Abstract
Crystallins, the highly abundant proteins of the ocular lens, are essential determinants of the transparency and refractivity required for lens function. Initially thought to be lens-specific and to have evolved as lens proteins, it is now clear that crystallins were recruited to the lens from proteins that existed before lenses evolved. Crystallins are expressed outside of the lens and most have been shown to have cellular functions distinct from their roles as structural elements in the lens. For one major crystallin group, the β/γ-crystallin superfamily, no such functions have yet been established. We have explored possible functions for the polypeptides (βA3- and βA1-crystallins) encoded by Cryba1, one of the 6 β-crystallin genes, using a spontaneous rat mutant and genetically engineered mouse models. βA3- and βA1-crystallins are expressed in astrocytes and retinal pigment epithelial (RPE) cells. In both cell types, these proteins appear to be required for the proper acidification of the lysosomes. In RPE cells, elevated pH in the lysosomes is shown to impair the critical processes of phagocytosis and autophagy, leading to accumulation of undigested cargo in (auto) phagolysosomes. We postulate that this accumulation may cause pathological changes in the cells resembling some of those characteristic of age-related macular degeneration (AMD). Our studies also suggest an important regulatory function of βA3/A1-crystallin in astrocytes. We provide evidence that the cellular function of βA3/A1-crystallin involves its interaction with V-ATPase, the proton pump responsible for acidification of the endolysosomal system.
Keywords: βA3/A1-crystallin, lysosome, astrocyte, retinal pigment epithelial cell, animal model, signaling
1. WHAT IS A CRYSTALLIN?
Crystallins are the very abundant, soluble proteins that are the major components of the ocular lens, the most protein-rich tissue in the body. The term crystallin has been traced as far back as 1830, when Berzelius (cited by Björk, 1964a) first studied the proteins of the lens. He found the bovine lens to be 36% protein and considered this protein to be a single, lens-specific entity. He called it ‘crystallin’, because it was the major constituent of the ‘crystalline lens’. Subsequently, it became apparent that more than one soluble protein was present in the lens; Mörner (1894) was able to separate 3 different soluble proteins, which he called α-crystallin, β-crystallin, and albumin. Mörner’s albumin fraction was later named γ-crystallin by Burky and Woods (1927). These 3 protein fractions were separated first on the basis of their differing net charge and later their differences in molecular mass. Each of these crystallins was initially thought to be a unique protein; however, by the latter half of the 20th century it became clear from the work of various laboratories that all 3 crystallin fractions were highly heterogeneous (Björk, 1964b, Spector et al., 1971, Zigler and Sidbury, 1973). The α-, β-, and γ-crystallins were found to constitute the full complement of crystallins in most vertebrate lenses and were considered to be unrelated to each other. In 1980, Driessen et al. produced the first primary sequence of a β-crystallin, the polypeptide now called βB2, and found it to be homologous to previously determined γ-crystallin sequences. As a result, the β- and γ-crystallins were combined into a single protein superfamily, the β/γ-crystallins, which together with the unrelated α-crystallins constitute the ubiquitous crystallins of the vertebrate lens. Collectively, proteins from these two groups represent over 90% of the total soluble protein in most vertebrate lenses. The relative amounts of the individual crystallins vary widely among vertebrate species; further, there are variations within any species as a function of age. As one example, using a species relevant to much of the data discussed below, Lampi et al. (2002) have analyzed the crystallin composition of the lenses of 16 day old rats. The β-crystallins constituted about 25% of the total, with γ-crystallins being about 60% and α-crystallins the remaining 15%. βA1-crystallin and βA3-crystallin, in aggregate, constitute between 25% and 30% of the β-crystallins or about 6% of the total crystallin complement.
Until about 25 years ago, it was generally accepted that the crystallins were evolutionarily highly conserved proteins, uniquely adapted to their role as structural elements in the lens, and that they were not present in any tissues except the lens. Several discoveries changed this concept. The first clue came in 1982 when Ingolia and Craig showed that the α-crystallins shared sequence homology with small heat shock proteins. Horwitz (1992) eventually was able to demonstrate that α-crystallin, like other members of the small heat shock protein family, has a chaperone-like activity that prevents the uncontrolled aggregation of partially unfolded proteins. At about the same time, it was discovered that in some vertebrate lineages, proteins not related to the α- or β/γ-crystallins were expressed at very high abundance in the lens (Hendriks et al., 1988; Huang et al., 1987). At least a dozen of these so-called taxon-specific crystallins have now been described, and shown to be identical to, or closely related to, cellular proteins such as lactate dehydrogenase, α-enolase, and glyceraldehyde-3-phosphate dehydrogenase (see Table 1 and literature cited therein). As a general (and very subjective) rule, in order to be considered a crystallin, a protein must be present in the lens of one or more species at a level of at least 5% of total soluble protein (de Jong et al, 1994). Since vertebrate lenses typically have soluble protein levels in the 300–450 mg/ml range this represents a substantial amount of bulk protein. With the discovery of the taxon-specific crystallins and as more sensitive analytical techniques revealed the presence of α- and β/γ-crystallins in tissues other than the lens, a new paradigm with respect to crystallins has evolved. As numerous authors have noted (Bloemendal et al., 2004, Piatigorsky, 2007), to function as a lens crystallin, a protein must (1) be highly soluble, so that it can be incorporated into the lens at high enough abundance to contribute to the required refractivity, while not being prone to aggregation; (2) be extremely stable, because the lack of protein turnover in mature lens fiber cells means crystallins are extraordinarily long-lived (Wannemaker and Spector, 1968); and (3) be capable of supra-molecular interactions with other crystallins in order to form a stable protein matrix with the high degree of short range order necessary for transparency (Delaye and Tardieu, 1983). These attributes have not changed, but what has changed is the original idea that crystallins are proteins unique to the lens which evolved specifically to meet these criteria. Under the new paradigm, it is understood that all crystallins originated in other cell types, pre-dating the evolution of the lens, and that they had a variety of functions, before they were recruited to the lens to function as ‘crystallins’. While some crystallins retain their original function in the lens, others do not. Crystallins are multi-functional proteins, giving rise to the concept of gene sharing (Piatigorsky, 2007). They are a diverse set of proteins that are called “crystallins” only because of their high abundance in the lens; while all crystallins must, at minimum, meet the 3 criteria listed above, the term crystallin does not imply any structural or evolutionary relationship among them.
Table 1.
Vertebrate Lens Crystallins
| Distribution | Identical to or (Related to) | |
|---|---|---|
| α | ubiquitous | small heat shock proteins |
| β,γ | ubiquitous | ? |
| λ | rabbit | L-gulonate-3-dehydrogenase |
| η | elephant shrew | retinaldehyde dehydrogenase |
| ζ | guinea pig, camelids, Japanese tree frog | NADPH: quinone oxidoreductase |
| μ | kangaroo | (ornithine cyclodeaminase) |
| ν | platypus | lactate dehydrogenase A |
| ψ | rhesus monkey | betaine-homocysteine methyltransferase |
| δ1, δ2 | birds, reptiles | argininosuccinate lyase |
| ε | birds, crocodiles | lactate dehydrogenase B4 |
| τ | turtle, lamprey, fish | α-enolase |
| π | diurnal geckos | glyceraldehyde 3-phosphate dehydrogenase |
| ι | diurnal geckos | cellular retinal binding protein type 1 |
| ρB | diurnal geckos | (aldose reductase) |
| ρ | frogs | (hydroxysteroid dehydrogenase) |
For references, see Piatigorsky, 2007 and literature cited therein.
2. β/γ-CRYSTALLINS: STRUCTURE AND EVOLUTION
The structures of the β/γ-crystallins have been extensively studied (see reviews by Bloemendal et al., 2004, Wistow, 1995). Suffice it to say here that the γ-crystallins are monomeric proteins with molecular masses of about 20 kDa, whereas the β-crystallins are a heterogeneous mixture of dimers and higher oligomers with native molecular masses ranging from about 50 kDa to 200 kDa. Our goal in this review is to explore what is known about the role(s) of these proteins outside the lens, focusing on βA3/A1-crystallin. We will provide background information on the β-crystallins, but for the most part will not discuss the γ-crystallin branch of the superfamily.
There are six β-crystallin genes scattered on several chromosomes, coding for homologous polypeptides with molecular masses of ~22–28 kDa (Table 2). Three of the genes code for polypeptides with slightly lower isoelectric points known as the βA(acidic)-crystallins, while the other 3 encode the βB(basic)-crystallins. The β-crystallin polypeptides, as with all members of the superfamily, have 2 domains, each domain being composed of 2 ‘Greek key’ motifs, common structural elements of proteins composed of 4 adjacent anti-parallel beta strands (Blundell et al., 1981). Slingsby et al. (2013) have described how the Greek key motif is the basic building block underlying the generation of the various monomeric and oligomeric β/γ-crystallins, and how the multiple forms contribute to the transparency and the refractive index gradient of the lens. β-crystallins, unlike the γ-crystallins, also have N-terminal extensions (acidic and basic polypeptides) and C-terminal extensions (basic polypeptides only). While the role of the terminal extensions is not well understood, they may be involved in the normal association into dimeric and oligomeric forms, or may protect against aggregation to larger forms during lens maturation and aging (Bloemendal et al., 2004; Dolinska et al., 2009). The core domains (excluding the extensions) of the various β-crystallin polypeptides are approximately 50% identical in primary sequence. With respect to the βA3- and βA1-crystallin polypeptides, several laboratories have done extensive work on their structures, association properties, and stability using recombinant proteins (Bateman et al., 2003; Dolinska et al., 2009; Takata et al., 2008). The structures of the genes for the β- and γ-crystallins are different. The two motifs comprising each domain in the β-crystallins are encoded by separate exons, while in the γ-crystallins each domain is encoded by a single exon (Lubsen et al., 1988).
Table 2.
β-crystallin polypeptides
| # Residues^ | Molecular Mass (Da)^ | Gene | Extra-lenticular expression | |
|---|---|---|---|---|
| βA1-crystallin | 198 | 23,191 | Cryba1 | retina, brain, RPE |
| βA2-crystallin | 196 | 21,964 | Cryba2 | testes, retina, brain |
| βA3-crystallin | 215 | 25,150 | *Cryba1 | retina, brain, RPE |
| βA4-crystallin | 195 | 22,243 | Cryba4 | retina |
| βB1-crystallin | 251 | 27,892 | Crybb1 | ciliary body, retina |
| βB2-crystallin | 204 | 23,249 | Crybb2 | testes, retina, brain, RPE |
| βB3-crystallin | 211 | 24,230 | Crybb3 | retina |
βA3- and βA1-crystallins encoded by a single gene (Cryba1)
Data for human polypeptides given (other vertebrates are similar)
As indicated above, all crystallins are believed to have been recruited from pre-existing genes when the lens evolved in early vertebrates (Piatigorsky, 2007). In the case of the β/γ-crystallin superfamily, not only are the functions of the protein precursors unknown, but the ancestral genes and the evolutionary path leading to current β/γ-crystallins remain unclear. It has been known for over 25 years that proteins distantly related to β/γ-crystallins exist in microbes, including Protein S in the soil bacterium Myxococcus xanthum (Wistow et al., 1985) and spherulin 3a in the slime mold Physarum polycephalum (Wistow, 1990). It has been postulated that these proteins are involved in resistance to stress, since both become abundant during formation of spores or cysts in response to adverse environmental conditions. These proteins and others found in various microbes (see Piatigorsky, 2007) have weak homology to vertebrate β/γ-crystallins and no known functions, although they are all composed of Greek key motifs. Of greater interest in terms of the evolutionary origin of lens β/γ-crystallins is a family member present in the urochordate, Ciona intestinalis (sea squirt). Urochordates are invertebrates, but are in the Phylum Chordata, as are vertebrates. Shimeld et al. (2005) reported that the Ciona β/γ-crystallin gene is expressed in a cell-specific manner in sensory organs of Ciona and that its promoter is capable of directing expression to the visual system in transgenic Xenopus tadpoles. Their data support the idea that a common ancestor of urochordates and vertebrates contained a single domain β/γ-precursor gene from which both the Ciona and vertebrate β/γ-crystallins have evolved. Interestingly, the invertebrate members of the superfamily are calcium binding proteins (Srivastava et al., 2014), while the vertebrate lens β/γ-crystallins and a few other members of the superfamily that are expressed in vertebrates have lost this trait or had it greatly attenuated. It seems likely that through a series of gene duplications and fusions in the early vertebrates, the β-crystallins were formed and that the γ-crystallins subsequently arose from a β-crystallin by loss of the intron separating the two motifs comprising the β-crystallin domain (Wistow, 1995).
