Summary
Alternative modes of metabolism enable cells to resist metabolic stress. Inhibiting these compensatory pathways may produce synthetic lethality. We previously demonstrated that glucose deprivation stimulated a pathway in which acetyl-CoA was formed from glutamine downstream of glutamate dehydrogenase (GDH). Here we show that import of pyruvate into the mitochondria suppresses GDH and glutamine-dependent acetyl-CoA formation. Inhibiting the mitochondrial pyruvate carrier (MPC) activates GDH and re-routes glutamine metabolism to generate both oxaloacetate and acetyl-CoA, enabling persistent tricarboxylic acid (TCA) cycle function. Pharmacological blockade of GDH elicited largely cytostatic effects in culture, but these effects became cytotoxic when combined with MPC inhibition. Concomitant administration of MPC and GDH inhibitors significantly impaired tumor growth compared to either inhibitor used as a single agent. Together, the data define a mechanism to induce glutaminolysis and uncover a survival pathway engaged during compromised supply of pyruvate to the mitochondria.
Cell survival and growth require metabolic pathways that produce energy, precursors for macromolecular synthesis, and substrates for other essential functions (Vander Heiden et al., 2009). In mammals, glucose and glutamine are two of the most abundant nutrients to support these functions (Cantor and Sabatini, 2012). Glucose is the most important source of carbon for energy-generating pathways, providing acetyl-CoA for oxidative metabolism in the mitochondria. Glutamine serves as an inter-organ shuttle of carbon and nitrogen and is the major source of nitrogen for non-essential amino acids, nucleotides, and hexosamines (Hensley et al., 2013). In culture, glucose and glutamine account for a high fraction of carbon and nitrogen metabolism.
The tricarboxylic acid (TCA) cycle coordinates energy production and biosynthesis. As typically rendered, the pathway begins with condensation of acetyl-CoA and oxaloacetate (OAA) to produce citrate. Oxidation of citrate generates reducing equivalents to drive ATP production via oxidative phosphorylation. Two carbons are released as CO2 per cycle, regenerating OAA. In proliferating cells, the TCA cycle operates in a different fashion characterized by the exit of intermediates from the cycle to supply various biosynthetic pathways. Under these conditions, OAA would become limiting unless it was produced from another pathway that did not flow from mitochondrial citrate. These OAA-producing pathways, termed anaplerosis, enable the TCA cycle to function as a biosynthetic pathway as opposed to a purely bioenergetic one (Owen et al., 2002).
In standard culture, many cancer cells use a form of the TCA cycle in which most of the acetyl-CoA is produced from glucose and most of the anaplerosis is supplied by glutamine (DeBerardinis et al., 2007). Glutamine is converted to glutamate by glutaminase (GLS), which releases the amide nitrogen of glutamine as ammonia (Mates et al., 2013), and by nitrogen-donating reactions involved in nucleotide synthesis. Glutamate is converted to α-ketoglutarate (AKG) by two types of reactions. In one, transaminases transfer the amino group from glutamate to a ketoacid, producing AKG and an amino acid. In the other, glutamate is deaminated by glutamate dehydrogenase (GDH), releasing ammonia and producing AKG without consuming a ketoacid. Under glucose-replete conditions, transamination predominates (Yang et al., 2009). GLS and transaminases are required for growth of glutamine-addicted cells (Cheng et al., 2011; Gao et al., 2009; Qing et al., 2012; Wang et al., 2010).
Glucose and glutamine are versatile, in some cases compensating for each other to maintain TCA cycle function. Although glutamine is the preferred anaplerotic precursor in many cancer cells, others use glucose to produce OAA via pyruvate carboxylase (PC). Glutamine-addicted cells can be converted to glutamine independence by reducing glutamine availability, a process which increases their dependence on PC (Cheng et al., 2011). Glutamine can also produce acetyl-CoA via reductive carboxylation, in which glutamine-derived AKG is carboxylated to produce isocitrate/citrate, which is then cleaved to generate OAA and acetyl-CoA. Reductive rather than oxidative metabolism is the dominant route by which glutamine produces acetyl-CoA to synthesize fatty acids in a brown adipocyte cell line (Yoo et al., 2008). Reductive labeling of citrate from 13C-glutamine is enhanced when electron transport chain (ETC) or TCA cycle function is altered by hypoxia or mutations (Metallo et al., 2012; Mullen et al., 2012; Scott et al., 2011; Wise et al., 2011).
Here we examined the mitochondrial effects of blocking entry of glucose-derived pyruvate into the mitochondria. We found that MPC blockade induces acetyl-CoA formation from glutamine using a pathway confined to the mitochondria. Activation of this glutaminolytic pathway increases reliance on GDH, which becomes essential when mitochondrial pyruvate import is inhibited.
Results
Glucose suppresses glutamine-dependent acetyl-CoA formation in numerous cell lines
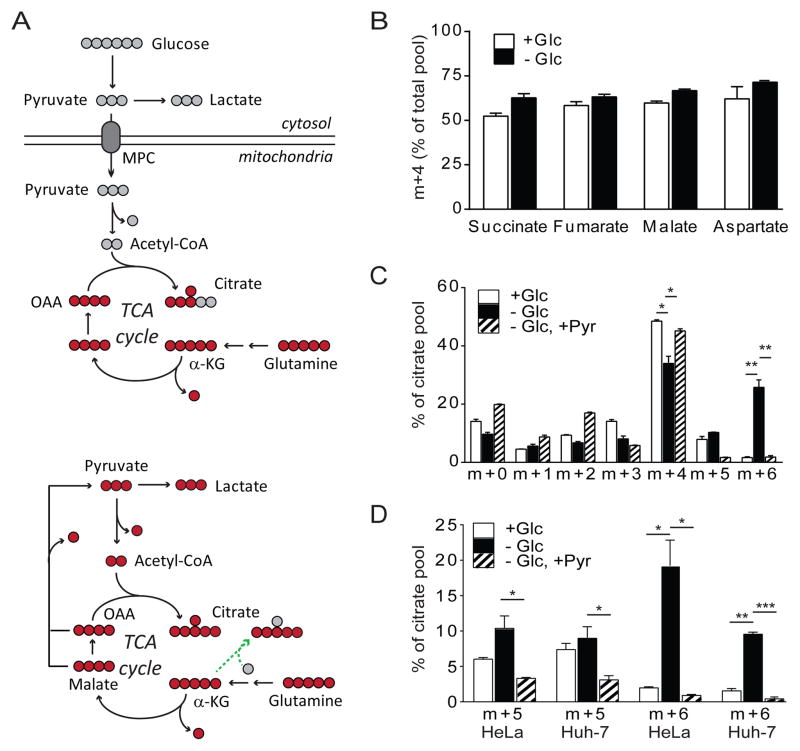
We previously showed that glucose withdrawal induces a pathway of of pyruvate formation from glutamine in SFxL glioma cells, which were derived from SF188 glioblastoma cells modified to express the anti-apoptotic protein Bcl-xL (Yang et al., 2009). To further characterize this pathway, SFxL cells were withdrawn from glucose and cultured with [U-13C]glutamine (glutamine containing all five carbons labeled as 13C). Typical [U-13C]glutamine metabolism is illustrated in Fig. 1A (top). In SFxL cells, under either glucose-replete or glucose-deprived conditions, the majority of succinate, fumarate, malate and aspartate (a surrogate for OAA labeling) were fully labeled from glutamine (m+4), indicating that glutamine was used as an anaplerotic precursor regardless of whether glucose was present (Fig. 1B). However, glucose deprivation caused significant changes in citrate, inducing the appearance of molecules in which all six carbons were labeled (m+6) (Fig. 1C). This pattern occurs if both OAA and acetyl-CoA are generated from glutamine, for example if a four-carbon TCA cycle intermediate is converted to pyruvate, then converted to acetyl-CoA (Fig. 1A, bottom). Citrate m+6 was barely detectable in the presence of glucose, but approached 30% when glucose was removed. Supplementing glucose-deprived medium with pyruvate reduced citrate m+6 to basal levels, indicating that induction of glutamine-dependent acetyl-CoA was caused by reduced pyruvate availability (Fig. 1C). Glucose deprivation also enhanced citrate m+6 in HeLa and Huh-7 cells, and this pattern was suppressed by pyruvate (Fig. 1D).
