efficacy of adoptive therapy (75, 76). Clinical studies are investigating if responses to tumor vaccines can be augmented in AML patients following Treg depletion using metronomic cyclophosphamide or/and anti-IL2 receptor (CD25) antibodies (NCT01513109, NCT01842139), or if immunomodulatory drugs such as pomalidomide may reduce Tregs following induction chemotherapy (NCT02029950).
Conclusion
The therapeutic armamentarium for AML is evolving as we increase our basic understanding of the diverse factors that play into leukemogenesis and leukemia cell biology. Given the complex interactions between AML cells and the many components of their environment, it is reasonable to surmise that the future of AML therapy lies in the combination of molecularly selective agents with traditional cytotoxics and/or with each other throughout induction and post-induction therapies. Such multi-directed approaches have the potential to overcome AML cell resistance by targeting crucial leukemic cell pathways and critical cellular and humoral components of the BM microenvironment, thereby preventing AML clonal expansion and survival. The successful introduction of diverse molecularly targeted agents into the clinic will require standardization of molecular tests, development of predictive biomarkers of response, proper timing, and integration with current therapies.
Acknowledgments
Grant Support
Research reported in this publication was supported in part by NCI of the NIH under award numbers U01CA70095 (to I. Gojo and J.E. Karp), UM1CA186691 (to I. Gojo), and P30CA006973 (to I. Gojo and J.E. Karp).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter MJ, Shen D, Ding L, Shao J, Koboldt DC, Chen K, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012;366:1090–1098. doi: 10.1056/NEJMoa1106968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grunwald MR, Lin M-T, Pratz KW, Gocke CD, Levis MJ. Tandem duplication PCR (TD-PCR) is a novel method of detecting minimal residual disease in FLT3/ITD AML and is highly predictive of relapse risk following allogeneic transplant [abstract]; In: Proceedings of the 54th ASH Annual Meeting and Exposition; Atlanta, GA. Washington (DC): ASH; Dec 8–11, 2012. 2012. Abstract nr 2479. [Google Scholar]
- 5.Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol. 2013;10:460–471. doi: 10.1038/nrclinonc.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121:2213–2223. doi: 10.1182/blood-2012-10-462879. [DOI] [PubMed] [Google Scholar]
- 7.Kronke J, Schlenk RF, Jensen KO, Tschurtz F, Corbacioglu A, Gaidzik VI, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29:2709–2716. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- 8.Yin JA, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120:2826–2835. doi: 10.1182/blood-2012-06-435669. [DOI] [PubMed] [Google Scholar]
- 9.Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27:5195–5201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 10.Felipe Rico J, Hassane DC, Guzman ML. Acute myelogenous leukemia stem cells: from Bench to Bedside. Cancer Lett. 2013;338:4–9. doi: 10.1016/j.canlet.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 12.Gerber JM, Smith BD, Ngwang B, Zhang H, Vala MS, Morsberger L, et al. A clinically relevant population of leukemic CD34(+)CD38(−) cells in acute myeloid leukemia. Blood. 2012;119:3571–3577. doi: 10.1182/blood-2011-06-364182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 14.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 15.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attar EC, Johnson JL, Amrein PC, Lozanski G, Wadleigh M, DeAngelo DJ, et al. Bortezomib added to daunorubicin and cytarabine during induction therapy and to intermediate-dose cytarabine for consolidation in patients with previously untreated acute myeloid leukemia age 60 to 75 years: CALGB (Alliance) study 10502. J Clin Oncol. 2013;31:923–929. doi: 10.1200/JCO.2012.45.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martelli AM, Evangelisti C, Chappell W, Abrams SL, Basecke J, Stivala F, et al. Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia. 2011;25:1064–1079. doi: 10.1038/leu.2011.46. [DOI] [PubMed] [Google Scholar]