3. EXPRESSION OF β-CRYSTALLINS OUTSIDE OF THE LENS
As noted above, mammalian crystallins were, until the 1990s, generally considered lens-specific proteins. With respect to the β/γ-crystallins, there had been suggestions as early as 1968 (Clayton et al.) that they were expressed at low levels in non-lens tissues of the chick embryo. Head et al. (1995) were the first to report clear evidence for expression of β-crystallin outside the lens in mammals, finding βB2-crystallin in both murine and feline neural retina and retinal pigmented epithelium (RPE). A number of other laboratories have reported the presence of β/γ-crystallins in the retina (Dirks et al., 1998, Magabo et al., 2000), including several that have found increased levels of these proteins following damage to the retina by intense light exposure, ocular hypertension, or retinal tears (Organisiak et al., 2006; Vazquez-Chona et al., 2004). Piri et al. (2007) found β-crystallins in retina to be predominantly in retinal ganglion cells and their expression to be affected by ocular hypertension. Crystallins, including βA3-crystallin, have been reported to be present in human drusen (Crabb et al., 2002). Reviews of crystallin gene expression in the retina exist (Xi et al., 2003; Andley, 2007). Expression of β/γ-crystallins outside of the eye appears to be quite limited. Magabo et al., (2000) demonstrated the presence of βB2-crystallin protein in both rat brain and testis, but found no detectable gene expression in a variety of other tissues tested. Recent studies from our laboratory using in situ hybridization and immunohistochemistry have analyzed the expression of βA3/A1-crystallin in the rat eye from embryonic stages to adulthood (Parthasarathy, et al., 2011). Besides robust expression in the lens, expression was also detected in the retinal astrocytes as well as in RPE, and in some retinal ganglion cells. Outside of the eye, we have detected expression only in astrocytes from the brain (Sinha, et al., 2008).
The presence of β/γ-crystallins outside of the lens strongly suggests that these proteins have functions other than their refractive function as a lens protein. This is also consistent with the concept that crystallins have all been recruited to the lens from among pre-existing proteins and with the fact that non-lens functions are now known for α-crystallin and for most, if not all, taxon-specific crystallins (Piatigorsky, 2007).
With respect to βA3/A1-crystallin, while no specific extra-lenticular function has been demonstrated, considerable evidence that such functions do exist has been presented, and several putative functions have been proposed. The first such function, put forth by Srivastava and Srivastava (1999), was that βA3/A1-crystallin has a proteolytic activity. The activity was detected in vitro and only under very specific, and seemingly non-physiological, conditions. This finding has not been independently reproduced, and its physiological significance remains to be established.
Another potential function for β/γ-crystallins relates to stimulation of axonal outgrowth from retinal ganglion cells. This idea originated in studies that demonstrated that injuring the lens was beneficial to optic nerve regeneration following axotomy. Two groups reported this remarkable effect of lens injury (Leon et al., 2000; Fischer et al., 2000; 2008). While the Benowitz laboratory focused on macrophage-derived factors produced in response to lens rupture, the Thanos group has produced evidence both in vivo and in vitro that β/γ-crystallins released by the injured lens stimulate axon regeneration. In 2007, they demonstrated expression of βB2-crystallin in cultured retinal ganglion cells, particularly in filopodia and growth cones, and reported that β-crystallins are secreted into the culture medium. βB2-crystallin, which is extremely stable and highly soluble, was shown to promote neurite outgrowth both in cultures of retinal ganglion cells and also in primary hippocampal neurons (Liedtke et al., 2007). Subsequently, Fischer et al., (2008) generalized these effects to the entire β/γ-crystallin superfamily, showing that purified β- or γ-crystallin fractions, but not α-crystallin or bovine serum albumin, could mimic the effects of lens injury on axon regeneration (Figure 1). They also showed that addition of exogenous β/γ-crystallins (1 mg injected intravitreally in the rat eye) activated retinal glial cells, stimulating astrocytes to secrete ciliary neurotrophic factor (CNTF) and up-regulating its downstream signaling pathway (JAK/STAT3). These studies, which have been recently reviewed by Thanos et al., (2012), suggest that β/γ-crystallins are able to alter the activity of signaling pathways by a mechanism, or mechanisms, yet to be identified.
Figure 1.
Effects of crystallins on axonal regeneration in the crushed optic nerve and survival of axotomized RGCs. (A–C) Longitudinal sections through the optic nerve showing GAP-43-positive axons distal to the injury site (asterisk) 2 weeks after optic nerve crush (ONC) and (A) two intravitreal injections of a solution containing BSA (1 mg) (B) lens injury or (C) two injections of β-crystallins (1 mg). Scale bar = 100 μm. (D) Quantitation of regeneration (number of axons growing ≥ 500 μm and ≥ 1000 μm beyond the injury site per optic nerve) 2 weeks after surgery. Scale bar: 100 μm. (E) Quantitation of surviving RGCs (βIII-tubulin-positive RGCs per retinal cross-section) 2 weeks after ONC. **p < 0.001 compared with BSA-treated controls. (reproduced with permission from Fischer et al, 2008)
4. βA3/A1-CRYSTALLIN AND LEAKY RIBOSOMAL SCANNING
βA3/A1-crystallin is unique amongst all crystallins. A single Cryba1 mRNA uses alternative translation by leaky ribosomal scanning to produce two closely related polypeptides, βA3 and βA1, from two different translation start sites (Werten et al., 1999). The two polypeptides are identical except that the longer one (βA3-crystallin) has 17 additional amino acid residues at its amino terminus. Leaky scanning occurs when the small ribosome subunit scanning over the mRNA molecule skips the first start codon (if the recognition site is poor) and subsequently initiates translation from a downstream alternate start codon. Translation start site selection is a highly regulated process and is critical for differential gene regulation in eukaryotes (Sonenberg and Hinnebusch, 2009).
5. THE NUC1 RAT: A SPONTANEOUS MUTATION IN βA3/A1-CRYSTALLIN
Further insight into the specific functions of the βA3- and βA1-crystallin polypeptides has been provided by our studies on a spontaneous mutant (Nuc1) in the Sprague-Dawley rat (Hose et al., 2005; Sinha et al., 2005; Zhang et al., 2005; Gehlbach et al., 2006; Sinha et al., 2008; Parthasarathy et al., 2011; Zhang et al., 2011). The Nuc1 mutation is a 27 base pair insertion in exon 6 of the Cryba1 gene that results in loss of an absolutely conserved glycine residue (G178 in βA3) and its replacement by 10 new amino acid residues (Sinha et al., 2008; Figure 2). The mutant protein is synthesized, but based on molecular modeling and analysis of proteins from mutant lenses, does not form normal associations with other β-crystallin polypeptides. The mutant polypeptides appear to aggregate and are found entirely in the void fraction following gel exclusion chromatography, indicating a molecular mass greatly exceeding that of normal β-crystallin oligomers (Sinha et al., 2008; Figure 3).
Figure 2.
The positional cloning of the βA3/A1-crystallin gene to rat chromosome 10. Markers used are designated by bisecting lines and are drawn to scale by physical distance. (A) shows the initial Nuc1 interval bounded by red lines (D10Rat125 and D10Rat98), D10Rat32 (green line) segregated perfectly with the disease and gave a LOD score of 6.02. The final Nuc1 interval is bounded by blue lines (D10Rat195 and D10Rat29) and this region is magnified in b. Scale bar = 5Mb. (B) shows magnification of final linkage interval and markers, in which position of βA3/A1 gene (Cryba1) is marked with a red line. Scale bar = 1 Mb. (C) shows the genomic structure of Cryba1 with the site of the Nuc1 mutation highlighted in red. Scale bar = 1 Kb. (D) displays the sequence at the site of mutation in +/+ and Nuc1/Nuc1 animals. Chromatograms display the sense strand of genomic amplimers. Grey highlight indicates the highly conserved glycine codon in normal Cryba1. Sequence inserted by the Nuc1 mutation is highlighted in yellow and the TGACTAT repeats are marked by reverse arrows. Agarose gel analysis of PCR products from the region of Cryba1 including the 27 base insertion is shown in E. Expected bands are observed for wildtype and homozygote. The 3rd (heavier) band seen in the heterozygote is the result of heteroduplex formation since it is also present in the wildtype plus homozygote heteroduplex control sample. (reproduced from Sinha et al., 2008)
Figure 3.

The effects of the βA3/A1-crystallin mutation on the composition of soluble crystallins in the lens. The elution patterns show trends in the distribution of soluble proteins (+/+ = green, +/− = red, −/− = blue). The pattern from normal lens has four major peaks: a void volume peak which contains α-crystallin plus any heavy molecular weight protein aggregates, two peaks of β-crystallins of different size which have been described in many species (βH and βL), and γ-crystallin which is the dominant peak in the rat lens. In the cataract lenses, the relative proportion of the void fraction increases from about 10% in the wildtype lens to 18% in the heterozygote lens and to 32% in the homozygote. γ-crystallin, which constitutes 47% of the soluble protein in the wildtype lens, is reduced to 37% in the heterozygote and to 16% in the homozygote. As a percentage of the soluble protein, β-crystallin is quite constant in all phenotypes, however there is a dramatic shift to the lower molecular weight species (dimers) in the heterozygote and especially the homozygous lens. The inset shows a dot blot in which an antibody specific for βA3/A1-crystallin (reactive with both normal and mutant forms) is used to localize those polypeptides in the chromatographically separated peaks. In the wildtype lens the protein is present only in the β-crystallin peaks as expected (fractions 24 and 28). In the heterozgote lens, reactivity is present in those fractions, but also in the void peak. In the homozygote, reactivity is essentially limited to the void peak. This indicates that the mutant protein aggregates and does not participate in the formation of stable oligomers as does normal βA3/A1-crystallin. (reproduced from Sinha et al., 2008)
Initial interest in the Nuc1 rats resulted from the obvious dense nuclear cataracts (Sinha et al., 2005). Subsequent study demonstrated that animals with the nuclear cataract phenotype were heterozygotes for the Nuc1 mutation, and that homozygotes had severe microphthalmia and lenses that ruptured at the posterior capsule before birth (Hose et al., 2005; Sinha et al., 2005; Figure 4). Since numerous crystallin mutations that cause cataracts had been described, both in human patients and in animals, the cataracts in Nuc1 were of limited interest. However, in contrast to these other mutations, which seemed to affect only the lens, Nuc1 had other ocular manifestations. These conditions, which were obvious only in the homozygotes, included, in addition to microphthalmia, an increase in retinal thickness associated with decreased apoptosis, and failure of the hyaloid vasculature to regress normally, a condition known as persistent fetal vasculature, or PFV (Goldberg, 1997; Sinha et al., 2005; Zhang et al., 2005; Gehlbach et al., 2006; Parthasarathy et al., 2008 and Zhang et al., 2011; Figures 5 and 6). Electroretinographic (ERG) deficits were observed in the Nuc1 homozygous animals (Figure 7) as well as abnormalities in the development of retinal neurons, astrocytes and the retinal vasculature (Gehlbach et al., 2006). The evidence, taken together, was consistent with the idea that mutation of βA3/A1-crystallin impaired the ability of the retina to successfully complete the cellular remodeling necessary to become mature and fully functional. Our data suggest that the effects in the Nuc1 homozygous retina result from total loss of the original or “non-crystallin” function of βA3/A1-crystallin. It is possible that this “non-crystallin” function of βA3/A1-crystallin is also necessary in the lens, since the orientation and organization of differentiating immature lens fibers is abnormal in Nuc1 homozygotes, but not in heterozygotes (Sinha et al., 2005).
Figure 4.

Structural disorganization of developing Nuc1 lens and identification of macrophage/macrophage-like cells. Histological analysis of lenses from wild-type (a) and homozygous (Nuc1/Nuc1) mutants (b and c). Representative hematoxylin and eosin-stained sections show protrusion at the posterior pole of the lens leading to rupture of the capsule with expulsion of lens material very early in post-natal development in Nuc1/Nuc1 (b and c). Lens abnormality in structure is clear in Nuc1/Nuc1 (b) at birth when compared to wild type (a) at post-natal day 1. The morphological damage to the lens becomes more severe as post-natal development progresses (c, post-natal day 3) compared to b (at birth). (Reproduced from Hose et al., 2005)
Figure 5.