Figure 1. Pyruvate depletion redirects glutamine metabolism to produce acetyl-CoA and citrate.
(A) Top, Anaplerosis supplied by [U-13C]glutamine. Glutamine supplies OAA via α-KG, while acetyl-CoA is predominantly supplied by other nutrients, particularly glucose. Bottom, Glutamine is converted to acetyl-CoA in the absence of glucose-derived pyruvate. Red circles represent carbons arising from [U-13C]glutamine, and gray circles are unlabeled. Reductive carboxylation is indicated by the green dashed line. Abbreviations: MPC, mitochondrial pyruvate carrier; α-KG, α-ketoglutarate; OAA, oxaloacetate.
(B) Fraction of succinate, fumarate, malate and aspartate containing four 13C carbons after culture of SFxL cells for 6 hours with [U-13C]glutamine in the presence or absence of 10 mM unlabeled glucose (Glc).
(C) Mass isotopologues of citrate after culture of SFxL cells for 6 hours with [U-13C]glutamine and 10 mM unlabeled glucose; no glucose; or no glucose plus 6 mM unlabeled pyruvate (Pyr).
(D) Citrate m+5 and m+6 after culture of HeLa or Huh-7 cells for 6 hours with [U-13C]glutamine and 10 mM unlabeled glucose; no glucose; or no glucose plus 6 mM unlabeled pyruvate.
Data are the average and SD of three independent cultures. *, p<0.05; **, p<0.01; ***, p<0.001.
Reductive carboxylation of AKG is induced by conditions that reduce production of citrate from acetyl-CoA (Fendt et al., 2013; Gameiro et al., 2013). Citrate formed through reductive carboxylation is labeled as m+5 from [U-13C]glutamine because of the incorporation of an unlabeled CO2 in the carboxylation reaction (Fig. 1A, bottom). In all three cell lines, the fractional amount of citrate m+5 was at most marginally increased by glucose withdrawal. This effect was small compared to the effect on citrate m+6 (Fig. 1C,D).
Mitochondrial metabolism is sufficient for glucose-independent formation of citrate
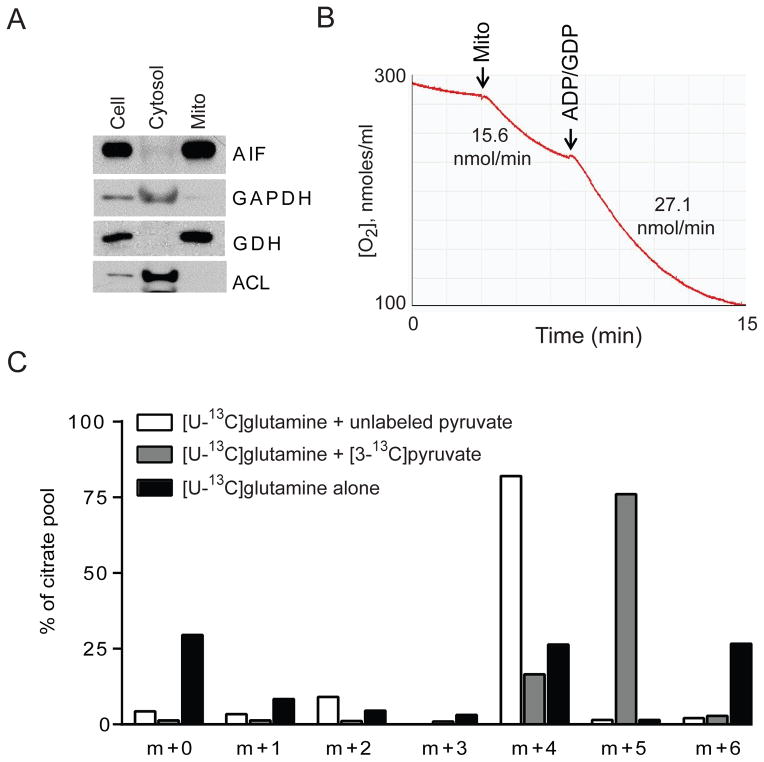
Some of the enzymatic reactions that might enable glutamine to be converted to acetyl-CoA exist in both the mitochondria and the cytosol. To test whether mitochondria contain all reactions necessary for this pathway of citrate formation, mitochondria were purified from SFxL cells (Fig. 2A). These preparations consumed oxygen when presented with respiratory substrates (Fig. 2B). When cultured with [U-13C]glutamine and unlabeled pyruvate, the mitochondria rapidly formed citrate m+4, indicating glutamine-dependent anaplerosis (Fig. 2C). Replacing unlabeled pyruvate with [3-13C]pyruvate reduced citrate m+4 and increased citrate m+5, indicating that pyruvate was used as a source of acetyl-CoA. Removing pyruvate from the medium shifted citrate from m+4 to m+6, indicating formation of glutamine-derived acetyl-CoA (Fig. 2C). Residual citrate m+4 indicates the presence of unlabeled acetyl-CoA; this was not surprising because the mitochondria contained unlabeled lactate and malate detected by mass spectrometry, and no attempts were made to inhibit fatty acid oxidation. Overall, the data demonstrate that isolated mitochondria are sufficient for glutamine-dependent anaplerosis to produce citrate in the presence of pyruvate. In the absence of pyruvate, mitochondria contain all activities necessary to produce citrate containing all six carbons from glutamine.
Figure 2. Isolated mitochondria convert glutamine to citrate.
(A) Western blot of whole cell lysates (Cell) and preparations of isolated mitochondria (Mito) or cytosol from SFxL cells. Abbreviations: AIF, apoptosis inducing factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GDH, glutamate dehydrogenase; ACL, ATP citrate lyase.
(B) Oxygen consumption in a representative mitochondrial sample. Rates before and after addition of ADP/GDP are indicated.
(C) Mass isotopologues of citrate produced by mitochondria cultured for 30 minutes with [U-13C]glutamine and with or without pyruvate.
Inhibiting mitochondrial pyruvate transport induces glutamine-dependent acetyl-CoA formation
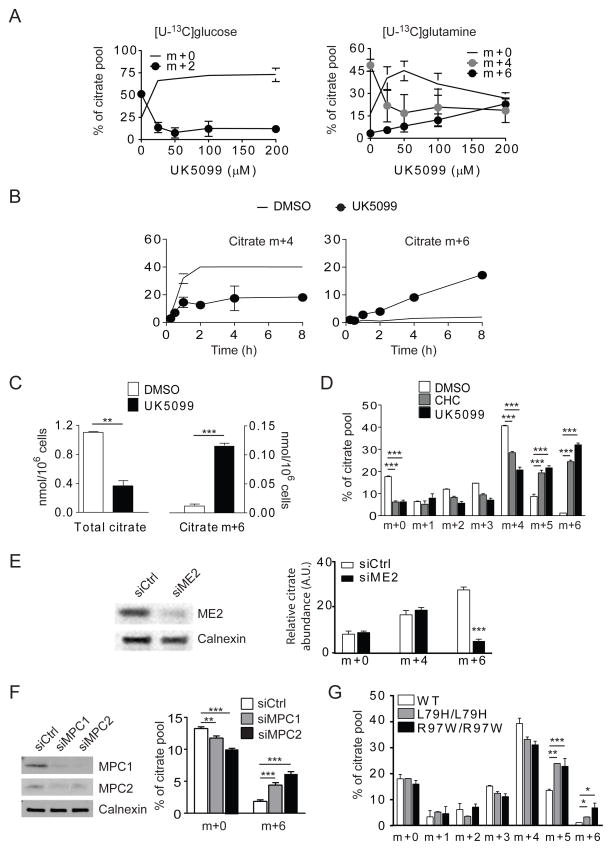
We next tested whether preventing pyruvate from entering the mitochondria in glucose-replete cells mimicked the effects of glucose withdrawal. α-cyano-β-(1-phenylindol-3-yl)acrylate (UK5099) inhibits mitochondrial pyruvate transport (Halestrap, 1976). UK5099 caused a dose-dependent reduction of incorporation of glucose carbon into citrate from [U-13C]glucose (Fig. 3A). Citrate m+0 persisted and the time-dependent accumulation of citrate m+2 was delayed, consistent with impaired mitochondrial pyruvate import (Fig. S1A). UK5099 also altered glutamine-dependent citrate labeling, causing a dose-dependent enhancement of citrate m+6 from [U-13C]glutamine (Fig. 3A). Over 8 hours, the fractional content of citrate m+6 was enhanced by UK5099 (Fig. 3B). The m+4 fraction was reduced, but was maintained at approximately 20% of the pool over several hours, indicating contribution of unlabeled acetyl-CoA (Fig. 3B). Although UK5099 suppressed total citrate abundance, it increased the absolute abundance of citrate m+6 (Fig. 3C). α-cyano-4-hydroxycinnamate (CHC), a monocarboxylate transport inhibitor which also inhibits mitochondrial pyruvate transport, had similar effects on citrate labeling (Fig. 3D and Fig. S1B). While CHC also suppressed lactate secretion, as expected from its effects on monocarboxylate transporter-1 (MCT1), UK5099 did not alter lactate secretion, indicating that its metabolic effects at these doses were related to MPC function (Fig S1C). We also examined lung adenocarcinoma cells (HCC4017) and non-transformed bronchial epithelial cells (HBEC30) obtained from the same patient (Kim et al., 2013). In both the transformed and non-transformed cells, UK5099 enhanced the fractional content of citrate m+6 from [U-13C]glutamine (Fig. S1D). The drug had a similar effect in 786-O renal carcinoma cells, in which mutation of the von-Hippel Lindau (VHL) tumor suppressor leads to a chronic pseudohypoxic state characterized by suppressed glucose oxidation and enhanced reductive carboxylation (Metallo et al., 2012) (Fig. S1E).