- 18.Hassane DC, Sen S, Minhajuddin M, Rossi RM, Corbett CA, Balys M, et al. Chemical genomic screening reveals synergism between parthenolide and inhibitors of the PI-3 kinase and mTOR pathways. Blood. 2010;116:5983–5990. doi: 10.1182/blood-2010-04-278044. [DOI] [PubMed] [Google Scholar]
- 19.Gojo I, Perl A, Luger S, Baer MR, Norsworthy KJ, Bauer KS, et al. Phase I study of UCN-01 and perifosine in patients with relapsed and refractory acute leukemias and high-risk myelodysplastic syndrome. Invest New Drugs. 2013;31:1217–1227. doi: 10.1007/s10637-013-9937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perl AE, Kasner MT, Tsai DE, Vogl DT, Loren AW, Schuster SJ, et al. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapy in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res. 2009;15:6732–6739. doi: 10.1158/1078-0432.CCR-09-0842. [DOI] [PubMed] [Google Scholar]
- 21.Rizzieri DA, Feldman E, Dipersio JF, Gabrail N, Stock W, Strair R, et al. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2008;14:2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- 22.Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344–355. doi: 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia. 2012;26:778–787. doi: 10.1038/leu.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Rahmani M, Aust MM, Attkisson E, Williams DC, Jr, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012;119:6089–6098. doi: 10.1182/blood-2011-09-378141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 28.Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Konopleva M, Hogge DE, Rizzieri DA, Cirrito TP, Kornblau SM, Borthakur G, et al. SL-401, a targeted therapy directed to the interleukin-3 receptor present on leukemia blasts and cancer stem cells, is active as a single agent in patients with advanced AML [abstract]; In: Proceeding of the 54th ASH Annual Meeting and Exposition; Atlanta, GA. Washington (DC): ASH; Dec 8–11, 2012. 2012. Abstract nr 3625. [Google Scholar]
- 30.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr., et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theocharides AP, Jin L, Cheng PY, Prasolava TK, Malko AV, Ho JM, et al. Disruption of SIRPalpha signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts. J Exp Med. 2012;209:1883–1899. doi: 10.1084/jem.20120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabe Y, Konopleva M. Advances in understanding the leukaemia microenvironment. Br J Haematol. 2014;164:767–778. doi: 10.1111/bjh.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rombouts EJ, Pavic B, Lowenberg B, Ploemacher RE. Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004;104:550–557. doi: 10.1182/blood-2004-02-0566. [DOI] [PubMed] [Google Scholar]
- 34.Tavor S, Petit I, Porozov S, Avigdor A, Dar A, Leider-Trejo L, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004;64:2817–2824. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- 35.Uy GL, Rettig MP, Motabi IH, McFarland K, Trinkaus KM, Hladnik LM, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119:3917–3924. doi: 10.1182/blood-2011-10-383406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 37.Williams K, Motiani K, Giridhar PV, Kasper S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med. 2013;238:324–338. doi: 10.1177/1535370213480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiegl M, Samudio I, Clise-Dwyer K, Burks JK, Mnjoyan Z, Andreeff M. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113:1504–1512. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konopleva M, Borthakur G, Thall PF, Coveler A, Ravandi F, Jabbour E, et al. Phase I/II study of PR104, a bioreductive prodrug, in patients with relapsed/refractory acute myeloid leukemia (AML) using patient-specific adaptive dose selection [abstract]; In: Proceedings of the 53rd ASH Annual Meeting and Exposition; San Diego, CA. Washington (DC): ASH; Dec, 2011. pp. 10–13. 2011. Abstract nr 1523. [Google Scholar]