Aberrant retinal morphology in Nuc1/Nuc1 mutation. a) Wild-type retinal tissue showing normal structure at post-natal day 21. b) In contrast, Nuc1/Nuc1 has a much thicker retina at the same age. More cells are present in Nuc1 homozygotes (b) compared to wild-type littermates (a). An additional nuclear layer (arrow in b) is present in Nuc1 homozygous retina. This layer is greatly diminished by 4 months after birth (c). The transient fiber layer of Chievitz (b, arrowheads) persists in Nuc1 retinas and only disappears around 4 months of age, indicative of delayed development. Photomicrographs in this figure are from the posterior region of the retina. Abbreviations: GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner retina (c) nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer. Bar=50μm. TUNEL labeling and H&E staining of postnatal day 9 wild type and Nuc1/Nuc1 rat retinas. The thickness of Nuc1/Nuc1 retina (e) was significantly increased compared to the wild type retina (d). TUNEL labeling showed more positive cells (arrows) in the wild type (f) than in the Nuc1/Nuc1 retina (g). Semi quantitative analysis also indicated reduced number of apoptotic cells in Nuc1/Nuc1 homozygotes compared to wild-type littermates (n=7; h). Also, every layer of Nuc1 retina was thicker than wild-type (i) (n=7; i). Bar=100μm. (reproduced from Sinha et al., 2005)
Figure 6.
Defective regression of embryonic vasculature in Nuc1 mutant rat. In wild type animals (a), the hyaloid artery had completely regressed by 5 weeks of age, showing a normal optic nerve head (ONH). In 5 week old Nuc1/Nuc1 rats (b,c), the hyaloid artery and adjacent tissue were still present on the surface of the optic nerve head projecting into the vitreous (arrow). Please note in (c), the thick vessel wall of the artery at higher magnification showing cellular morphology of the retained vessel. Representative H & E stained sections from 25-day-old animals (d) show normal lens (L), iris (I), ciliary body (CB) and cornea (C). In (e) the pupillary membrane is still evident in the Nuc1 homozygous animals (arrows) whereas it has fully regressed in the wild type eye (d). Iris hyperplasia was also noted in the Nuc1/Nuc1 eyes (arrowheads). The lens shows abnormal shape and disorganization of structure. The normal eye structure at 120 days in wild-type eyes is shown in (f). In Nuc1 homozygote (g), the ciliary process (arrow) is dragged centrally towards the disrupted lens, resulting in traction, which causes peripheral retinal dragging and folding (arrowhead). Scale bar= 50μm. (reproduced from Zhang et al., 2005)
Figure 7.
Wave-form morphology shows diminished a-wave and b-wave amplitudes in homozygous but not heterozygous animals, as compared with wild-type at 10 weeks. Light-adapted wave forms are preserved in all animals (a). ERG amplitude vs. flash intensity (V-log I) curves for the dark adapted a-wave (b), dark-adapted b-wave (c) and light adapted b-wave (d) in each group of rats: wildtype (open circles, n=6), Nuc1 heterozygotes (filled squares, n=6) and Nuc1 homozygotes (filled triangles, n=5) are presented. Error bars indicate standard deviation. The dark-adapted b-wave amplitudes in the Nuc1 homozygous rats were significantly smaller than the wildtype and the Nuc1 heterozygous rats (P<0.01 at all intensities). The dark adapted a-wave amplitudes of the Nuc1 homozygous rats were smaller at the highest flash intensities relative to the wildtype (P<0.05) and the Nuc1 heterozygous (P<0.05) rats. The light-adapted b-wave amplitudes of the Nuc1 homozygous rats were comparable to the wildtype and the Nuc1 heterozygous rats (P>0.05). (e) Indicates the dark-adapted ERG b-wave/a-wave ratio of the homozygous Nuc1 rats was smaller than that of the control (P<0.05) and the heterozygous (P<0.05) rats. (reproduced from Gehlbach et al., 2006)
To establish which cell types in the retina express βA3/A1-crystallin, laser capture micro-dissection was performed on wild type (WT) rat retina; Cryba1 message was detected only in the ganglion cell layer and in the RPE (Sinha et al., 2008). Immunohistochemistry with antibodies specific to βA3/A1-crystallin confirmed its presence in RPE cells, and confocal microscopy showing colocalization of staining in the ganglion cell layer with Glial Fibrillary Acidic Protein (GFAP) demonstrated its presence in astrocytes (Sinha et al., 2008; Figure 8). Subsequent studies utilizing in situ hybridization and immunohistochemistry demonstrated expression of βA3/A1-crystallin in some ganglion cells as well (Parthasarathy et al., 2011). While no studies have been conducted on the possible role of βA3/A1-crystallin in ganglion cells, some progress has been made toward understanding its function(s) in RPE cells and in ocular astrocytes.
Figure 8.
Localization of βA3/A1-crystallin in the retina. Top-panel: Laser-capture microdissection studies. (A), (B) and (C) respectively show the location of samples taken from the ganglion cell layer (GCL), outlined in black, the inner nuclear layer (INL) outlined in green and the photoreceptor layer (PRL) outlined in pink. Scale bar = 50μm. For each region quantitative RT-PCR demonstrates the purity of the dissected tissue by measuring specific markers for each cell type. In (D), quantitative RT-PCR shows the relative expression of βA3/A1-crystallin in the 3 retinal regions of wildtype Sprague-Dawley rat at 24 days, 4 months and 10 months of age. Data shown as mean +/− SD. Middle-panel: Confocal microscopy showing GFAP positive staining at the internal limiting membrane of 4 week old wildtype (A) and Nuc1 (D) retinas. (B) and (E) show βA3/A1-crystallin staining on the same section, while (C) and (F) are merged images showing co-localization of GFAP and βA3/A1-crystallin in the wildtype and Nuc1 retinas, respectively. The fluorescence is enhanced because of the reduced amount of GFAP present in the mutant cells. Scale bar = 50μm Bottom-panel: Wildtype and Nuc1 astrocytes cultured from brain of neonatal rats. Note βA3/A1-crystallin (red) is expressed strongly in the cell nuclei of both normal and mutant astrocytes but also in the cytoplasm. The astrocytes from Nuc1 homozygous rats show striking changes in cell morphology; they are larger in size with disrupted processes and reduced expression of GFAP (green). Scale bar = 40μm (reproduced from Sinha et al., 2008)
6. βA3/A1-CRYSTALLIN IN GLIA OR GLIA-LIKE CELLS OF THE RETINA
6.1 Astrocytes
Astrocytes, glial cells unique to the central nervous system (CNS), were first visualized in 1913 by Ramon y Cajal using a gold sublimate stain (Kimelberg, 2004). Astrocytes have usually been viewed as having a passive role in the CNS; only in the past decade has emerging research suggested an active role in brain function and information processing (Parpura and Haydon, 2009; Allen and Barres, 2009). The role of astrocytes in the retina, however, remains to be determined.
Astrocytes are one of the two types of macroglial cells found in mammalian retinas. Unlike Muller cells, which span the entire thickness of the retina and are present in all mammals, astrocytes are mainly confined to the retina’s inner surface (Ling et al., 1989; Watanabe and Raff, 1988) and are closely associated with retinal blood vessels. Avascular retinas contain no astrocytes (Stone and Dreher, 1987; Schnitzer, 1988). Retinas that are diffusely vascularized contain diffusely distributed astrocytes (Stone and Dreher, 1987; Schnitzer, 1988), and those that are vascularized in a restricted region contain astrocytes only in that region (Stone and Dreher, 1987; Schnitzer, 1988).
Retinal astrocytes are immigrant cells in the retina, arising from the neuroepithelial cells of the optic stalk, the primordium of the optic nerve (Small et al, 1987). During retinal histogenesis, they migrate from the optic nerve into the inner retina where they become closely associated with retinal blood vessels (Stone and Dreher, 1987; Watanabe and Raff, 1988; Dorrell and Friedlander, 2006). Astrocytes first appear in the developing rat optic nerve at E (embryonic day) 16 and increase in number until 6 weeks after birth (Miller et al., 1985). They form a corona of processes around the optic nerve head at E18, cover approximately 35% of the retina at birth, and reach the periphery of the retina by P (postnatal day) 8 (Ling et al., 1989).
Our studies to date indicate that βA3/A1-crystallin has an important regulatory function in astrocytes (Sinha et al., 2008; Zhang et al., 2011; Ma et al., 2011; Valapala et al., 2013). There were definite morphological and functional abnormalities in the Nuc1 astrocytes as compared to WT astrocytes. In addition, comparison of retinal flat mounts from WT and Nuc1 animals demonstrated defective formation in the mutants of the normally highly organized honeycomb-like astrocyte network (Sinha et al., 2008; Figure 9, A–D). This network serves as a template for the proper formation of the retinal vasculature in rodents. The network of vessels in the Nuc1 retina was much less dense than in the normal animals, and there was leakage from some vessels in the Nuc1 retina, consistent with a failure in the process of retinal remodeling (Gehlbach et al., 2006; Sinha et al., 2008; Figure 9, E–I and Figure 10). Retinal astrocytes with the Nuc1 mutation were shown to migrate faster than WT astrocytes in vitro. In Nuc1 homozygous animals the mutant astrocytes migrated abnormally out of the retina, ensheathing the hyaloid artery where they likely were a primary cause of the PFV observed in these animals (Zhang et al., 2011; Figure 11).
Figure 9.
Retinal blood vessel abnormalities in Nuc1 homozygous rats. GFAP (red) and GSA lectin blood vessel staining (green) in wildtype (A, C) and Nuc1 retinas (B, D) at postnatal day 6 (A, B) and 10 weeks of age (C, D). In the wildtype (A), there is a dense capillary plexus (top) and a capillary-free zone around arteries. The capillary-free zones are missing in Nuc1 (B) and the capillary plexus pattern is abnormal. By 10 weeks, the wildtype retina is remodeled and the astrocyte processes are extensive and enwrapping the blood vessels (C). In Nuc1, the number of astrocytes and their processes are greatly reduced (D) suggesting fewer interactions between astrocytes and endothelial cells. In the insets of B and D, it is apparent that the Nuc1 blood vessels have too many endothelial cells suggesting that remodeling during maturation of the retinal vasculature has not occurred. Evans blue injections at 4 weeks of age demonstrate that the wildtype retinal vasculature does not leak (E) but the Nuc1 retinal blood vessels do leak (F and G at higher magnification). Using the ADPase flat-embedding technique, the normal vascular pattern is apparent in the 10 month old wildtype (H) while the Nuc1 vascular pattern is sparse, appears to be lacking a deep capillary plexus and has an abnormal pattern (I). Scale bars = 20μm (A–G) and 0.25mm (H–I). (reproduced from Sinha et al,. 2008)
Figure 10.
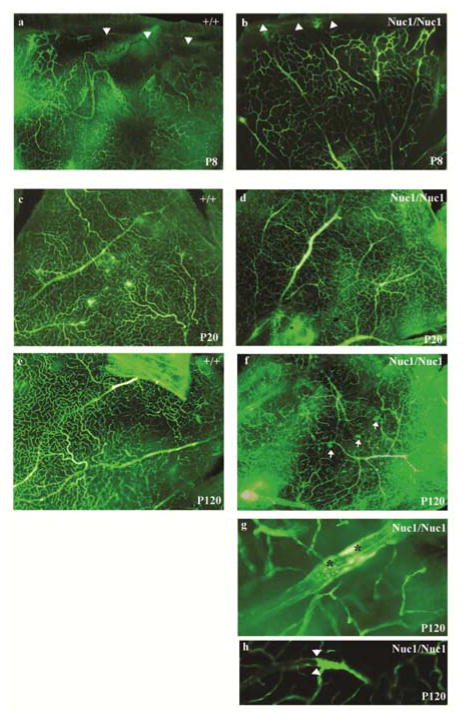
Fluorescence microscopy of flatmounts from FITC–dextran perfused wildtype and Nuc1 homozygous retinas show that vascularization of the Nuc1 homozygous retina reaches the ora serrata in advance of wildtype (a and b, arrowheads). However, by P20 the vascular patterning of both Nuc1 homozygous and wildtype appears to be similar with more large caliber vessels present in the Nuc1 homozygotes (c and d). By P120, the Nuc1 homozygote shows clear difference in the morphology and patterning of the vasculature compared with wildtype (e and f). We find evidence of microaneurysm formation in Nuc1 homozygotes (f, small arrows) and intravascular deposits (g, asterisks). Confocal microscopy (h) shows blockage of blood flow inside some vessels (arrowheads). (reproduced from Gehlbach et al., 2006)
Figure 11.