Figure 3. Blockade of mitochondrial pyruvate transport activates glutamine-dependent citrate formation.
(A) Dose-dependent effects of UK5099 on citrate labeling from [U-13C]glucose and [U-13C]glutamine in SFxL cells.
(B) Time course of citrate labeling from [U-13C]glutamine with or without 200 μM UK5099.
(C) Abundance of total citrate and citrate m+6 in cells cultured in [U-13C]glutamine with or without 200 μM UK5099.
(D) Mass isotopologues of citrate in cells cultured for 6 hours in [U-13C]glutamine with or without 10 mM CHC or 200 μM UK5099.
(E) Effect of silencing ME2 on citrate m+6 after 6 hours of culture in [U-13C]glutamine. Relative abundances of citrate isotopologues were determined by normalizing total citrate abundance measured by mass spectrometry against cellular protein for each sample, then multiplying by the fractional abundance of each isotopologue.
(F) Effect of silencing MPC1 or MPC2 on formation of citrate m+6 after 6 hours of culture in [U-13C]glutamine.
(G) Citrate isotopologues in primary human fibroblasts of varying MPC1 genotypes after culture in [U-13C]glutamine.
Data are the average and SD of three independent cultures. *, p<0.05; **, p<0.01; ***, p<0.001.
See also Fig. S1.
We next sought to understand how TCA cycle intermediates were converted to pyruvate in the mitochondria to enable glutamine-dependent formation of acetyl-CoA. This could presumably involve malic enzyme, which decarboxylates malate to pyruvate, or mitochondrial phosphoenolpyruvate carboxykinase (PEPCK), which converts OAA to PEP. The latter seemed unlikely because conversion of PEP to pyruvate is thought to occur primarily in the cytosol. However, mitochondrial isoforms of malic enzyme (ME2 or ME3) could produce a local pyruvate pool for pyruvate dehydrogenase (PDH). ME2 is reported to supply pyruvate to the TCA cycle when glucose is limiting (Pongratz et al., 2007), and silencing ME2 alters TCA cycle metabolism and growth in lung cancer cells (Ren et al., 2014). ME3 mRNA and protein were barely detectable in SFxL cells. However, silencing ME2 reduced the fractional content of citrate m+6 from [U-13C]glutamine during UK5099 treatment, indicating ME2’s involvement in this pathway of glutamine metabolism (Fig. 3E).
Molecular components of a human mitochondrial pyruvate carrier (MPC) were recently reported (Bricker et al., 2012; Herzig et al., 2012). This carrier consists of at least two subunits, MPC1 and MPC2, both of which are required for maximal pyruvate transport. Mutations in human MPC1 encoded by the gene BRP44L were identified in patients with lactic acidosis and neurodevelopmental abnormalities (Bricker et al., 2012; Brivet et al., 2003). To determine whether activity of this carrier influences glutamine metabolism, MPC1 and MPC2 were silenced using siRNA. Suppressing either protein reduced glucose-dependent citrate labeling (Fig. S1F) and enhanced citrate m+6 from [U-13C]glutamine (Fig. 3F). We also tested primary fibroblasts from patients with modest or severe MPC1 deficiency resulting from homozygous BRP44LI mutations (L79H or R97W, respectively). Compared to fibroblasts from a healthy control, cells from MPC1-deficient patients displayed a higher fractional abundance of citrate m+6 from [U-13C]glutamine (Fig. 3G).
Kinetic analysis of MPC inhibition using hyperpolarized 13C
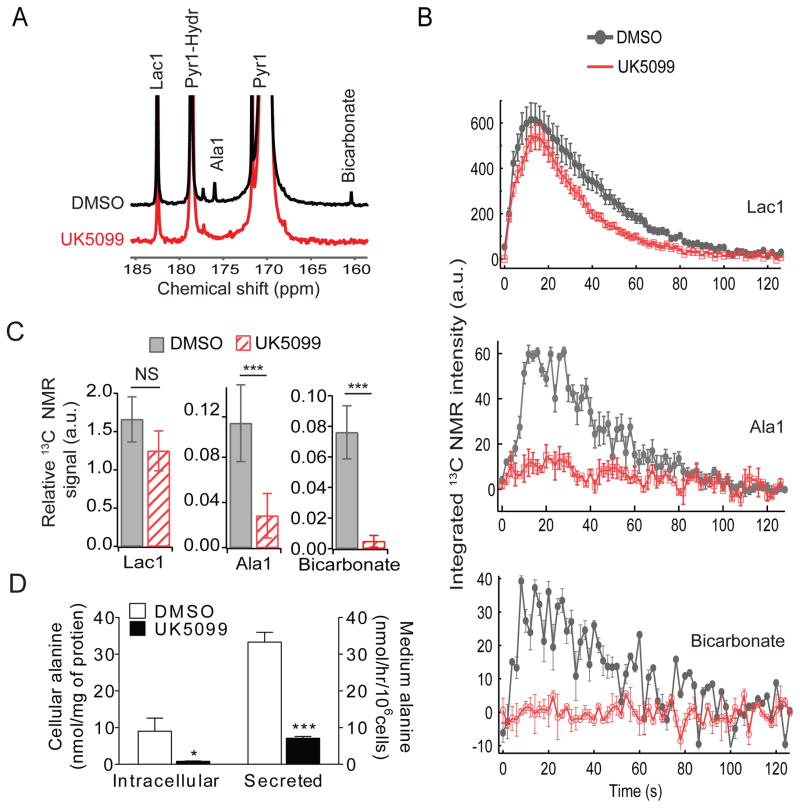
To obtain dynamic views of UK5099’s effects on pyruvate metabolism, cells were cultured with hyperpolarized [1-13C]pyruvate. This allows metabolic activities to be monitored by NMR with extremely fine temporal resolution (Golman et al., 2006). Cells were treated with DMSO or UK5099, and cultured in an NMR tube with hyperpolarized [1-13C]pyruvate. To maximize detection of metabolic products, we used a cryogenic probe that reduces electronic noise of the NMR system. In control cells, spectra acquired over two minutes revealed transfer of hyperpolarized 13C from pyruvate to lactate and alanine, two anaerobic products of glucose metabolism (Fig. 4A). These activities require that pyruvate be imported into the cell and encounter lactate dehydrogenase (LDH) and alanine aminotransferase (ALT), respectively. The control spectrum also contained a peak from H[13C]O3−, reflecting activity of PDH in the mitochondria (Fig. 4A)(Yang et al., 2014). These three products were present in individual spectra, enabling time evolution to be observed for each signal (Fig. 4B). Cumulative 13C signals derived from [1-13C]lactate, [1-13C]alanine and H[13C]O3− were expressed as a fraction of the total 13C signal over the time course (Fig. 4C). Cells treated with UK5099 contained no significant alterations of lactate labeling, indicating that UK5099 did not prevent hyperpolarized [1-13C]pyruvate from entering the cells or serving as a substrate for LDH (Fig. 4B,C). However, both [1-13C]alanine and H[13C]O3− were almost completely eliminated by UK5099 (Fig. 4B,C). Elimination of H[13C]O3− was expected, because PDH resides in the mitochondrial matrix. ALT, on the other hand, can exist in both compartments. Despite the fact that ALT does not require oxygen, the data suggest that most ALT activity requires mitochondrial pyruvate import in these cells. Indeed, UK5099 suppressed intracellular abundance and secretion of alanine (Fig. 4D). Among other amino acids analyzed from the intracellular pool, aspartate levels were enhanced approximately four fold, and others were not altered (Fig. S2).