- 40.Craddock C, Quek L, Goardon N, Freeman S, Siddique S, Raghavan M, et al. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia. 2013;27:1028–1036. doi: 10.1038/leu.2012.312. [DOI] [PubMed] [Google Scholar]
- 41.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yen K, Wang F, Travins J, Chen Y, Yang H, Straley K, et al. AG-221 offers a survival advantage in a primary human IDH2 mutant AML xenograft model [abstract]; In: Proceedings of the 55th ASH Annual Meeting and Exposition; New Orleans, LA. Washington (DC: ASH; Dec, 2013. pp. 7–10. 2013. Abstract nr 240. [Google Scholar]
- 45.Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 46.Metzeler KH, Walker A, Geyer S, Garzon R, Klisovic RB, Bloomfield CD, et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2012;26:1106–1107. doi: 10.1038/leu.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lancet JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tibes R, Bogenberger JM, Chaudhuri L, Hagelstrom RT, Chow D, Buechel ME, et al. RNAi screening of the kinome with cytarabine in leukemias. Blood. 2012;119:2863–2872. doi: 10.1182/blood-2011-07-367557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schenk EL, Koh BD, Flatten KS, Peterson KL, Parry D, Hess AD, et al. Effects of selective checkpoint kinase 1 inhibition on cytarabine cytotoxicity in acute myelogenous leukemia cells in vitro. Clin Cancer Res. 2012;18:5364–5373. doi: 10.1158/1078-0432.CCR-12-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karp JE, Thomas BM, Greer JM, Sorge C, Gore SD, Pratz KW, et al. Phase I and pharmacologic trial of cytosine arabinoside with the selective checkpoint 1 inhibitor Sch 900776 in refractory acute leukemias. Clin Cancer Res. 2012;18:6723–6731. doi: 10.1158/1078-0432.CCR-12-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowenberg B, Muus P, Ossenkoppele G, Rousselot P, Cahn JY, Ifrah N, et al. Phase 1/2 study to assess the safety, efficacy, and pharmacokinetics of barasertib (AZD1152) in patients with advanced acute myeloid leukemia. Blood. 2011;118:6030–6036. doi: 10.1182/blood-2011-07-366930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kantarjian HM, Sekeres MA, Ribrag V, Rousselot P, Garcia-Manero G, Jabbour EJ, et al. Phase I study assessing the safety and tolerability of barasertib (AZD1152) with low-dose cytosine arabinoside in elderly patients with AML. Clin Lymphoma Myeloma. 2013;13:559–567. doi: 10.1016/j.clml.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maertens J, Lubbert M, Fiedler W, Fouillard L, Haaland A, Brandwein JM, et al. Phase I/II study of volasertib (BI 6727), an intravenous polo-like kinase (Plk) inhibitor, in patients with acute myeloid leukemia (AML): results from the randomized phase II part for volasertib in combination with low-dose cytarabine (LDAC) versus LDAC monotherapy in patients with previously untreated AML ineligible for intensive treatment [abstract]; In: Proceeding of the 54th ASH Annual Meeting and Exposition; Atlanta, GA. Washington (DC): ASH; Dec 8–11, 2012. 2012. Abstract nr 411. [Google Scholar]
- 54.D’Andrea AD. Targeting DNA repair pathways in AML. Best Pract Res Clin Haematol. 2010;23:469–473. doi: 10.1016/j.beha.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Gaymes TJ, Mufti GJ, Rassool FV. Myeloid leukemias have increased activity of the nonhomologous end-joining pathway and concomitant DNA misrepair that is dependent on the Ku70/86 heterodimer. Cancer Res. 2002;62:2791–2797. [PubMed] [Google Scholar]
- 56.Gaymes TJ, Shall S, MacPherson LJ, Twine NA, Lea NC, Farzaneh F, et al. Inhibitors of poly ADP-ribose polymerase (PARP) induce apoptosis of myeloid leukemic cells: potential for therapy of myeloid leukemia and myelodysplastic syndromes. Haematologica. 2009;94:638–646. doi: 10.3324/haematol.2008.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDevitt MA, Koh BD, Patel A, Moliterno AR, Poh W, Herman JG, et al. Genetic and epigenetic defects in DNA repair lead to synthetic lethality of poly (ADP-ribose) polymerase (PARP) inhibitors in aggressive myeloproliferative disorders [abstract]; In: Proceeding of the 53rd ASH Annual Meeting and Exposition; San Diego, CA. Washington, DC: ASH; Dec 10–13, 2011. 2011. Abstract nr 400. [Google Scholar]