Double labeling with the macrophage marker ED1 (red) and GFAP (green) in 20-day-old wild type and Nuc1 rat eyes. A. In the wild type rat, some GFAP-positive cells (arrow) are seen at the base of the regressing hyaloid artery (HA). Clusters of ED1-positive cells (arrowheads) are also observed at the base of the involuting hyaloid artery. B. In the Nuc1 rat, the persistent hyaloid artery is surrounded by a layer of GFAP-positive astrocytes (arrows), with abundant ED1-positive cells (arrowheads) in the vicinity of the hyaloid artery. A fragment of GFAP-positive cells (arrow) is also observed in the vitreous, presumably from a tangential section of the hyaloid artery, surrounded by ED1-positive cells (arrowhead). Scale bar=50 μm, n=5 wild type, n=5 Nuc1 homozygote. (reproduced from Zhang et al., 2011)
Further, in loss- and gain-of-function mouse models for βA3/A1-crystallin, as well as in the Nuc1 mutant rat, we showed that this protein is required to form the organized astrocyte template that is essential for the remodeling of retinal vessels (Sinha et al., 2008; Sinha et al., 2012). Remodeling is a complex developmental process in which programmed cell death (PCD) plays a major role. PCD of astrocytes in the rodent retina peaks between P0 and P5 and declines by P15, coinciding with the time of astrocyte template for mation (Chan-Ling et al., 2009). We have shown that βA3/A1-crystallin regulates anoikis (Ma et al., 2011), a type of apoptotic (programmed) cell death, which is initiated by the loss of cell anchorage (Gilmore, 2005; Chiarugi and Giannoni, 2008). PCD is carefully coordinated to control the number and organization of cells in tissues and organs. Our data indicate that loss of βA3/A1-crystallin induces IGF-II and increases cell survival by regulating the PI3K/AKT/mTOR and ERK pathways, thereby protecting astrocytes from anoikis-mediated cell death (Ma et al., 2011).
To better understand which signaling mechanism βA3/A1-crystallin might regulate during the formation of the astrocyte template, we studied the Notch signaling pathway in optic nerve astrocytes. We chose Notch because it is the most significantly enriched signaling pathway in developing tissues and is known to regulate pattern formation in other systems (Collier et al., 1996; Lai, 2004). Moreover, Notch signaling has an important role during differentiation and maturation of brain astrocytes (Givogri et al., 2006; Carlen et al., 2009). We found that optic nerve astrocytes express the receptor Notch 1, as well as the ligand, Jagged1 (Valapala et al., 2013; Figure 12). This suggests that in astrocytes, Notch is involved in juxtacrine signaling, where Jagged1 from one astrocyte activates the Notch receptor on a neighboring astrocyte. This juxtacrine signaling may be critical to the proper organization of astrocytes in the developing retina. Binding of the ligand activates two sets of proteolytic cleavages in the Notch receptor. The second set of proteolytic cleavages, by the γ-secretase multiprotein complex (including presenilin, nicastrin, APH1 and PEN2), results in the release of the Notch intracellular domain (NICD), which translocates to the nucleus to activate Notch target genes (Spasic and Annaert, 2008; Kopan and Ilagan, 2009). Surprisingly, Nuc1 optic nerve astrocytes were found to express Notch1 transcript at twice the concentration of the normal cells (Valapala et al., 2013). However, when a specific antibody for NICD was used, the Nuc1 cells were shown to have less NICD than the control cells, a finding confirmed by immunofluorescence and confocal microscopy. Expression of the Notch target genes Hey1 and Hes1 was also reduced by 65–75% in the Nuc1 cells. When studies were undertaken to determine the mechanism whereby Notch signaling is decreased in the Nuc1 cells, it was found that the proteolytic processing of Notch was decreased in the Nuc1 cells, leading to decreased generation of NICD. When NICD was generated exogenously in the Nuc1 cells, it was translocated efficiently to the nucleus and activated transcription of target genes normally (Valapala et al., 2013).
Figure 12.

Levels of Notch1 and Jagged1 in immunopanned, postnatal day 2 WT and Nuc1 optic nerve astrocytes. a, b. Quantitative reverse transcriptase (qRT) PCR using Taqman expression probes shows that Notch1 was elevated by >2 fold in Nuc1 astrocytes (*P=0.027), relative to WT astrocytes. WT and Nuc1 astrocytes had similar levels of Jagged1. c. Western analysis for Jagged1 shows similar protein levels in WT and Nuc1 astrocytes. d. Retinal flat mounts from a 3-day old WT rat showing significant co-localization (yellow) of Jagged1 (red) with GFAP (green) positive astrocytes. e, f. Immunostaining and western analysis for active Notch (NICD) using anti-V1744 antibody shows that NICD was decreased by 76% in Nuc1 astrocytes compared to WT astrocytes (*P=0.0031). g. Expression and distribution of NICD and Jagged1 as revealed by immunostaining with anti-V1744 active Notch (red) and anti-Jagged1 antibodies (green). Decreased nuclear staining for NICD was evident in Nuc1 cells, but Jagged1 staining was similar in WT and Nuc1 astrocytes. Scale bar = 20μm. In all panels, graphs show mean values and error bars represent s.d. from a triplicate experiment representative of at least three independent experiments. Statistical analysis was performed by a two-tailed Student’s t-test: *P <0.05. (reproduced from Valapala et al., 2013)
Notch processing occurs in the lysosome, and the activity of γ-secretase, an acid protease, is dependent upon proper lysosomal acidification (Vaccari et al., 2010). The acidic luminal environment of endosomes, lysosomes and secretory vesicles is established by the vacuolar-type proton ATPase (V-ATPase), which pumps protons into the lumen (Yan et al., 2009; Sun-Wada et al., 2003; Vaccari et al., 2010). Interestingly, in Drosophila, various mutants that show impaired acidification due to alteration of V-ATPase activity affect Notch signaling (Yan et al., 2009; Vaccari et al., 2010). The lysosomes in the retinal astrocytes of Nuc1 animals had a significantly higher pH than did those in the control cells and the activity of V-ATPase was reduced (Valapala et al., 2013; Figure 13).
Figure 13.
Impaired V-ATPase activity in astrocytes lacking functional βA3/A1-crystallin a. V-ATPase activity was measured by acridine orange fluorescence in WT and Nuc1 astrocytes after intravesicular H+ uptake was initiated by the addition of Mg-ATP. Increase in acridine orange fluorescence upon addition of ATP was significantly greater in WT astrocytes compared to Nuc1 astrocytes (*P=0.017). During the measurement of lysosomal pH, bafilomycin a1 was added to the ATP treated lysosomes for control purposes. b. In Nuc1 astrocytes, over-expression of βA3/A1-crystallin rescued activity to >80% of normal level (*P=0.020). Empty vector had no effect. c. V-ATPase activity in heterozygote astrocytes was significantly reduced by Cryba1 siRNA knockdown (*P=0.033), but was rescued to normal levels when βA3/A1-crystallin was subsequently over-expressed in the same astrocytes (*P=0.024). d. In astrocytes from mice with floxed Cryba1, deletion of Cryba1 by Cre-recombinase decreased V-ATPase activity relative to control astrocytes (*P=0.018). The V-ATPase activity was rescued to normal levels by subsequent over-expression of βA3/A1-crystallin in Cryba1 knockout mouse astrocytes (*P=0.027). e. Measurement of endolysosomal pH in WT and Nuc1 astrocytes was performed using a fluid phase fluorescent probe, FITC-dextran. 3 hours after incubation of WT and Nuc1 astrocytes with FITC-dextran the fluorescence emission was measured at 520 nm with excitation at 450 nm and 495 nm. The fluorescence excitation ratio at 495 nm and 450 nm (I495nm/I450nm) was calculated, and endolysosomal pH in WT and Nuc1 astrocytes was measured to be ~4.5 and 5.7 respectively (*P=0.031). f. The elevated pH in Nuc1 homozygous astrocytes was restored to near normal acidic range by over-expression of βA3/A1-crystallin (*P=0.026). Empty vector had no effect. g. Further knockdown of βA3/A1-crystallin in Nuc1 heterozygote astrocytes by Cryba1 siRNA elevated the endolysosomal pH to near Nuc1 homozygous levels (*P=0.036). Subsequent over-expression of βA3/A1-crystallin decreased pH to the level characteristic of WT astrocytes (*P=0.021). Scrambled siRNA had no effect. h. pH was elevated to near Nuc1 homozygote level in Cryba1 knockout mouse astrocytes (*P=0.029) and was rescued to near normal levels by subsequent over-expression of βA3/A1-crystallin to same cells (*P=0.024). In all panels, graphs show mean values and error bars represent s.d. from a triplicate experiment representative of at least three independent experiments. Statistical analysis was performed by a two-tailed Student’s t-test: *P <0.05. (reproduced from Valapala et al., 2013)
To completely eliminate any possible effects secondary to damage to the lens or other ocular structures affected in the Nuc1 model, astrocytes isolated from the βA3/A1-crystallin floxed mice were transfected with a Cre-recombinase adenoviral construct to ablate Cryba1 expression (Valapala et al., 2013). Data from studies on these cells confirmed the results with the Nuc1 astrocytes: lysosomal pH was elevated, V-ATPase activity was decreased, NICD levels decreased, and expression of Notch target genes also decreased relative to cells transfected with empty vector. All of these abnormalities could be reversed, both in the floxed astrocytes transfected with Cre and in primary cultures of Nuc1 astrocytes, by over-expression of βA3/A1-crystallin (Valapala et al., 2013). Thus, βA3/A1-crystallin has a fundamental effect on Notch signaling in astrocytes (Figure 14), and the improper template formation and patterning by retinal astrocytes in the Nuc1 animals may be the result of defective Notch signaling.
Figure 14.

Schematic model of Notch signaling and βA3/A1-crystallin. Steps affected by loss of βA3/A1-crystallin shown by red dotted arrows. Impaired endolysosomal acidification inhibits Notch receptor processing and degradation, decreases NICD, ultimately reducing transcription of Notch target genes. (reproduced from Valapala et al., 2013)
Previous studies have shown a direct link between Notch signaling and STAT3 (Signal transducers and activators of transcription 3) activation to be important for astroglial differentiation and regulation of GFAP expression (Nagao et al., 2007; Chenn, 2009). Notch signaling induces astrocyte differentiation in gliogenic cells by STAT3-mediated activation of the GFAP promoter (Ge et al., 2002). Thus, Notch and its downstream signaling mediators are involved in the regulation of GFAP, and this pathway is also important for the regulation of astrocyte-derived VEGF (Vascular Endothelial Growth Factor). This prompted us to determine if STAT3 activation might also be affected by βA3/A1-crystallin in astrocytes, as we have shown for Notch (Valapala et al., 2013). Our data suggests that in astrocytes, STAT3 and βA3/A1-crystallin are co-regulated (Valapala et al., unpublished observations). This could lead to a positive feedback loop in astrocytes, with βA3/A1-crystallin participating in the phosphorylation of STAT3 in the cytosol and, in turn, STAT3 regulating the transcription of Cryba1 in the nucleus. This modulation of the Notch/STAT3 signaling axis by βA3/A1-crystallin appears to be involved in regulating GFAP, as well as secretion of VEGF. Loss of Cryba1 in astrocytes reduces gene promoter activity for GFAP and decreases GFAP protein expression (Gehlbach et al., 2006; Sinha et al., 2008; Valapala et al; 2013). We postulate that βA3/A1-crystallin is a local mediator in astrocytes, regulating the Notch/STAT3 signaling axis and stimulating the secretion of VEGF required for remodeling of retinal vessels.
6.2 Retinal Pigment Epithelium
The retinal pigmented epithelium (RPE) is a single layer of pigmented cells situated between the neurosensory retina and the choroid, which is the vascular layer at the back of the eye (Strauss, 2005). The RPE serves many crucial physiological roles that are vital for the normal functioning of the retina (Kevany and Palczewski, 2010). One such function is critical to the well-being of the light sensitive cells of the retina, called the photoreceptors (Kevany and Palczewski, 2010). Photoreceptors continually grow new outer segments (OS), where the photosensitive proteins are deployed to absorb incident light. As the OS are renewed from the base, the terminal portions become damaged and are released into the space between the photoreceptors and RPE. The RPE cells must engulf this material, digest it by a process called phagocytosis, and recycle the constituent molecules back to the retina so the photoreceptors can produce new OS (Kevany and Palczewski, 2010). In a related process known as autophagy, damaged proteins or organelles within the cell are collected, degraded, and removed (Feng et al., 2014). Both phagocytosis and autophagy depend upon the activity of lysosomal hydrolases for final degradation and are essential for keeping the photoreceptors healthy through the constant recycling of OS (Kaarniranta et al., 2013). We recently provided novel evidence that βA3/A1-crystallin, acting through V-ATPase/mTORC1 signaling, is essential for normal lysosome-mediated clearance in the RPE (Valapala et al., 2014).