Figure 4. Kinetic analysis of the metabolic effects of blocking mitochondrial pyruvate transport.
(A) Summation of 13C spectra acquired over 2 minutes of exposure of SFxL cells to hyperpolarized [1-13C]pyruvate. Resonances are indicated for [1-13C]pyruvate (Pyr1), the hydrate of [1-13C]pyruvate (Pyr1-Hydr), [1-13C]lactate (Lac1), [1-13C]alanine (Ala1) and H[13C]O3− (Bicarbonate).
(B) Time evolution of appearance of Lac1, Ala1 and Bicarbonate in control and UK5099-treated cells.
(C) Relative 13C NMR signals for Lac1, Ala1 and Bicarbonate. Each signal is summed over the entire acquisition and expressed as a fraction of total 13C signal.
(D) Quantity of intracellular and secreted alanine in control and UK5099-treated cells.
Data are the average and SD of three independent cultures. *, p<0.05; ***, p<0.001.
See also Fig. S2.
Inhibition of mitochondrial pyruvate import induces glutamine-dependent lipid synthesis
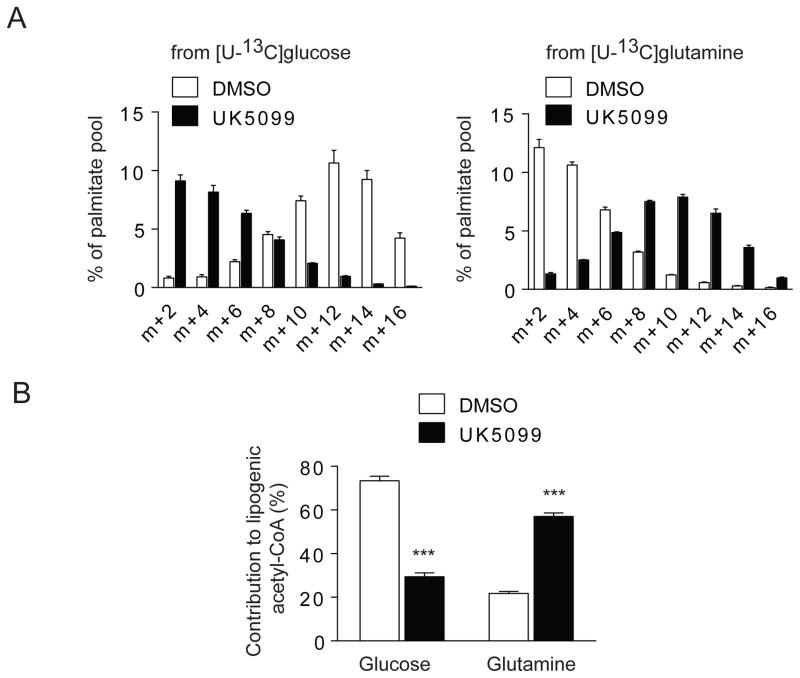
Glucose provides carbon for fatty acid synthesis. Normally this occurs through import of mitochondrial pyruvate followed by PDH-dependent conversion to acetyl-CoA, condensation with OAA to produce citrate, export of citrate to the cytosol and cleavage to produce cytosolic acetyl-CoA, the substrate for fatty acid synthesis. Thus, suppressing MPC should reduce the contribution of glucose to lipogenesis. SFxL cells were cultured in complete medium containing [U-13C]glucose and unlabeled glutamine or unlabeled glucose and [U-13C]glutamine. Lipids were extracted and analyzed by GC/MS to determine the contribution of each substrate to the fatty acid palmitate. Carbon from both nutrients was transferred to palmitate regardless of whether UK5099 was present (Fig. 5A). However, UK5099 altered the relative contributions of glucose and glutamine. In the absence of UK5099, glucose and glutamine summed to more than 90% of the acetyl-CoA used to produce palmitate, with glucose accounting for 70% and glutamine less than 30% (Fig. 5B). UK5099 reversed this pattern so that glutamine-derived carbon was much more prominent (Fig. 5B). To determine whether glutamine-derived fatty acid synthesis occurred via oxidative or reductive metabolism, SFxL cells were cultured in either [5-13C]glutamine or [3-13C]glutamine, with or without UK5099, and lipids were analyzed by 13C NMR to determine the positions of 13C within fatty acids. This positional specificity defines which pathway or pathways the label traversed to reach fatty acids, with [5-13C]glutamine and [3-13C]glutamine primarily reporting reductive and oxidative metabolism, respectively. Although fatty acids were robustly labeled by [5-13C]glutamine, there was no enhancement upon UK5099 treatment (Fig. S3). By contrast, labeling from [3-13C]glutamine increased substantially, indicating that oxidative acetyl-CoA formation accounted for the majority of enhanced glutamine-dependent fatty acid labeling induced by UK5099.
Figure 5. Inhibiting mitochondrial pyruvate transport enhances the contribution of glutamine to fatty acid synthesis.
(A) Mass isotopologues of palmitate extracted from cells cultured with [U-13C]glucose or [U-13C]glutamine, with or without 200 μM UK5099. For simplicity, only even-labeled isotopologues (m+2, m+4, etc.) are shown.
(B) Fraction of lipogenic acetyl-CoA derived from glucose or glutamine with or without 200 μM UK5099.
Data are the average and SD of three independent cultures. ***, p<0.001.
See also Fig. S3.