- 58.Horton TM, Jenkins G, Pati D, Zhang L, Dolan ME, Ribes-Zamora A, et al. Poly(ADP-ribose) polymerase inhibitor ABT-888 potentiates the cytotoxic activity of temozolomide in leukemia cells: influence of mismatch repair status and O6-methylguanine-DNA methyltransferase activity. Mol Cancer Ther. 2009;8:2232–2242. doi: 10.1158/1535-7163.MCT-09-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel AG, Flatten KS, Schneider PA, Dai NT, McDonald JS, Poirier GG, et al. Enhanced killing of cancer cells by poly(ADP-ribose) polymerase inhibitors and topoisomerase I inhibitors reflects poisoning of both enzymes. J Biol Chem. 2012;287:4198–4210. doi: 10.1074/jbc.M111.296475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121:734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer. 2013;1:pii. doi: 10.1186/2051-1426-1-1. 1/1/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation. Blood. 2011;118:5084–5095. doi: 10.1182/blood-2011-07-365817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong RK, Loken M, Lane TA, Ball ED. CTLA-4 blockade by a human MAb enhances the capacity of AML-derived DC to induce T-cell responses against AML cells in an autologous culture system. Cytotherapy. 2006;8:3–12. doi: 10.1080/14653240500499507. [DOI] [PubMed] [Google Scholar]
- 66.Perez-Garcia A, Brunet S, Berlanga JJ, Tormo M, Nomdedeu J, Guardia R, et al. CTLA-4 genotype and relapse incidence in patients with acute myeloid leukemia in first complete remission after induction chemotherapy. Leukemia. 2009;23:486–491. doi: 10.1038/leu.2008.339. [DOI] [PubMed] [Google Scholar]
- 67.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 68.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christiansson L, Soderlund S, Svensson E, Mustjoki S, Bengtsson M, Simonsson B, et al. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PloS One. 2013;8:e55818. doi: 10.1371/journal.pone.0055818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanakry CG, Hess AD, Gocke CD, Thoburn C, Kos F, Meyer C, et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood. 2011;117:608–617. doi: 10.1182/blood-2010-04-277939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A, et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15:3325–3332. doi: 10.1158/1078-0432.CCR-08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Q, Bucher C, Munger ME, Highfill SL, Tolar J, Munn DH, et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114:3793–3802. doi: 10.1182/blood-2009-03-208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chamuleau ME, van de Loosdrecht AA, Hess CJ, Janssen JJ, Zevenbergen A, Delwel R, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. 2008;93:1894–1898. doi: 10.3324/haematol.13113. [DOI] [PubMed] [Google Scholar]
- 79.Curti A, Aluigi M, Pandolfi S, Ferri E, Isidori A, Salvestrini V, et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. Leukemia. 2007;21:353–355. doi: 10.1038/sj.leu.2404485. [DOI] [PubMed] [Google Scholar]
- 80.Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood. 2009;113:2394–2401. doi: 10.1182/blood-2008-07-144485. [DOI] [PubMed] [Google Scholar]
- 81.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 82.Coles SJ, Hills RK, Wang EC, Burnett AK, Man S, Darley RL, et al. Increased CD200 expression in acute myeloid leukemia is linked with an increased frequency of FoxP3+ regulatory T cells. Leukemia. 2012;26:2146–2148. doi: 10.1038/leu.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coles SJ, Wang EC, Man S, Hills RK, Burnett AK, Tonks A, et al. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25:792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Braciak TA, Wildenhain S, Roskopf CC, Schubert IA, Fey GH, Jacob U, et al. NK cells from an AML patient have recovered in remission and reached comparable cytolytic activity to that of a healthy monozygotic twin mediated by the single-chain triplebody SPM-2. J Transl Med. 2013;11:289. doi: 10.1186/1479-5876-11-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]