In RPE cells of normal rats, it was demonstrated by immunoelectron microscopy that βA3/A1-crystallin specifically localizes to lysosomes (Zigler et al., 2011; Figure 15). In contrast, in rats homozygous for the Nuc1 mutation, which do express the larger mutant forms of βA3- and βA1-crystallins, immunoreactivity was randomly distributed within the cytoplasm of RPE cells, with no indication of lysosomal localization. Ultrastructural analysis revealed abnormalities in the RPE of young Nuc1 mutant animals, including disorganization of basal infoldings and an increased number of vacuoles. By one year of age, lipofuscin-like aggregates and large vacuoles containing undigested cellular organelles and debris as well as photoreceptor outer segment discs were abundant in the RPE of the mutant animals (Figures 16 and 17). Decreased activity of the lysosomal hydrolase, Cathepsin D, and a decrease in the concentration of the active form of microtubule-associated protein 1A/1B light chain 3 (LC3-II, an indicator of autophagy activity) were observed in the mutant RPE. These findings are consistent with the observed decrease in the lysosome-mediated degradation of cargo contained in phagosomes and autophagosomes (Figure 18). This suggests that βA3/A1-crystallin has an important function in the lysosomes of the RPE cell. Two factors must be considered when comparing this putative function with the structural role of βA3/A1-crystallin in the lens. First, the concentration of the protein in the lens is extremely high compared to its concentration in RPE. Siezen et al (1986) and later investigators have clearly demonstrated that in vitro, protein concentration markedly affects the state of oligomerization of β-crystallin. Secondly, the function in the lysosome must occur at the very acidic (~pH 4.5) environment of this organelle, as compared to the neutral pH in the lens. The increased acidity could be expected to cause conformational change in the protein. Thus, it is likely that not only the function, but also the structure, of βA3/A1-crystallin is different in RPE as compared to lens.
Figure 15.

Immuno-electron microscopy to determine the intracellular localization of βA3/A1-crystallin in RPE cells. In Panel A is shown a representative image from a 5.5 month old wild type animal. The arrows indicate lysosomes which contain nearly all the gold particles. In contrast, Panel B is an image from an age-matched Nuc1 animal showing several lysosomes which lack gold particles. In this sample, the gold is randomly distributed within the cytoplasm, the arrow indicates an example. Image C is from a 22 month old Nuc1 animal. Part of a large lysosome is visible at the lower right and does not contain gold particles. As in B, gold particles are distributed throughout the cytoplasm. In this section they are not as apparent because a shorter silver enhancement step was used. To demonstrate the particles the area indicated by the arrow is shown at higher magnification in the insert. Scale bar = 500nm (A–C) and 125nm (C, inset) (reproduced from Zigler et al., 2011)
Figure 16.
Demonstration of abnormal lipid accumulation in the Nuc1 RPE. At 10M of age, minimal autofluorescence is seen in the RPE cells of the wild type animals (A). However, abundant autofluorescence (arrows) is observed in the RPE of 10M Nuc1 animals, consistent with accumulation of lipofuscin. (B). Autofluorescence viewed with red filter (560–620nm). Oil red O staining, indicating presence of neutral lipids, shows abundant intense staining in the RPE cells of the 10M old Nuc 1 retinas (arrows, D), but not in the control wild type animals (C). Scale bar = 50 μm (reproduced from Zigler et al., 2011)
Figure 17.

At higher magnification prominent features of RPE cells from 1 year old Nuc1 animals are shown: A. RPE cell showing large aggregates of lipofuscin-like material (arrow). A large vacuole contains many degenerated cellular organelles (arrowheads) intermixing with lipofuscin-like aggregates (arrows). B. Abundant lipofuscin-like aggregates (arrows) and a large phagosome containing undigested outer segment discs (arrowhead) are present in the cytoplasm of an RPE cell. Scale bar = 500 nm. (reproduced from Zigler et al., 2011)
Figure 18.
Phagosomes and Autophagosomes in conditional knockout of βA3/A1- crystallin in RPE cells. (A) Transmission electron microscopy image from a 2-month old RPE of cryba1 cKO mouse showing phagosomes containing shed outer segment discs (arrows). Bar, 2 μm. (B) TEM showing the same phagosomes (as in A) at higher magnification to demonstrate that they are enclosed by a single membrane. Bar, 500 nm. (C) A 5-month old Cryba1 cKO mouse RPE shows autophagosomes with double membranes (arrows). M designates mitochondria, which also have double membranes. Bar, 500 nm. (D) TEM images from RPE of a 7-month old Cryba1 cKO mouse showing an autophagosome with double membrane (arrows). Bar, 500 nm. (E) A 12-month old starved Cryba1fl/fl mouse RPE shows a phagosome containing shed outer segment discs (arrow). Bar, 500 nm. (F) TEM showing an autophagosome with double membrane (arrows) in a 12-month old Cryba1fl/fl mouse following starvation. Bar, 500 nm. (reproduced from Valapala et al., 2014)
To definitively demonstrate that βA3/A1-crystallin has cellular functions outside the lens that are distinct from its refractive function in the lens, a genetic approach was adopted. We generated a conditional knockout mouse in which Cryba1 was deleted specifically from RPE using the Cre-loxP technology. Cryba1 floxed mice (Valapala et al., 2013) were mated with Best1-cre mice (Iacovelli et al., 2011) that express Cre recombinase specifically in RPE to produce Cryba1 cKO mice (Valapala et al., 2014). Live cell imaging of cultured primary RPE cells showed accumulation of (auto)phagosomes in the cells isolated from cKO animals as compared to those isolated from control animals. Over-expression of βA3/A1-crystallin in the cKO cells alleviated this accumulation, while SiRNA knockdown of Cryba1 expression in control cells mimicked the effect seen in cKO cells. Sub-cellular fractionation of normal RPE cells revealed that βA3/A1-crystallin is a lysosomal lumenal protein (Figure 19, A-B). Immunoprecipitation studies suggest that it binds to the V0-ATPase a1 subunit (Figure 20); if this result is confirmed, βA3/A1-crystallin would be the first binding partner identified for the V0 domain (a1 subunit) of V-ATPase in a mammalian system. The a1 subunit is known to mediate phagosome-lysosome fusion in the brain (Peri and Nusslein-Volhard, 2008; Valapala et al., 2014). Since acidification of the endolysosomal compartments is essential for lysosomal clearance of (auto)phagosome cargo, the endolysosomal pH was determined in RPE cells from Cryba1 floxed and cKO mice. Consistent with the findings in astrocytes, the pH was higher in the knockout cells (Figure 21), and the activity of V-ATPase, the proton pump responsible for the acidification, was significantly decreased (Figure 22). Again, over-expression of βA3/A1-crystallin in the cKO cells rescued both the pH and the V-ATPase activity to the normal range (Valapala et al., 2014; Figures 21 and 22).
Figure 19.

Loss of Cryba1 affects lysosomal acidification. (A) Subcellular fractionation as assessed by measuring the activity of HEXA, a lysosomal lumenal enzyme. The purification factor is calculated from the ratio of the specific activities by normalizing the specific activity of the post-nuclear supernatant (PNS) to 1. (B) Western blotting of lysosomal fractions showed βA3/A1-crystallin to be in the lysosomal lumen fraction (fraction 1), with minimal immunostaining in the lysosomal membrane fraction (fraction 2). LAMP1 and HEXA indicate the purity of the fractions. (reproduced from Valapala et al., 2014)
Figure 20.

(A) Coimmunoprecipitation of lysosomal lumen and membrane extracts from Cryba1fl/fl cells with βA3/A1-crystallin antibody and immunoblotting with V0-ATPase a1 antibody, demonstrating pull-down of V0-ATPase a1 subunit by the crystallin antibody. Non-specific IgG was used as negative control. (B) Coimmunoprecipitation of lysosomal lumen and membrane extracts from Cryba1 cKO cells with βA3/A1-crystallin antibody and immunoblotting with V0-ATPase a1 antibody, demonstrating that the crystallin antibody does not pull-down V0-ATPase a1 as there is no detectable band on the gel. Non-specific IgG was used as negative control. (adapted from Valapala et al., 2014)
Figure 21.

(A) Lysosomal pH in cultured primary RPE cells from Cryba1fl/fl mice was ~ 4.5, while in cKO cells it was ~ 5.2. The lysosomal pH was not significantly changed when cells were starved (St) to induce autophagy. (B) Overexpression of βA3/A1-crystallin in cKO cells reduced lysosomal pH to the normal value of 4.5, while empty vector had no effect. Graphs show mean values and error bars represent s.d. from a triplicate experiment, representative of at least 3 independent experiments. Statistical analysis was performed by a two-tailed Student t-test: *P<0.05. (reproduced from Valapala et al., 2014)
Figure 22.

V-ATPase is regulated by βA3/A1-crystallin in RPE cells. Left panel: V- ATPase activity in isolated lysosomes from Cryba1fl/fl RPE cells is about twice that of cKO cells. Bafilomycin A1 (Baf-1) reduced activity markedly in both cell types. Right panel: Overexpression of βA3/A1-crystallin in cultured RPE cells from cKO mice restored lysosomal V-ATPase activity to near normal level. The point of addition of ATP (1.4 μM final concentration) to activate the enzyme is indicated. Each point represents the average of 3 determinations; error bars represent mean ± s.d. (reproduced from Valapala et al., 2014)
Recently, V-ATPase has been shown to be an important component of the mTOR (mechanistic target of rapamycin) signaling pathway (Bar-Peled and Sabatini, 2014). mTOR is an atypical serine/threonine kinase that has been conserved throughout evolution. It interacts with many proteins to form at least two distinct multiprotein complexes called mTORC1 and mTORC2 (Laplante and Sabatini, 2013). On the basis of physical interactions with rapamycin, mTORC1 and mTORC2 have been respectively characterized as rapamycin-sensitive and rapamycin-insensitive complexes (Laplante and Sabatini, 2013). The mTOR-containing complexes also have different upstream inputs and downstream outputs (Zoncu et al., 2011). mTORC1 integrates multiple signals either to promote cellular growth when growth factors, nutrients and energy are available, or to induce catabolic processes during stress (Zoncu et al., 2011). The downstream effects of active mTORC1 include translation of mRNA, suppression of autophagy, ribosome biogenesis and activation of transcription leading to mitochondrial metabolism (Zoncu et al., 2011). mTORC2 promotes cellular survival, in part through regulating cytoskeletal dynamics, ion transport, and growth (Zoncu et al., 2011). V-ATPase has been shown to play a direct role in amino acid signaling to mTORC1 (mTOR complex 1) and to be a component of the mTOR pathway in lysosomes (Zoncu et al., 2011). Previous studies have shown the existence of bidirectional regulatory loops between mTORC1 and V-ATPase (Pena-Llopis et al., 2011), and it is known that lumenal pH is involved in mTORC1 regulation and has important implications for its ability to control autophagy (Korolchuk et al., 2011). Following induction of autophagy in vivo by nutrient starvation, there was a significant increase in the level of p-mTOR (active mTOR) in the cKO cells relative to control cells. Further, there was a significant decrease in p-AKT in the knock-out cells, although total AKT and mTOR levels were similar in the cKO and control cells. When cultured cKO astrocytes were transfected with a vector construct over-expressing βA3/A1 crystallin, both of these effects were reversed. Downregulation of Cryba1 in control cell cultures decreased the level of p-AKT and increased p-mTOR, mimicking the findings in the cKO cells in vivo (Valapala et al., 2014; Figure 23). Therefore, crosstalk between the V-ATPase and mTORC1 appears to be necessary for regulating autophagy, and Cryba1 is required for proper functioning of this signaling axis (Valapala et al., 2014). Based on our studies to date, we speculate that βA3/A1-crystallin may regulate mTORC1 activity in RPE cells by modulating V-ATPase in the lysosomal lumen (Figure 24).
Figure 23.
Cryba1 regulates the mTORC1 signaling pathway. (A) Following induction of autophagy in vivo by starvation, RPE/choroid was isolated and levels of signaling intermediates determined. In the cKO samples p-Akt was decreased compared to Cryba1fl/fl, while p-MTOR was increased. Total Akt and total mTOR were not changed. (B) In starved RPE cells cultured from cKO mice, overexpression of βA3/A1-crytallin increased p-Akt and p-Raptor expression, while p-MTOR decreased. (C) Conversely, downregulation of βA3/A1-crystallin expression in Cryba1fl/fl cells using siRNA, decreased p-Akt, and p-Raptor while p-mTOR was upregulated. (D) Rapamycin, an inhibitor of mTOR, was effective at decreasing p-mTOR and increasing p-Raptor levels in the cKO cells. (adapted from Valapala et al., 2014)
Figure 24.