Mitochondrial pyruvate import suppresses glutamate dehydrogenase
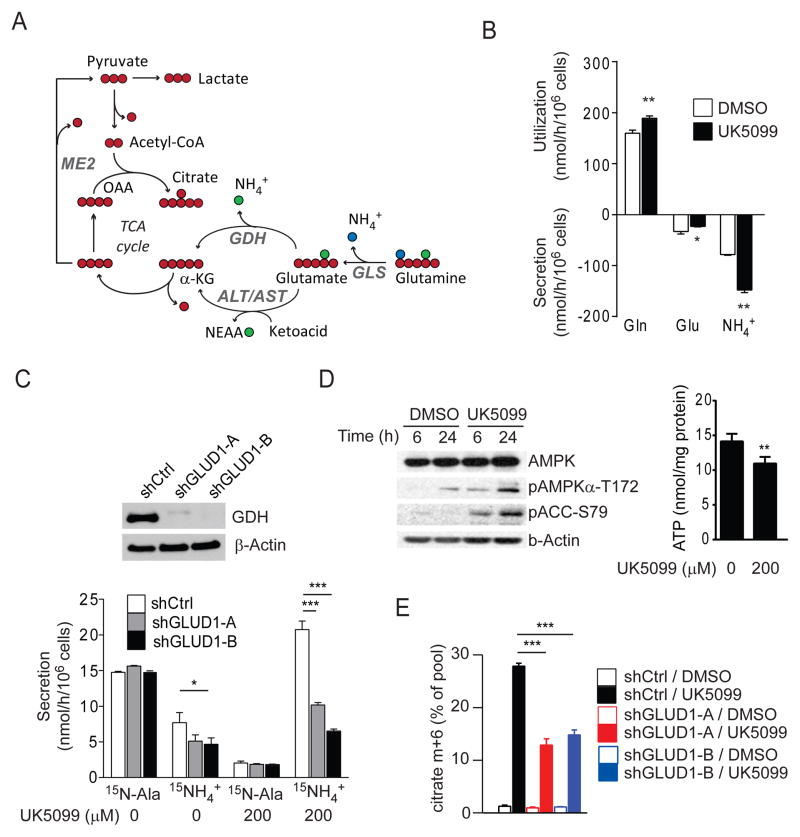
Considering the suppression of alanine synthesis upon UK5099 treatment, we tested whether blocking mitochondrial pyruvate import impacted how glutamate was converted to AKG (Fig. 6A). UK5099 modestly increased glutamine consumption and suppressed glutamate secretion, while ammonia secretion nearly doubled (Fig. 6B). Because over 90% of ammonia released by these cells is derived from glutamine (DeBerardinis et al., 2007; Yang et al., 2009), these changes suggested enhanced GDH activity to release glutamine’s amino (α) nitrogen as ammonia to complement the large amount of amido (γ) nitrogen already released by glutaminase (Fig. 6A). Activation of GDH would have the benefit of producing AKG in the face of diminished alanine synthesis. To test this possibility, control SFxL cells were cultured with [α-15N]glutamine. In this scheme, [α-15N]glutamine is converted to [α-15N]glutamate, then [α-15N]glutamate is converted to AKG by either transaminases or GDH. Labeled ammonium (15NH4+) released by GDH can be monitored indirectly by GC/MS (Brosnan et al., 2001). Basal rates of 15NH4+ production were low and were suppressed further by shRNAs against GLUD1, the gene encoding GDH (Fig. 6C). The rate of 15NH4+ release was far exceeded by the rate of 15N-alanine release (Fig. 6C). In the presence of UK5099, release of 15N-alanine declined precipitously, and 15NH4+ release nearly tripled, indicating a metabolic shift from mitochondrial ALT towards GDH. This effect was blunted in cells expressing GLUD1 shRNAs (Fig. 6C). UK5099 also enhanced 15NH4+ release from [α-15N]glutamine in HBEC30 and HCC4017 cells (Fig. S4A).
Figure 6. Blockade of mitochondrial pyruvate transport induces glutamate dehydrogenase.
(A) Two routes by which glutamate can be converted to AKG. Blue and green symbols are the amide (γ) and amino (α) nitrogens of glutamine, respectively. Abbreviations: GLS, glutaminase; ALT/AST, alanine aminotransferase/aspartate aminotransferase; GDH, glutamate dehydrogenase.
(B) Utilization and secretion of glutamine (Gln), glutamate (Glu) and ammonia (NH4+) by SFxL cells with and without 200 μM UK5099.
(C) Secretion of 15N-alanine and 15NH4+ derived from [α-15N]glutamine in SFxL cells expressing a control shRNA (shCtrl) or either of two shRNAs directed against GLUD1 (shGLUD1-A and shGLUD1-B).
(D) Left, Phosphorylation of AMPK (S172) and acetyl-CoA carboxylase (ACC, S79) during treatment with 200 μM UK5099. Right, Steady-state levels of ATP 24 hrs after addition of vehicle or 200 μM UK5099.
(E) Fractional contribution of the m+6 isotopologue to total citrate in shCtrl, shGLUD1-A and shGLUD1-B SFxL cells cultured in [U-13C]glutamine with or without 200 μM UK5099.
Data are the average and SD of three independent cultures. *, p<0.05; **, p<0.01; ***, p<0.001.
See also Fig. S4.
GDH is responsive to the mitochondrial energetic state, as ATP and GTP inhibit GDH and ADP activates it (Li et al., 2012; Wanders et al., 1983). To test whether UK5099 altered bioenergetics, we evaluated ATP and activation of the energy sensor AMP-activated protein kinase (AMPK). UK5099 decreased ATP levels and enhanced phosphorylation of AMPK and its target acetyl-CoA carboxylase-1, both of which indicate a compromised energetic state (Fig. 6D). Consistent with the altered status of AMPK signaling, UK5099 treatment also enhanced fatty acid oxidation (Fig. S4B).
We also tested whether GDH contributed to glutamine-dependent citrate formation. Control and GLUD1-silenced SFxL cells were cultured in medium containing unlabeled glucose and [U-13C]glutamine. In absence of UK5099, neither GLUD1 shRNA impacted citrate labeling (Fig. S4C). When treated with UK5099, however, the large increase in citrate m+6 was blunted by GLUD1 shRNAs (Fig. 6E), indicating that GDH is involved in allowing cancer cells to produce acetyl-CoA and citrate from glutamine.
Blockade of mitochondrial pyruvate import uncovers a dependence on GDH for cell survival and tumor growth
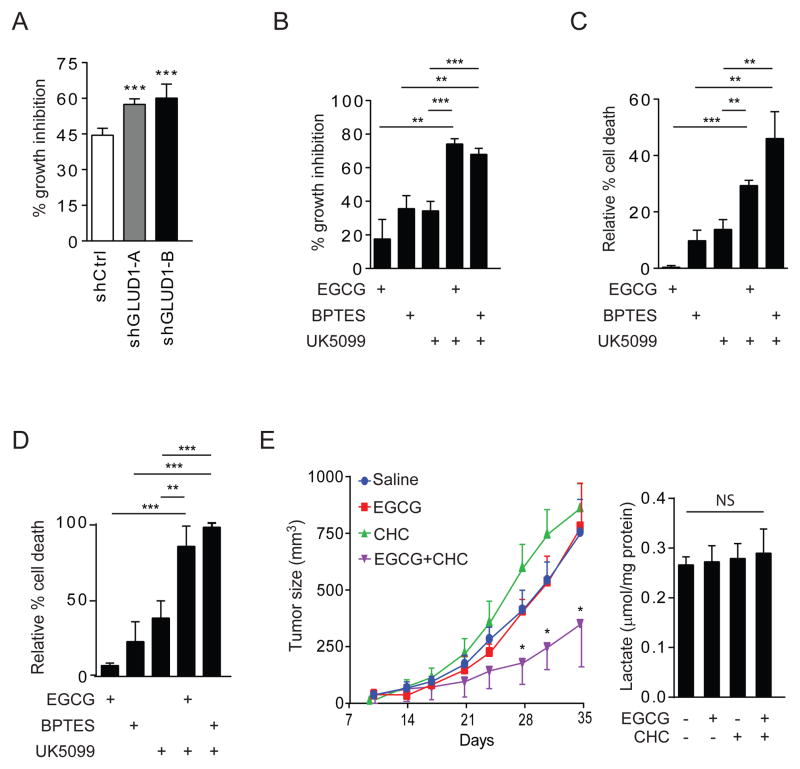
Given the importance of acetyl-CoA and the TCA cycle for bioenergetics and biosynthesis, we tested whether cells became more dependent on glutamine metabolism when mitochondrial pyruvate uptake was inhibited. In SFxL cells, constitutive GLUD1 silencing caused a modest but significant increase in sensitivity to UK5099 (Fig. 7A). UK5099 modestly suppressed cell proliferation and caused little cell death when used as a single agent (Fig. 7B, C). Similar effects were observed with the glutaminase inhibitor BPTES and the GDH inhibitor epigallocatechin gallate (EGCG) (Fig. 7B, C). However, combining UK5099 with inhibition of either GLS or GDH substantially increased both growth suppression and cell death (Fig. 7B,C, S5). In parental SF188 cells lacking Bcl-xL, the effects of combined treatment was even more prominent, killing essentially all the cells within 48 hours (Fig. 7D). Thus, blockade of mitochondrial pyruvate import results in an enhanced reliance on glutaminase and GDH for survival and proliferation.
Figure 7. GDH sustains growth and viability during suppression of mitochondrial pyruvate transport.