A schematic diagram showing the mechanism of mTORC1 activation at the surface of the lysosomes and a possible role for βA3/A1-crystallin in the process. In the “inside-out” model of amino acid sensing postulated by Zoncu et al. (2011), the proton pump, vacuolar H (+)-adenosine triphosphate ATPase (V-ATPase) interacts in an amino acid sensitive manner with pentameric Ragulator, a scaffolding complex that anchors the heterodimeric Rag GTPases to the lysosomes. This leads to the translocation of the inactive mTORC1 to the lysosomal surface. Once mTORC1 is on the surface of the lysosomes, it is activated by Rheb that is also localized to the lysosomal surface. It has been postulated that amino acids are probably translocated to the lysosomal lumen by V-ATPase and the amino acid signaling from the lysosomal lumen plays an important role in the complex process of recruiting mTORC1 to the lysosomal surface and activating it. We have recently shown that βA3/A1-crystallin is localized to the lysosomal lumen of RPE cells and is a binding partner of V-ATPase. We speculate that βA3/A1-crystallin may regulate the activity of mTORC1 by modulating V-ATPase and thereby amino acid signaling from the lysosomal lumen of RPE cells.
Finally, to determine whether conditional knockout of βA3/A1-crystallin in the RPE had an effect on retinal function, electroretinography was performed on cKO and control mice (Figure 25). At 2 months of age, only the photopic b-wave amplitudes were decreased in the cKO animals, whereas by 7 months of age the a-wave amplitudes were also decreased. This indicates that the cone function is affected first, but that both rods and cones are ultimately affected by loss of Cryba1 expression in RPE (Valapala et al., 2014).
Figure 25.
Decreased lysosomal clearance leads to loss of cellular homeostasis in RPE of Cryba1 cKO mice. (A) Scotopic ERGs from eyes of 4-month old Cryba1 cKO mice showed similar a-wave amplitudes as those in Cryba1fl/fl mice (n = 10 for each group), indicating that rod photoreceptor function was not affected. (B) Photopic ERGs of cKO mice from the same age (4 months) showed smaller b-wave amplitudes in comparison to Cryba1fl/fl mice (n = 10 for each group), indicating reduced cone photoreceptor function (*P≤0.018 by ANOVA with Dunnett correction for multiple comparisons). (C) Scotopic ERGs from 7-month old cKO mice showed decreased a-wave amplitudes when compared to Cryba1fl/fl mice (n = 10 for each group), demonstrating that rod photoreceptor function was reduced significantly (*P≤0.0002 by ANOVA with Dunnett correction for multiple comparisons). (D) Photopic ERGs from these 7-months old cKO mice also showed further decrease in b-wave amplitudes compared to Cryba1fl/fl mice (n = 10 for each group), indicating the cone function had been reduced (*P≤0.0001 by ANOVA with Dunnett correction for multiple comparisons). (reproduced from Valapala et al., 2014)
7. βA3/A1-CRYSTALLIN IN OCULAR HEALTH AND DISEASE
There is abundant evidence, both from human mutations and from experimental animal studies, that β/γ-crystallins play a critical role in the maintenance of lens transparency. Numerous mutations in these proteins cause cataracts (see reviews by Hejtmancik, 2008 and Graw, 2009) with a variety of phenotypes. Almost all of these cataracts are dominant traits, so the human patients studied will be heterozygotes for the mutation; thus abnormal phenotypes resulting from loss or modification of “non-crystallin” functions of the protein in these patients would not be present if such a trait were recessive. In the Philly mouse, which has a deletion mutation in the βB2-crystallin gene, cataract is a dominant phenotype, but sub-fertility, presumably associated with the fact that the protein was detected in both sperm and ovary, was reported only in mice homozygous for the mutation (DuPrey et al, 2007). A single report in the human genetic literature of an individual presumed to be homozygous for a βB2-crystallin mutation is suggestive (Litt et al, 1997). While heterozygotes for this mutation developed cataracts requiring extraction in adulthood, the homozygous individual required cataract surgery by age 5 and lost all visual perception by adolescence. Thus, while the function or functions of β/γ-crystallins outside of the lens remain unclear, evidence is accumulating that such functions do exist and that in certain cell types they may be important.
In the Nuc1 rat, which has a mutation in the βA3/A1-crystallin gene, heterozygotes have congenital cataracts (Sinha et al., 2005), whereas non-lens ocular effects are apparent only in the homozygotes. One such abnormality that we have studied is the persistence of the fetal vasculature (PFV) (Zhang et al., 2005; Zhang et al., 2011). During early development of the mammalian eye, there is a transient network of blood vessels arising from the area of the optic nerve, extending through the vitreous, and surrounding the developing lens. Called the fetal vasculature, these blood vessels nourish the lens and retina before formation of the retinal vasculature. When vessels begin to appear in the retina, the fetal vasculature normally degenerates and disappears completely, prior to birth in humans and within the first few weeks of post-natal development in rodents, to provide an optically clear path between the cornea and the retina. One of the more common congenital, developmental disorders of the eye, persistent fetal vasculature, results from the complete or partial failure of this vascular regression (Goldberg, 1997). PFV is a disease that leads to blindness or serious loss of vision, with few treatment options at present, in otherwise normal children. The Nuc1 animal model provides unique opportunities to investigate molecular mechanisms underlying PFV disease, thereby helping us to better understand the disease etiology and potentially to develop therapies to prevent or ameliorate the condition. Our findings suggest that abnormalities in Nuc1 astrocytes cause them to migrate faster and to ensheath the hyaloid vasculature, thereby inhibiting its regression. Further, the Nuc1 mutant rats, with an aberrant astrocyte template and defective retinal remodeling, show an age-dependent increase in the expression of colony stimulating factor-1 receptor (CSF-1R) and infiltration of microglia, suggesting the activation of an immune response (unpublished observations). Microaneurysm formation, intravascular deposits, vessel leakage and blockage of vessels, characteristic clinical features of diabetic retinopathy, have been observed in Nuc1 rats (Gehlbach et al., 2006). The Nuc1 model may provide mechanistic insights as to how abnormalities in astrocytes contribute to both neuronal cell death and vascular changes in diabetic retinopathy.
As noted above, we have reported evidence suggesting that βA3/A1-crystallin interacts directly with the V0-ATPase a1 subunit (Valapala et al., 2014). In vivo, V-ATPase activity is regulated by several mechanisms, including reversible dissociation of the V1 and V0 domains (Forgac, 2007). We speculate that binding of βA3/A1-crystallin to V0-ATPase a1 regulates H+-pumping activity by assembly/disassembly of the holoenzyme. Interestingly, presenilin 1, a protein that is often mutated in Alzheimer’s disease, is required for the V0 a1 subunit of V-ATPase to be translocated to lysosomes (Lee et al., 2010). In neurodegenerative diseases of the brain, such as Alzheimer’s and Parkinson’s, a number of studies suggest that defective lysosomal clearance is involved in the disease pathogenesis (Bergamini et al., 2004; Keller, 2004; Shintani and Klionsky, 2004). It should also be noted that in Alzheimer’s disease, optic nerve degeneration and loss of retinal ganglion cells have been reported (Hinton et al., 1986). Further knowledge of the binding complex between V-ATPase and βA3/A1-crystallin in the RPE could identify a specific role of βA3/A1-crystallin in the acidification of the endolysosomal system, thereby revealing new therapeutic targets for retinal degenerative diseases that result from abnormalities in lysosomal function.
It is also likely that the accumulation of undegraded intracellular material disturbs RPE homeostasis and triggers a parainflammatory response in an attempt to restore normal RPE function. Tissue homeostasis is monitored by resident tissue macrophages that normally remain in a basal homeostatic state. When cellular homeostasis is disrupted, a state of para-inflammation will be initiated (Medzhitov, 2008). However, when tissue malfunction is excessive, the support provided by resident macrophages may be insufficient, requiring recruitment of additional macrophages and thereby, induction of chronic inflammation. We believe that prolonged impairment of lysosomal-mediated clearance in RPE, as seen in our Cryba1 cKO model, can convert para-inflammation to chronic inflammation (Valapala et al., 2014b). Indeed, dysregulated para-inflammation, leading to a chronic inflammatory state, has been proposed to play an important role in other diseases, including type 2 diabetes, cardiovascular disease and age-related diseases of the retina (Medzhitov, 2008; Xu et al., 2009).
Aging is the main risk factor for the development of various diseases, including age-related macular degeneration (AMD); therefore, understanding the mechanisms regulating aging may lead to new avenues of treatment that could delay the development of such diseases. The mTOR pathway regulates many major cellular processes and is implicated in an increasing number of pathological conditions, including cancer, obesity, type 2 diabetes and neurodegeneration (Efeyan et al., 2012). In an elegant study (Zoncu et al., 2011), V-ATPase was shown to be a component of the mTOR pathway in lysosomes. Previous studies have shown the existence of bidirectional regulatory loops between mTOR and V-ATPase (Pena-Llopis et al., 2011). This crosstalk between V-ATPase and mTOR is necessary for regulating cellular processes, including autophagy, and βA3/A1-crystallin may be an essential component of this signaling axis (Valapala et al., 2014). A multicenter study (Interventions Testing Program) conducted by the National Institute of Aging, NIH, reported that inhibition of mTOR with rapamycin expands maximal and median life spans in mice (Harrison et al., 2009). The study suggested that mTORC1 inhibition may be effective in treating age-related diseases even when the treatment is initiated in middle-aged humans. However, the mechanism by which mTORC1 inhibition increases longevity in mammals is unresolved. It should also be noted that strong inhibition of mTORC1 would have considerable adverse effects, particularly if treatment were required long term for chronic conditions. A better understanding of the functions of the m-TOR interacting proteins might permit the development of more specific modulators of the mTOR complexes that perturb their function only toward specific effectors. Since defective lysosomal homeostasis in RPE has been suggested to be involved in the pathogenesis of AMD disease (Bird, 2010; Chen et al., 2011; Tseng et al., 2013), one could speculate that βA3/A1-crystallin might be one such effector that could be targeted in an effort to restore or maintain normal lysosomal function.
8. FUTURE DIRECTIONS
In loss and gain-of-function mouse models for βA3/A1-crystallin and in the Nuc1 mutant rat, we show that this protein is needed to establish the specific mechanisms and signaling molecules involved in crucial physiological processes. It must be recognized that these mechanisms could be different in the different cell types where the protein is expressed. Complicating the efforts to get at this most important question are several other issues which remain unresolved and which could have major impact on our understanding of these functions. First, two distinct proteins (βA3- and βA1-crystallin) are produced from the Cryba1 gene by leaky ribosomal scanning. Are they equivalent and inter-changeable in terms of their role as crystallins in the lens and with respect to their putative functions in other cell types? Alternatively, does each protein have an independent function in some or all of these situations? Secondly, do βA3- and βA1-crystallins exert their functions independently or only when associated with other β-crystallin polypeptides. The dogma from studies on the lens is that the β-crystallins exist only as dimers or higher oligomers of the seven polypeptides constituting the family. This conclusion is based on in vitro studies of lens homogenates and association studies using dilute solutions of isolated or recombinant proteins, which may not reflect the situation in the intact lens with its extremely high protein concentration. Recent studies have cast doubt on the long established concept of α-crystallin structure in the lens (Gangalum et al., 2012), and the same concerns regarding extrapolation from in vitro to in vivo status could apply in this situation as well. With respect to the situation in other cell types, it is not even clear at this point what complement of β-crystallins, aside from βA3/A1-crystallin, is expressed in RPE cells, astrocytes, or ganglion cells. In summary, a growing body of evidence supports the hypothesis that β/γ crystallins, and in particular βA3/A1-crystallin, have specific cellular functions distinct from their role in the lens as structural elements contributing to refractivity and transparency. In the case of βA3/A1-crystallin it appears that these “non-crystallin” functions are mediated via signaling pathways. It should be noted that it is highly likely that the βA3/A1-crystallin in lysosomes, where the “non-crystallin” function occurs, is different not only in function, but also in structure from the protein in the lens. Studies on recombinant proteins in vitro have shown that at low concentration, βA3/A1-crystallin exists as a mixture of monomers and dimers rather than the dimers and higher oligomers believed to be present in the lens (Sergeev et al., 2000). The very acidic conditions within lysosomes could also be expected to affect the conformation of the polypeptides.
It is likely that βA3/A1-crystallin regulates multiple cellular processes in astrocytes and RPE by modulating specific signaling pathways mediated by V-ATPase. Our in silico docking and co-immunoprecipitation results suggest that βA3/A1-crystallin and V0-ATPase a1 subunit may interact, with formation of a complex. Future Nuclear Magnetic Resonance (NMR) studies may establish that such binding actually occurs and may also further our understanding of the structure-function relationship of this binding. Should the binding of βA3/A1-crystallin and V0-ATPase a1 be confirmed by NMR, it could be speculated that the putative lysosomal sorting signal present on the surface of βA3/A1-crystallin (Zigler et al., 2011) might be important in the trafficking of V-ATPase to lysosomes in astrocytes and RPE cells, since previous studies reported that the V0-ATPase a1 subunit is important in organelle targeting (Lee et al., 2010). We have also demonstrated that βA3/A1-crystallin participates in the mTOR signaling pathway in the lysosomes of RPE cells (Valapala et al., 2014). To understand how βA3/A1-crystallin affects lysosomal function, it will be necessary to elucidate its interaction with mTORC1. V-ATPase, previously shown to be a component of the mTORC1 pathway in lysosomes (Zoncu et al., 2011), may be the source of this interaction. Other studies have shown the existence of bi-directional regulatory loops between mTOR and V-ATPase (Pena-Llopis, 2011). This crosstalk is necessary for regulating cellular processes and βA3/A1-crystallin may be an essential component of this signaling axis.
To better understand the “crystallin” function of βA3/A1-crystallin, we will determine how its mutation, or deletion, affects the expression, oligomerization, and interactions of the other β-crystallins in the lens. Further, we will determine whether the loss of these two major polypeptide constituents from the highly ordered lens protein matrix affects the expression levels and distribution of the other major lens proteins, the α- and γ-crystallins, and investigate how changes in the complement of lens crystallins affects lens growth and transparency. Moreover, βA3/A1-crystallin may have other cellular functions in lens, apart from its structural role. Based on our studies (Sinha et al., 2005), we will investigate whether it is involved in the orientation of elongating lens fiber cells and the subsequent loss of organelles from the terminally differentiated fibers.
Finally, it remains to be determined whether mutations in βA3/A1-crystallin can contribute to, or cause, human ocular disease aside from cataract. Multiple mutations in Cryba1 are known to cause autosomal dominant cataracts of various descriptions (Hejtmancik, 2008; Graw, 2009). These are undoubtedly due to disruption of the “crystallin” function of the proteins, leading to protein aggregation and light scattering in the lens. Based on animal studies, effects of βA3/A1-crystallin mutations, other than cataract, are apparent only in homozygotes; to date, such homozygous individuals have not been identified among human patients. Since βA3/A1-crystallin has been shown to be expressed in the human retina and RPE (unpublished observations), the animal studies suggest that some mutations would negatively affect these tissues, perhaps producing retinal degenerative changes.
Acknowledgments
We thank Drs. Morton F. Goldberg and Eric W. Wawrousek for critical reading and discussion of this review. We thank Ms. Stacey Hose for able technical assistance and for preparation of the figures. DS is a recipient of the Carolyn K. McGillvray Memorial Award for Macular Degeneration Research from BrightFocus Foundation and the Sybil B. Harrington Special Scholar Award for Macular Degeneration from Research to Prevent Blindness. The authors would like to acknowledge funding support from National Institutes of Health: EY019037 (DS), EY019037-S (DS), EY018636 (DS), EY01765 (Wilmer Imaging Core); IPA from National Eye Institute (DS); Research to Prevent Blindness (unrestricted grant to The Wilmer Eye Institute); Wilmer Pooled Professors Fund (DS); Knights Templar Eye Foundation (DS); Helena Rubinstein Foundation (DS); Raymond Kwok Research Fund (from Walter Stark) and Intramural Research Program, National Eye Institute (JSZ). The authors would also like to thank the following for their contributions to the earlier studies leading up to this review: Souvonik Adhya, Mille Arora, Laura Ashaghi, Walid Barbour, Colin Barnstable, Imran A. Bhutto, Lawrence Brako, Karl W. Broman, Peter Campochiaro, Marisol Cano, Aling Dong, Lijin Dong, Melinda K. Duncan, Charles G. Eberhart, Masoud Ashaei Fard, Robert N. Fariss, Peter Gehlbach, Madhumita Ghosh, Morton F. Goldberg, Celine Gongora, Rhonda Grebe, William R. Green, Seth Greenbaum, Limin Gu, Sean Hackett, Laszlo Hackler, Jr., James T. Handa, J. Fielding Hejmancik, Stacey Hose, Andrew Klise, Bo Lei, Woo-Kuen Lo, Gerard A. Lutty, Bo Ma, Tanya Malpic-Ilanos, D. Scott McLeod, Avindra Nath, Rachel Neal, Terrence P. O’Brien, Geetha Parthasarathy, Bonnie Patek, W. Gerald Robison, Jr., Paul Russell, Sonia Samtani, Gitanjali Sehrawat, Tanusree Sen, Samhita Sengupta, Yuri Sergeev, Kamaljeet Singh, Walter J. Stark, Olof Sundin, Mallika Valapala, Eric F. Wawrousek, Christine Wilson, Goutong Xu, Fang Yang, Donald J. Zack and Cheng Zhang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen NJ, Barres BA. Glia-more than just brain glue. Nature Neuroscience. 2009;451:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- Andley U. Crystallins in the eye: Function and pathology. Prog Retinal Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24:400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman OA, Sarra R, van Genesen ST, Kappe G, Lubsen NH, Slingsby C. The stability of human acidic β-crystallin oligomers and hetero-oligomers. Exp Eye Res. 2003;77:409–422. doi: 10.1016/s0014-4835(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Bergamini E, Cavallini G, Donati A, Gori Z. The role of macroautophagy in the ageing process, antiageing intervention and age-associated diseases. Int J Biochem Cell Biol. 2004;36:2392–2404. doi: 10.1016/j.biocel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010;120:3033–3041. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I. Thesis. University of Uppsala, Department of Medical Chemistry; 1964a. Studies on the soluble proteins of the bovine lens. [Google Scholar]
- Björk I. Studies on γ-crystallin from calf lens II. Purification and some properties of the main components. Exp Eye Res. 1964b;3:254–261. doi: 10.1016/s0014-4835(64)80018-x. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Blundell TL, Lindley P, Miller L, Moss D, Slingsby C, Tickley I, Turnell B, Wistow G. The molecular structure and stability of the eye lens: X-ray analysis of γ-crystallin II. Nature. 1981;289:771–777. doi: 10.1038/289771a0. [DOI] [PubMed] [Google Scholar]
- Burky EL, Woods AC. Lens protein—the isolation of a third (gamma) crystallin. Arch Opthal. 1928;57:464–466. [Google Scholar]
- Carlen M, Meletis K, Göritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabé-Heider F, Yeung MS, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisén J. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Chu Y, Baxter L, Weible M, II, Hughes S. In vivo characterization of astrocyte precursor cells (APCs) and astrocytes in developing rat retinae: differentiation, proliferation, and apoptosis. Glia. 2009;57:39–53. doi: 10.1002/glia.20733. [DOI] [PubMed] [Google Scholar]
- Chen PM, Gombart ZJ, Chen JW. Chloroquine treatment of ARPE-19 cells leads to lysosome dilation and intracellular lipid accumulation: possible implications of lysosomal dysfunction in macular degeneration. Cell Biosci. 2011;1:1–10. doi: 10.1186/2045-3701-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A. A top-NOTCH way to make astrocytes. Dev Cell. 2009;16:158–159. doi: 10.1016/j.devcel.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Clayton RM, Campbell JC, Truman DES. A re-examination of the organ specificity of lens antigens. Exp Eye Res. 1968;7:11–29. doi: 10.1016/s0014-4835(68)80022-3. [DOI] [PubMed] [Google Scholar]
- Collier JR, Monk NA, Maini PK, Lewis JH. Pattern formation by lateral inhibition with feedback: a mathematical model of delta-notch intercellular signaling. J Theor Biol. 1996;183:429–446. doi: 10.1006/jtbi.1996.0233. [DOI] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong WW, Lubsen NH, Kraft HJ. Molecular evolution of the eye lens. Prog Ret Eye Res. 1994;13:391–442. [Google Scholar]
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Dirks RP, van Genesen ST, Kruse JJ, Jorissen L, Lubsen NH. Extralenticular expression of the rodent βB2-crystallin gene. Exp Eye Res. 1998;66:276–269. doi: 10.1006/exer.1997.0439. [DOI] [PubMed] [Google Scholar]
- Dolinska MB, Sergeev YV, Chan MP, Palmer I, Wingfield PT. N-terminal extension of βB1-crystallin: Identification of a critical region that modulates protein interaction with βA3-crystallin. Biochem. 2009;48:9684–9695. doi: 10.1021/bi9013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006;25:277–295. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Driessen HP, Herbrink P, Bloemendal H, de Jong WW. The β-crystallin Bp chain is internally duplicated and homologous to γ-crystallin. Exp Eye Res. 1980;31:243–246. doi: 10.1016/0014-4835(80)90082-2. [DOI] [PubMed] [Google Scholar]
- DuPrey KM, Robinson KM, Wang Y, Taube JR, Duncan MK. Subfertility in mice harboring a mutation in βB2-crystallin. Mol Vision. 2007;13:366–373. [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18:524–533. doi: 10.1016/j.molmed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Ophthalmol Vis Res. 2000;41:3943–3954. [PubMed] [Google Scholar]
- Fischer D, Hauk TG, Muller A, Thanos S. Crystallins of the β/γ-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci. 2008;37:471–479. doi: 10.1016/j.mcn.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- Gangalum RK, Horwitz J, Kohan SA, Bhat SP. Alpha A-crystallin and alpha B-crystallin reside in separate subcellular compartments in the developing lens. J Biol Chem. 2012;287:42407–42416. doi: 10.1074/jbc.M112.414854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Martinowich K, Wu X, He F, Miyamoto A, Fan G, Weinmaster G, Sun YE. Notch signaling promotes astrogliogenesis via direct CSL-mediated glial gene activation. J Neurosci Res. 2002;69:848–860. doi: 10.1002/jnr.10364. [DOI] [PubMed] [Google Scholar]
- Gehlbach P, Hose S, Zhang C, Cano M, Lei B, Barnstable C, Goldberg MF, Zigler S, Sinha D. Developmental abnormalities in the Nuc1 rat retina: a spontaneous mutation that affects neuronal and vascular remodeling and retinal function. Neuroscience. 2006;137:447–461. doi: 10.1016/j.neuroscience.2005.08.084. [DOI] [PubMed] [Google Scholar]
- Gilmore AP. Anoikis. Cell Death Differ. 2005;12:1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1997;124:587–626. doi: 10.1016/s0002-9394(14)70899-2. [DOI] [PubMed] [Google Scholar]
- Graw J. Genetics of crystallins: cataract and beyond. Exp Eye Res. 2009;88:173–189. doi: 10.1016/j.exer.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head MW, Sedowofia K, Clayton RM. βB2-crystallin in the mammalian retina. Exp Eye Res. 1995;61:423–428. doi: 10.1016/s0014-4835(05)80137-x. [DOI] [PubMed] [Google Scholar]
- Hejmancik JF. Congenital cataracts and their molecular genetics. Sem Cell Dev Biol. 2008;19:134–149. doi: 10.1016/j.semcdb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks W, Mulders JWM, Bibby MA, Slingsby C, Bloemendal H, de Jong W. Duck lens ε-crystallin and lactate dehydrogenase B4 are identical: Single copy gene product with two distinct functions. Proc Natl Acad Sci USA. 1988;85:7114–7118. doi: 10.1073/pnas.85.19.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton DR, Saudin AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315:485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose S, Zigler S, Sinha D. A novel rat model to study the functions of macrophages during normal development and pathophysiology of the eye. Immunology Letters. 2005;96:299–302. doi: 10.1016/j.imlet.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Huang QL, Russell P, Stone S, Zigler JS., Jr ζ-crystallin, a novel lens protein from the guinea pig. Curr Eye Res. 1987;6:725–732. doi: 10.3109/02713688709034836. [DOI] [PubMed] [Google Scholar]