(A) Relative growth inhibition of shCtrl, shGLUD1-A and shGLUD1-B SFxL cells treated with 50 μM UK5099 for 3 days.
(B) Relative growth inhibition of SFxL cells treated with combinations of 50 μM of the GDH inhibitor EGCG, 10 μM of the GLS inhibitor BPTES, and 200 μM UK5099 for 3 days.
(C) Relative cell death assessed by trypan blue staining in SFxL cells treated as in panel B.
(D) Relative cell death assessed by trypan blue staining in SF188 cells treated as in panel B for 2 days.
(E) Left, Growth of A549-derived subcutaneous xenografts treated with vehicle (saline), EGCG, CHC, or EGCG plus CHC (n=4 for each group). Data are the average and SEM. Right, Lactate abundance in extracts of each tumor harvested at the end of the experiment.
Data in (A)–(D) are the average and SD of three independent cultures. NS, not significant; *, p<0.05; **, p<0.01; ***, p<0.001.
See also Fig. S5.
Finally, we tested whether MPC/GDH inhibition impaired tumor growth. Although to our knowledge UK5099 has not been used for in vivo models of tumorigenesis, CHC can be administered to mice and alters tumor growth in some settings (Zhao et al., 2014). A549 lung cancer cells were implanted into the flanks of nude mice, and cohorts were established to receive vehicle, EGCG, CHC, or both EGCG and CHC. Only tumors receiving both EGCG and CHC displayed reduced growth (Fig. 7E). Because CHC is also an MCT1 inhibitor, lactate levels were measured in all tumors. None of these treatments altered lactate content (Fig. 7E). Together, these results indicate a mode of cellular metabolic flexibility, and uncover a specific function for GDH in a pathway that results in the oxidative production of citrate from glutamine.
Discussion
Mitochondrial metabolism complements glycolysis as a source of energy and biosynthetic precursors. Precursors for lipids, proteins and nucleic acids are derived from the TCA cycle. Maintaining pools of these intermediates is essential, even under circumstances of nutrient limitation or impaired supply of glucose-derived pyruvate to the mitochondria. Glutamine’s ability to produce net amounts of both acetyl-CoA and OAA allow it to support TCA cycle activity as a sole carbon source and imposes a greater cellular dependence on glutamine metabolism when MPC function or pyruvate supply is impaired. Other anaplerotic amino acids could also supply both OAA and acetyl-CoA, providing flexible support for the TCA cycle when glucose is limiting. Although fatty acids are an important fuel in some cancer cells (Caro et al., 2012), and fatty acid oxidation is induced upon MPC inhibition, this pathway produces acetyl-CoA but not OAA. Thus, fatty acids would need to be oxidized along with an anaplerotic nutrient in order to enable the cycle to function as a biosynthetic hub. Notably, enforced MPC over-expression also impairs growth of some tumors (Schell et al., 2014), suggesting that maximal growth may require MPC activity to be maintained within a narrow window.
Despite decades of research on mitochondrial pyruvate transport, molecular components of the MPC were only recently reported (Halestrap, 2012; Schell and Rutter, 2013). MPC1 and MPC2 form a heterocomplex in the inner mitochondrial membrane, and loss of either component impairs pyruvate import, leading to citrate depletion (Bricker et al., 2012; Herzig et al., 2012). Mammalian cells lacking functional MPC1 display normal glutamine-supported respiration (Bricker et al., 2012), consistent with our observation that glutamine supplies the TCA cycle in absence of pyruvate import. We also observed that isolated mitochondria produce fully-labeled citrate from glutamine, indicating that this pathway operates as a self-contained mechanism to maintain TCA cycle function. Recently, two well-known classes of drugs have unexpectedly been shown to inhibit MPC. First, thiazolidinediones, commonly used as insulin sensitizers, impair MPC function in myoblasts (Divakaruni et al., 2013). Second, the phosphodiesterase inhibitor Zaprinast inhibits MPC in the retina and brain (Du et al., 2013b). Zaprinast also induced accumulation of aspartate, suggesting that depletion of acetyl-CoA impaired the ability of a new turn of the TCA cycle to be initiated from OAA; as a consequence, OAA was transaminated to aspartate. We noted a similar phenomenon in cancer cells, suggesting that UK5099 elicits a state in which acetyl-CoA supply is insufficient to avoid OAA accumulation. Unlike UK5099, Zaprinast did not induce glutamine-dependent acetyl-CoA formation. This may be related to the reliance of isolated retinas on glucose rather than glutamine to supply TCA cycle intermediates or the exquisite system used by retinas to protect glutamate from oxidation (Du et al., 2013a). Zaprinast was also recently shown to inhibit glutaminase (Elhammali et al., 2014), which would further reduce the contribution of glutamine to the acetyl-CoA pool.
A similar pathway to the one described here was identified in c-Myc-expressing lymphoma cells under glucose deprivation (Le et al., 2012). However, these lymphoma cells were reported to utilize a more complex pathway in which the acetyl-CoA used to produce citrate was generated by ATP-Citrate Lyase (ACL) in the cytosol (Le et al., 2012). We demonstrate that glutamine-dependent citrate formation can occur in isolated mitochondria lacking ACL. This difference is unlikely related to c-Myc, as the SFxL cells used in our study also display c-Myc-dependent glutamine metabolism (Wise et al., 2008). Thus, there may be alternative routes to supply the citrate pool from glutamine, and it remains to be seen whether cell-intrinsic factors dictate pathway preference.
In nutrient-rich culture, cells have access to abundant pyruvate for mitochondrial metabolism. However, survival in the nutrient-limited conditions of actual tumors may require cells to engage alternative metabolic pathways, including degradation of proteins to supply carbon to the TCA cycle (Commisso et al., 2013). The GDH-dependent pathway described here may function in such nutrient-limited conditions where glucose is insufficient to supply a large pool of pyruvate for mitochondrial import. If glutamine and/or glutamate are obtained through protein degradation, GDH could supply both OAA and acetyl-CoA to maintain TCA cycle activity regardless of glucose availability. Unlike transamination reactions, GDH does not require a ketoacid to produce AKG from glutamate, and this would provide a significant advantage during nutrient limitation. This likely explains why simultaneous blockade of GDH and mitochondrial pyruvate transport enhances the growth-suppressive and cytotoxic effects of inhibiting either activity alone. Although both CHC and EGCG may have multiple effects in vivo, it is interesting that combining these inhibitors suppressed tumor growth at doses for which neither drug had any effect on its own. Notably, GDH is dispensable for growth of some glutamine-dependent cells in standard culture (Yang et al., 2009), but is important for growth of Myc-amplified tumors in vivo (Qing et al., 2012). GDH facilitates carbon flow into the TCA cycle during restricted nutrient access.
Experimental Procedures
Reagents
2-cyano-3-(1-phenyl-1H-indol-3-yl)-2-propenoic acid (UK5099), α-cyano-4-hydroxycinnamate (CHC), epigallocatechin gallate (EGCG), GDP, ADP and sodium pyruvate were from Sigma. Isotope-labeled nutrients were from Cambridge Isotope Laboratories. Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES) was from Takashi Tsukamoto at Johns Hopkins School of Medicine.
Cell culture, cell growth, and viability
SFxL cells were generated by infecting SF188 glioma cells with a retoviral vector to express human Bcl-xL (pBabe-Hygro-Bcl-xL). SF188, SFxL, Hela, A549, 786-O, Huh-7 and human fibroblast cells were maintained in DMEM with 10% fetal calf serum. HBEC30 and HCC4017 cells were cultured in ACL4 medium (Kim et al., 2013). To monitor proliferation, cells were seeded at 5,000/well in 48-well plates. The next day, cells were replenished with 0.5 ml of medium with 50 μM UK5099 or DMSO (final 0.1% v/v). After 72 hours, water (0.25 ml) was added to each well and frozen at −80°C for 2 hours. Cells were then warmed to room temperature and 0.5 ml of 0.1 μg/ml Hoechst 33258 in TNE buffer (2 M NaCl, 10 mM Tris-HCl pH 7.4, 1 mM EDTA) was added. Fluorescence was measured using a plate reader. For viability measurements, cells were stained with trypan blue and counted with a hemocytometer. Alternatively, 0.5 × 106 cells were washed with PBS/4% BSA and stained with FITC-conjugated Annexin-V antibody and propidium iodide (PI) in 0.4 ml staining buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH7.4) for 15 min. Annexin-V-positive and PI-positive cells were detected by flow cytometry.