- Iacovelli J, Zhao C, Wolkow N, Veldman P, Gollomp K, Ojha P, Lukinova N, King A, Feiner L, Esumi N, Zack DJ, Pierce EA, Vollrath D, Dunaief JL. Generation of Cre transgenic mice with postnatal RPE-specific ocular expression. Invest Ophthalmol Vis Sci. 2011;52:1378–1383. doi: 10.1167/iovs.10-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Vereb Z, Salminen A, Bourlton ME, Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, Ding Q. Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol. 2004;36:2376–2391. doi: 10.1016/j.biocel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology. 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. The problem of astrocyte identity. Neurochemistry International. 2004;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Shih M, Ueda Y, Shearer TR, David LL. Lens proteomics: analysis of rat crystallin sequences and two-dimensional electrophoresis map. Invest Ophthalmol Vis Sci. 2002;43:216–224. [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedke T, Schwamborn JC, Schroer U, Thanos S. Elongation of axons during regeneration involves retinal crystalline βb2 (Crybb2) Mol Cell Proteomics. 2007;6(5):895–907. doi: 10.1074/mcp.M600245-MCP200. [DOI] [PubMed] [Google Scholar]
- Ling TL, Mitrofanis J, Stone J. Origin of retinal astrocytes in the rat: evidence of migration from the optic nerve. J Comp Neurol. 1989;286:345–352. doi: 10.1002/cne.902860305. [DOI] [PubMed] [Google Scholar]
- Litt M, Carrero-Valenzuela R, LaMorticella DM, Schultz DW, Mitchell TN, Kramer P, Maumenee IH. Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human β-crystallin gene CRYBB2. Hum Mol Genet. 1997;6:665–668. doi: 10.1093/hmg/6.5.665. [DOI] [PubMed] [Google Scholar]
- Lubsen NH, Aarts HJM, Schoenmakers JGG. The evolution of lenticular proteins: the beta- and gamma-crystallin superfamily. Prog Biophys Mol Biol. 1988;51:47–76. doi: 10.1016/0079-6107(88)90010-7. [DOI] [PubMed] [Google Scholar]
- Ma B, Sen T, Asnaghi L, Valapala M, Yang F, Hose S, McLeod DS, Lu Y, Eberhart C, Zigler JS, Jr, Sinha D. βA3/A1-crystallin controls anoikis-mediated cell death in astrocytes by modulating PI3K/AKT/mTOR and ERK survival pathways via the PKD/Bit1 signaling axis. Cell Death Dis. 2011;2:e217. doi: 10.1038/cddis.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magabo KS, Horwitz J, Piatigorsky J, Kantorow M. Expression of βB2-crystallin mRNA and protein in retina, brain and testis. Invest Ophthalmol Vis Sci. 2000;41:3056–3060. [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Miller RH, David S, Patel R, Abney R, Raff MC. A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve: in vivo evidence for two distinct astrocyte lineages. Dev Biol. 1985;111:35–41. doi: 10.1016/0012-1606(85)90432-4. [DOI] [PubMed] [Google Scholar]
- Mörner CT. Untersuchen der Proteinsubstanzen in licht-brechenden Medien des Auges. Hoppe-Seyler’s Z physiol Chem. 1894;18:61–106. [Google Scholar]
- Nagao M, Sugimori M, Nakafuku M. Cross talk between notch and growth factor/cytokine signaling pathways in neural stem cells. Mol Cell Biol. 2007;27:3982–3994. doi: 10.1128/MCB.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisiak D, Darrow R, Gu X, Barsalou L, Crabb JW. Genetic, age and light mediated effects on crystallin protein expression in the retina. Photochem Photobiol. 2006;82:1088–1096. doi: 10.1562/2005-06-30-RA-599. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG, editors. Astrocytes in (Patho)Physiology of the nervous system. Spring Science and Business Media, LLC; New York: 2009. [Google Scholar]
- Parthasarathy G, Ma B, Zhang C, Gongora C, Zigler JS, Jr, Duncan MK, Sinha D. Expression of βA3/A1-crystallin in the developing and adult rat eye. Journal of Molecular Histology. 2011;42:59–69. doi: 10.1007/s10735-010-9307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TA, Zou L, Xie XJ, Corey DR, Brugarolas J. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 2011;30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri F, Nüsslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–27. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Gene Sharing and Evolution: The diversity of protein functions. Harvard University Press; Cambridge, MA: 2007. [Google Scholar]
- Piri N, Song M, Kwong JMK, Caprioli J. Modulation of alpha and beta crystallin expression in rat retinas with ocular hypertension-induced ganglion cell degeneration. Brain Res. 2007;1141:1–9. doi: 10.1016/j.brainres.2006.11.095. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Astrocytes in the guinea pig, horse, and monkey retina: their occurrence coincides with the presence of blood vessels. Glia. 1988;1:74–89. doi: 10.1002/glia.440010109. [DOI] [PubMed] [Google Scholar]
- Sergeev YV, Wingfield PT, Hejtmancik JF. Monomer-dimer equilibrium of normal and modified beta A3-crystallins: experimental determination and molecular modeling. Biochemistry. 2000;39:15799–15806. doi: 10.1021/bi001882h. [DOI] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld SM, Purkiss AG, Dirks RPH, Bateman OA, Slingsby C, Lubsen NH. Urochordate β/γ-crystallin and the evolutionary origin of the vertebrate eye lens. Current Biology. 2005;15:1684–1689. doi: 10.1016/j.cub.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Siezen RJ, Anello RD, Thomson JA. Interactions of lens proteins. Concentration Dependence of β-crystallin aggregation. Exp Eye Res. 1986;43:293–303. doi: 10.1016/s0014-4835(86)80067-7. [DOI] [PubMed] [Google Scholar]
- Sinha D, Hose S, Zhang C, Neal R, Ghosh M, O’Brien TP, Sundin O, Goldberg MF, Robison GW, Russell P, Lo WK, Zigler S. A rat spontaneous mutation affects programmed cell death during the early development of the eye. Experimental Eye Research. 2005;80:323–335. doi: 10.1016/j.exer.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sinha D, Klise A, Sergeev Y, Hose S, Bhutto IA, Hackler L, Jr, Malpic-llanos T, Samtani S, Grebe R, Goldberg MF, Hejtmancik JF, Nath A, Zack DJ, Fariss RN, McLeod DS, Sundin O, Broman KW, Lutty GA, Zigler JS., Jr βA3/A1-Crystallin in astroglial cells regulates retinal vascular remodeling during development. Molecular and Cellular Neuroscience. 2008;37:85–95. doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D, Valapala M, Patek B, Bhutto I, Zhang C, Hose S, Fang Y, Cano M, Stark WJ, Lutty GA, Zigler JS, Wawrousek E., Jr βA3/A1-crystallin is required for proper astrocytes template formation and vascular remodeling in the retina. Transgenic Research. 2012;21:1033–1042. doi: 10.1007/s11248-012-9608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingsby C, Wistow GJ, Clark AR. Evolution of crystallins for a role in the vertebrate eye lens. Protein Science. 2013;22:367–380. doi: 10.1002/pro.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small RK, Riddle P, Noble M. Evidence for migration of oligodendrocytes-type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987;328:155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic D, Annaert W. Building gamma-secretase: the bits and pieces. J Cell Sci. 2008;121:413–420. doi: 10.1242/jcs.015255. [DOI] [PubMed] [Google Scholar]
- Spector A, Li LK, Augusteyn RC, Schneider A, Freund T. α-Crystallin: isolation and characterization of distinct macromolecular fractions. Biochem J. 1971;124:327–343. doi: 10.1042/bj1240337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SS, Mishra A, Krishnan B, Sharma Y. Ca2+-binding motif of β/γ-crystallins. J Biol Chem. 2014;289:10958–10966. doi: 10.1074/jbc.O113.539569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava OP, Srivastava K. Characterization of a sodium deoxcholate-activatable proteinase activity associated with beta A3/A1-crystallin of human lenses. Biochim Biophys Acta-Prot, Structure and Mol Enzymology. 1999;1434:331–346. doi: 10.1016/s0167-4838(99)00183-1. [DOI] [PubMed] [Google Scholar]
- Stone J, Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol. 1987;255:35–49. doi: 10.1002/cne.902550104. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Phys Rev. 2005;85:841–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Sun-Wada GH, Wada Y, Futai M. Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct Funct. 2003;28:455–463. doi: 10.1247/csf.28.455. [DOI] [PubMed] [Google Scholar]
- Takata T, Oxford JT, Demeler B, Lampi KJ. Deamidation destabilizes and triggers aggregation of a lens protein, beta A3-crystallin. Prot Sci. 2008;17:1565–1575. doi: 10.1110/ps.035410.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos S. Traumatology of the optic nerve and contribution of crystallins to axonal regeneration. Cell Tissue Res. 2012;349:49–69. doi: 10.1007/s00441-012-1442-4. [DOI] [PubMed] [Google Scholar]
- Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D’Amore PA, Ksander BR. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:110–120. doi: 10.1167/iovs.12-10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Duchi S, Cortese K, Tacchetti C, Bilder D. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development. 2010;137:1825–1832. doi: 10.1242/dev.045484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valapala M, Hose S, Gongora C, Dong L, Wawrousek E, Zigler JS, Sinha D. Impaired endolysosomal function disrupts Notch signaling in optic nerve astrocytes. Nature Communications. 2013;4:1629. doi: 10.1038/ncomms2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valapala M, Wilson C, Hose S, Bhutto IA, Grebe R, Dong A, Greenbaum S, Gu L, Sengupta S, Cano M, Hackett S, Xu G, Lutty GA, Dong L, Sergeev Y, Handa JT, Campochiaro P, Wawrousek E, Zigler JS, Sinha D., Jr Lysosomal-mediated waste clearance in retinal pigmented epithelial cells is regulated by CRYBA1/βA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10:480–496. doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valapala M, Edwards M, Hose S, Grebe R, Bhutto IA, Cano M, Berger T, Mak TW, Wawrousek E, Handa JT, Lutty GA, Zigler JS, Jr, Sinha D. Increased lipocalin-2 in the retinal pigment epithelium of cryba1 cKO mice is associated with a chronic inflammatory response. Aging Cell. 2014b doi: 10.1111/acel.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Chona F, Song BK, Geisert EE., Jr Temporal changes in gene expression after injury in the rat retina. Invest Ophthalmol Vis Sci. 2004;45:2737–2746. doi: 10.1167/iovs.03-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemaker CF, Spector A. Protein synthesis in the core of calf lens. Exp Eye Res. 1968;7:623–625. doi: 10.1016/s0014-4835(68)80018-1. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature. 1988;332:834–837. doi: 10.1038/332834a0. [DOI] [PubMed] [Google Scholar]
- Werten PJL, Stege GJJ, de Jong WW. The short 5′ untranslated region of the βA3/A1-crystallin mRNA is responsible for leaky ribosomal scanning. Molecular Biology Reports. 1999;26:201–205. doi: 10.1023/a:1007046926233. [DOI] [PubMed] [Google Scholar]
- Wistow G. Molecular Biology and Evolution of Crystallins: Gene Recruitment and Multifunctional Proteins in the Eye Lens. R. G. Landes Company; Austin, TX: 1995. [Google Scholar]
- Wistow G. Evolution of a protein superfamily: relationships between vertebrate lens crystallins and micro-organism dormancy proteins. J Mol Evol. 1990;30:140–145. doi: 10.1007/BF02099940. [DOI] [PubMed] [Google Scholar]
- Wistow G, Summers L, Blundell T. Myxococcus xanthus spore coat protein S may have a similar structure to vertebrate lens β/γ-crystallins. Nature. 1985;315:771–773. doi: 10.1038/315771a0. [DOI] [PubMed] [Google Scholar]
- Xi J, Farjo R, Yoshida S, Kern TS, Swaroop A, Andley UP. A comprehensive analysis of the expression of crystallins in mouse retina. Mol Vis. 2003;9:410–419. [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yan Y, Denef N, Schupbach T. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell. 2009;17:387–402. doi: 10.1016/j.devcel.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Gehlbach P, Gongora C, Cano M, Fariss Robert, Hose S, Nath A, Green WR, Goldberg MF, Zigler S, Sinha D. A potential role for β- and γ-crystallins in the vascular remodeling of the eye. Developmental Dynamics. 2005;234:36–47. doi: 10.1002/dvdy.20494. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ashaghi L, Gongora C, Patek B, Hose S, Ma B, Aghsaei-Fard M, Brako L, Singh K, Goldberg MF, Handa JT, Lo WK, Eberhart CG, Zigler JS, Sinha D., Jr A developmental defect in astrocytes inhibits programmed regression of the hyaloid vasculature in the mammalian eye. European Journal of Cell Biology. 2011;90:440–8. doi: 10.1016/j.ejcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler JS, Sidbury JB., Jr Structure of calf lens β-crystallins. Exp Eye Res. 1973;16:207–214. doi: 10.1016/0014-4835(73)90215-7. [DOI] [PubMed] [Google Scholar]
- Zigler JS, Jr, Zhang C, Grebe R, Sehrawat G, Hackler L, Jr, Adhya S, Hose S, McLeoc DS, Bhutto I, Barbour W, Parthasarathy G, Zack DJ, Sergeev Y, Lutty GA, Handa JT, Sinha D. Mutation in the βA3/A1-crystallin gene impairs phagosome degradation in the retinal pigmented epithelium of the rat. J Cell Sci. 2011;124:523–531. doi: 10.1242/jcs.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–83. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]