RNA interference
SFxL-derived sub-lines expressing GLUD1 shRNAs were described previously (Yang et al., 2009). Short interfering RNA (siRNA) oligos against MPC1, MPC2, and ME2 and a nontargeting siRNA were obtained from Dharmacon Inc., reconstituted in water to 20 μM, and transfected using Effectene (Qiagen). After 72 hours, 80–90% confluent cells in 6-cm dishes were used for western blot and stable isotope tracing assays.
Metabolic assays and stable isotope tracing
Glucose, glutamine and glutamate were measured with an electrochemical analyzer (BioProfile Basic-4 analyzer, NOVA). Ammonia was measured enzymatically (Megazyme). Amino acids were measured by HPLC (Hitachi L8900). Consumption/secretion rates were determined by normalizing absolute changes in metabolite abundance to cell number and time. Stable isotope tracing of TCA cycle intermediates (Cheng et al., 2011) and ammonia (Yang et al., 2009) were performed as described previously. To assay 13C enrichment in palmitate, cells were cultured in 6-cm dishes with DMEM containing [U-13C]glucose or [U-13C]glutamine for 48 hours, then lysed in 0.4 ml 0.1% triton X-100. Lipids were extracted in methanol:chloroform:water (2:1:1.4, v/v/v). The organic phase was evaporated at 42°C under nitrogen and trans-esterified in 2 ml methanol:toluene (4:1, v/v) containing 0.01% butylated hydroxytoluene and 15 μl acetyl chloride at 100°C for 1 hour. Samples were cooled and 5 ml 6% K2CO3 was added. The toluene phase was analyzed using an Agilent 6970 gas chromatograph networked to an Agilent 5975 mass selective detector. Each palmitate mass isotopologue from m+0 to m+16 was converted into a percentage of the total pool. Fractional enrichment of lipogenic acetyl-CoA was calculated by a regression model. Fatty acid oxidation was measured as described using [U-14C]palmitate (Fediuc et al., 2006).
Lipid NMR
SFxL cells were cultured in 150-mm dishes until 80% confluent. The medium contained 25 mM glucose plus 4 mM unlabeled glutamine, [3-13C]glutamine or [5-13C]glutamine, with or without 200 μM UK5099. After 24 hours, cells from 2 dishes for each condition were collected by trypsinization, washed with PBS and re-suspended in 1ml of 0.1% Triton X-100. Lipids were extracted as described (Bligh and Dyer, 1959) dried down under nitrogen, reconstituted in 180 μl of a 90/10 mixture of CDCl3/CH2Cl2 and loaded into 3 mm NMR tubes. NMR was performed on a Bruker Avance 3 HD 600 MHz instrument equipped with a 10-mm 1H/13C dual cryoprobe operating at 15 K. Sample temperature was held at 27°C. The 13C spectra were recorded with bi-level broadband proton decoupling and NOE enhancement. Carbon spectra were acquired as follows: pulse flip angle, 60°; repetition time, 2 s; acquisition time 1.84 s for 132,768 points over a spectral width of 36 kHz. The total Tr was 3.84 seconds. CDCl3 signal at 77 ppm was used as the chemical shift reference. The total scans acquired for each experiment was 4,096. Spectra were analyzed using the ACD NMR software (ACD Labs, Toronto, CA).
ATP assays
Cells (5×105) were plated into a 6-cm culture dish and allowed to adhere. Approximately 24 hours later, the medium was replaced with 2 mL complete DMEM containing DMSO or UK5099 (200 μM). After another 24 hours, the cells were lysed in lysis buffer (10 mM Tris pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.01% Triton X-100). This lysate was diluted 1:40 and a 25 μl aliquot was combined with 75 μl of reaction solution for ATP measurement (Invitrogen A22066).
Isolated mitochondria
Mitochondria were prepared essentially as described (Pongratz et al., 2009) at 4°C, starting with eight 150-mm dishes of 80–90% confluent SFxL cells. Fresh mitochondrial pellets were reconstituted in 0.5 ml assay buffer (130 mM KCl, 10 mM MOPS, 1 mM MnCl2, 2 mM potassium phosphate, 0.5 mM ADP, 0.5 mM GDP, 2 mM MgCl2, 4 mM [U-13C]glutamine, and 0.2 mM pyruvate or [3-13C]pyruvate). O2 consumption was determined with an Oxygraph water-jacketed oxygen electrode (Hansatech). Isotope tracing was performed by culturing mitochondria in 13C-containing medium at 30°C with agitation at 500 rpm in a heat block. After 30 min, cultures were terminated with 0.5 ml methanol.
Western blots
Protein lysates were prepared in RIPA buffer and quantified using the BCA Protein Assay (Thermo Scientific). Protein was separated on 4–20% SDS-PAGE gels, transferred to PVDF membranes, and probed with antibodies against GDH (Novus Biologicals), β-actin, ME2 (Sigma), calnexin (Stressgen), AIF (Santa Cruz Biotechnology), GAPDH (Millipore), ACL, total AMPK, phospho-AMPK, phosphor-ACC (Cell Signaling), MPC1 (Abcam) and MPC2 (Sigma).
Hyperpolarized 13C-NMR spectroscopy and data analysis
As described (Harrison et al., 2012), cells were resuspended in fresh medium with or without UK5099 and kept at 37°C. Hyperpolarization of 1.4 M [1-13C]sodium pyruvate was performed in an Oxford HyperSense polarizer (Oxford Instruments, UK) as described (Lumata et al., 2012). Polarized samples were dissolved through an automated process using 4 mL of superheated PBS. Approximately 4 ml of hyperpolarized liquid (pH ~7) was collected in a beaker within 8 seconds of dissolution. NMR experiments were immediately carried out at 14.1 T using a Bruker CryoProbe (Bruker Biospin, Billerica, MA). A 200 μl aliquot of hyperpolarized [1-13C]pyruvate was placed in the bottom of a 5 mm NMR tube. Upon insertion into the magnet, 50 million SFxL cells in suspension were injected into the NMR tube using a syringe, resulting in a final concentration of 6 mM hyperpolarized [1-13C]pyruvate in 1 ml. Acquisition was queued, and 13C spectra were acquired using 18-degree inspection radiofrequency pulses with a 2 second repetition time. NMR data were analyzed using ACD 1D NMR processor (Advanced Chemistry Development, Toronto, Canada) and Igor Pro version 6 (Wavemetrics Inc., OR).
Xenografts
Animal procedures were performed with the approval of the UT Southwestern IACUC. A549 cells were suspended in RPMI (107/ml), mixed 1:1 with Matrigel (Becton-Dickinson), and 1 million cells were implanted subcutaneously into 6–8 week old male NCRNU mice. Mice received daily intraperitoneal injections of normal saline or an isotonic solution of CHC (40 μmol in 200 μl, pH adjusted to 7.0). EGCG was provided in the drinking water at 0.5 mg/ml. Tumor size was measured with electronic calipers. To measure lactate, tumors were frozen in liquid nitrogen within 30 seconds of euthanasia. Tissues were ground under liquid nitrogen in a mortar. About 100 mg of each sample was homogenized in a polytron in 1ml of cold 80% methanol to extract metabolites. The samples were centrifuged at 20,000g for 10 min at 4°C. Pellets were re-suspended in 0.1 N NaOH for determination of protein content, and supernatants were subjected to an enzymatic assay for lactate (Vassault, 1991).
Supplementary Material
Acknowledgments
We thank Aron Jaffe for critically evaluating the paper. Kumar Pichumani assisted with hyperpolarization experiments involving the cryoprobe, and Takashi Tsukamoto provided BPTES. This work was supported by the N.I.H. (CA157996 and RR02584) and the Cancer Prevention and Research Institute of Texas (RP140021-P3 and RP130272). C.T.H. was supported by an N.I.H. Training Grant (5T32GM007062-38), A.T.W. was supported by the 1 Million 4 Anna Foundation and the Children’s Cancer Research Fund, and M.A.C. was supported by Fondazione Umberto Veronesi, Milan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivet M, Garcia-Cazorla A, Lyonnet S, Dumez Y, Nassogne MC, Slama A, Boutron A, Touati G, Legrand A, Saudubray JM. Impaired mitochondrial pyruvate importation in a patient and a fetus at risk. Molecular genetics and metabolism. 2003;78:186–192. doi: 10.1016/s1096-7192(03)00016-7. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME, Yudkoff M, Nissim I, Daikhin Y, Lazarow A, Horyn O, Nissim I. Alanine metabolism in the perfused rat liver. Studies with (15)N. The Journal of biological chemistry. 2001;276:31876–31882. doi: 10.1074/jbc.M103890200. [DOI] [PubMed] [Google Scholar]
- Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer discovery. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Cleghorn W, Contreras L, Linton JD, Chan GC, Chertov AO, Saheki T, Govindaraju V, Sadilek M, Satrustegui J, et al. Cytosolic reducing power preserves glutamate in retina. Proceedings of the National Academy of Sciences of the United States of America. 2013a;110:18501–18506. doi: 10.1073/pnas.1311193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Cleghorn WM, Contreras L, Lindsay K, Rountree AM, Chertov AO, Turner SJ, Sahaboglu A, Linton J, Sadilek M, et al. Inhibition of mitochondrial pyruvate transport by zaprinast causes massive accumulation of aspartate at the expense of glutamate in the retina. The Journal of biological chemistry. 2013b;288:36129–36140. doi: 10.1074/jbc.M113.507285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhammali A, Ippolito JE, Collins L, Crowley J, Marasa J, Piwnica-Worms D. A high-throughput fluorimetric assay for 2-hydroxyglutarate identifies zaprinast as a glutaminase inhibitor. Cancer discovery. 2014;4:828–839. doi: 10.1158/2159-8290.CD-13-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fediuc S, Gaidhu MP, Ceddia RB. Regulation of AMP-activated protein kinase and acetyl-CoA carboxylase phosphorylation by palmitate in skeletal muscle cells. Journal of lipid research. 2006;47:412–420. doi: 10.1194/jlr.M500438-JLR200. [DOI] [PubMed] [Google Scholar]
- Fendt SM, Bell EL, Keibler MA, Olenchock BA, Mayers JR, Wasylenko TM, Vokes NI, Guarente L, Vander Heiden MG, Stephanopoulos G. Reductive glutamine metabolism is a function of the alpha-ketoglutarate to citrate ratio in cells. Nature communications. 2013;4:2236. doi: 10.1038/ncomms3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro PA, Yang J, Metelo AM, Perez-Carro R, Baker R, Wang Z, Arreola A, Rathmell WK, Olumi A, Lopez-Larrubia P, et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell metabolism. 2013;17:372–385. doi: 10.1016/j.cmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golman K, in ‘t Zandt R, Thaning M. Real-time metabolic imaging. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11270–11275. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP. The mechanism of the inhibition of the mitochondrial pyruvate transportater by alpha-cyanocinnamate derivatives. The Biochemical journal. 1976;156:181–183. doi: 10.1042/bj1560181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP. The mitochondrial pyruvate carrier: has it been unearthed at last? Cell metabolism. 2012;16:141–143. doi: 10.1016/j.cmet.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Harrison C, Yang C, Jindal A, DeBerardinis RJ, Hooshyar MA, Merritt M, Dean Sherry A, Malloy CR. Comparison of kinetic models for analysis of pyruvate-to-lactate exchange by hyperpolarized 13 C NMR. NMR in biomedicine. 2012;25:1286–1294. doi: 10.1002/nbm.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. The Journal of clinical investigation. 2013;123:3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- Kim HS, Mendiratta S, Kim J, Pecot CV, Larsen JE, Zubovych I, Seo BY, Kim J, Eskiocak B, Chung H, et al. Systematic identification of molecular subtype-selective vulnerabilities in non-small-cell lung cancer. Cell. 2013;155:552–566. doi: 10.1016/j.cell.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell metabolism. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li C, Allen A, Stanley CA, Smith TJ. The structure and allosteric regulation of mammalian glutamate dehydrogenase. Archives of biochemistry and biophysics. 2012;519:69–80. doi: 10.1016/j.abb.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumata L, Merritt ME, Malloy CR, Sherry AD, Kovacs Z. Impact of Gd3+ on DNP of [1-13C]pyruvate doped with trityl OX063, BDPA, or 4-oxo-TEMPO. The journal of physical chemistry A. 2012;116:5129–5138. doi: 10.1021/jp302399f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates JM, Segura JA, Martin-Rufian M, Campos-Sandoval JA, Alonso FJ, Marquez J. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Current molecular medicine. 2013;13:514–534. doi: 10.2174/1566524011313040005. [DOI] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. The Journal of biological chemistry. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- Pongratz RL, Kibbey RG, Cline GW. Investigating the roles of mitochondrial and cytosolic malic enzyme in insulin secretion. Methods in enzymology. 2009;457:425–450. doi: 10.1016/S0076-6879(09)05024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz RL, Kibbey RG, Shulman GI, Cline GW. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. The Journal of biological chemistry. 2007;282:200–207. doi: 10.1074/jbc.M602954200. [DOI] [PubMed] [Google Scholar]
- Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, Mayes PA, Wise DR, Thompson CB, Maris JM, et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer cell. 2012;22:631–644. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JG, Seth P, Clish CB, Lorkiewicz PK, Higashi RM, Lane AN, Fan TW, Sukhatme VP. Knockdown of malic enzyme 2 suppresses lung tumor growth, induces differentiation and impacts PI3K/AKT signaling. Scientific reports. 2014;4:5414. doi: 10.1038/srep05414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell JC, Rutter J. The long and winding road to the mitochondrial pyruvate carrier. Cancer & metabolism. 2013;1:6. doi: 10.1186/2049-3002-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, Earl EG, Egnatchik RA, DeBerardinis RJ, Rutter J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg Effect and colon cancer cell growth. Molecular Cell. doi: 10.1016/j.molcel.2014.09.026. this issue. [DOI] [PMC free article] [PubMed]
- Scott DA, Richardson AD, Filipp FV, Knutzen CA, Chiang GG, Ronai ZA, Osterman AL, Smith JW. Comparative metabolic flux profiling of melanoma cell lines: beyond the Warburg effect. The Journal of biological chemistry. 2011;286:42626–42634. doi: 10.1074/jbc.M111.282046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassault A, Bonnefont JP, Specola N, Saudubray JM. Lactate, pyruvate and ketone bodies. In: Hommes EA, editor. Techniques in diagnostic human biochemical genetics. New York: Wiley-Liss; 1991. pp. 285–308. [Google Scholar]
- Wanders RJ, Meijer AJ, Groen AK, Tager JM. Bicarbonate and the pathway of glutamate oxidation in isolated rat-liver mitochondria. European journal of biochemistry/FEBS. 1983;133:245–254. doi: 10.1111/j.1432-1033.1983.tb07455.x. [DOI] [PubMed] [Google Scholar]
- Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Harrison C, Jin ES, Chuang DT, Sherry AD, Malloy CR, Merritt ME, DeBerardinis RJ. Simultaneous steady-state and dynamic 13C NMR can differentiate alternative routes of pyruvate metabolism in living cancer cells. The Journal of biological chemistry. 2014;289:6212–6224. doi: 10.1074/jbc.M113.543637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer research. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H, Antoniewicz MR, Stephanopoulos G, Kelleher JK. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. The Journal of biological chemistry. 2008;283:20621–20627. doi: 10.1074/jbc.M706494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Wu MS, Zou C, Tang Q, Lu J, Liu D, Wu Y, Yin J, Xie X, Shen J, et al. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-kappaB pathway. Cancer letters. 2014;342:150–158. doi: 10.1016/j.canlet.2013.08.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.