Abstract
Fischer 344 × Brown Norway F1 (F344×BN-F1) hybrid rats express greater longevity with improved health relative to aging rodents of other strains; however, few behavioral reports have thoroughly evaluated cognition across the F344×BN-F1 lifespan. Consequently, this study evaluated spatial reference memory in F344×BN-F1 rats at 6, 18, 24 or 28 months (mo) of age in the Morris water maze. Reference memory decrements were observed between 6 mo and 18 mo and between 18 mo and 24 mo. At 28 mo, spatial learning was not worse than 24 mo, but swim speed was significantly slower. Reliable individual differences revealed that ~50% of 24-28 mo performed similarly to 6 mo while others were spatial learning-impaired. Aged rats were impaired at learning within daily training sessions, but not impaired at retaining information between days of training. Aged rats were also slower to learn to escape onto the platform, regardless of strategy. In summary, these data clarify the trajectory of cognitive decline in aging F344×BN-F1 rats and elucidate relevant behavioral parameters.
1. Introduction
Aging is associated with decline in a number of cognitive domains; most notable is an age-related decrement in medial temporal lobe-dependent declarative memory (reviewed in (Mishkin et al., 1997; Squire and Zola, 1996; Milner et al., 1998). There is considerable heterogeneity in the onset and severity of cognitive impairment in older humans. This variability is due, in part, to differences in lifestyle and general health over the course of one's lifespan (Colcombe et al., 2004; Biessels et al., 2006; Kidd, 2008; Peters et al., 2008; Ahlskog et al., 2011; Siervo et al., 2011). Consequently, well-controlled studies of aging animal models that incorporate sensitive and reliable behavioral measures are invaluable to the understanding of normal cognitive aging. In this context, there has been a proliferation of aging rat models characterized for spatial reference memory using the Morris water maze, a robust behavioral probe of the rodent medial temporal lobe system (Morris, 1981; Morris et al., 1982; Moser et al., 1993; Steffenach et al., 2005).
The Fischer 344 × Brown Norway F1 hybrid rat (F344×BN-F1) was developed by the National Institute on Aging (NIA) to supply a genetically defined rat model with an improved health profile at older ages relative to the more widely used F344 strain (Sprott, 1991; Sprott and Ramirez, 1997). F344×BN-F1 rats are vigorous hybrids that exhibit increased lifespan and delayed onset of typical age-associated pathologies (Spangler et al., 1994; Lipman et al., 1996; Turturro et al., 1999). Although young adult F344×BN-F1 rats outperform age-matched F344 rats on the Morris water maze (van der Staay and Blokland, 1996), the effect of advancing age on spatial reference memory in the F344×BN-F1 rat is poorly characterized relative to the F344 model. Previous studies demonstrate that spatial reference memory is impaired in older F344×BN-F1 rats, but the onset of this impairment is variously reported as early as 18 months or as late as 31 months of age (Hebda-Bauer et al., 1999; Markowska and Savonenko, 2002; Adams et al., 2008). Apart from disagreement in the timing or trajectory of spatial reference memory decrements, there has been minimal consideration of specific behavioral changes that could explain the nature of memory impairments in aging F344×BN-F1 hybrids.
A survey of spatial reference memory studies using the parent F344 model reveals a variety of explanations for age-related memory impairment. The first category of hypotheses addresses the role of variability in aging. Whereas some report individual variability of reference memory impairment is minimal among aged F344 rats (Lindner et al., 1992; Frick et al., 1995) or at least not greater as a function of age (Lindner, 1997), others demonstrate that group differences may be ascribed to robust impairment that manifests in a subset of aged rats (Tombaugh et al., 2002; Bizon et al., 2009). An alternate explanation posits that unreliable trial-to-trial performance by aged rats, or greater within-subject variability, is the mediator of differences between age groups (Barnes et al., 1997; but see Bizon et al., 2009). The second category of hypotheses proposes distinct roles for acquisition and retention in age-related memory impairment. Spatial working memory is compromised in aged F344 rats, and an inability to rapidly acquire trial-specific information may impair the rate of reference memory acquisition(Lindner et al., 1992; Lindner, 1997; Guidi et al., 2014 but see Frick et al., 1995; Bizon et al., 2009). In contrast, others argue that aged rats are impaired in retention of previously acquired spatial information over long delays (i.e. at least 24 h; Shukitt-Hale et al., 1998; Foster et al., 2001; Foster and Kumar, 2007). Finally, the third category of hypotheses centers on non-cognitive variables. Aged F344 rats do not swim as quickly as young adults (Frick et al., 1995; Lindner, 1997; Bizon et al., 2009), are susceptible to retinal degeneration (Lindner and Gribkoff, 1991), and are impaired at acquiring navigational procedures even when spatial information is not necessary to facilitate escape (Lindner, 1997; Burke et al., 2010; Guidi et al., 2014 but see Bizon et al., 2009). The former two observations suggest that very old F344 rats may not readily meet basic sensorimotor criteria for water maze testing, whilst the latter could indicate aging modulates fronto-striatal circuits that mediate procedural learning in spatial tasks (Whishaw et al., 1987; Devan et al., 1996, 1999, 1999; DeCoteau and Kesner, 2000 and reviewed in Devan et al., 2011).
As the F344×BN-F1 hybrid is bred for health and longevity, behavioral data accurately depicting the trajectory of age-dependent decline of reference memory in this model could provide important insight into the normal cognitive aging process. Accordingly, the present study examines spatial reference memory at key aging time-points in the F344×BN-F1 lifespan. Furthermore, insight from behavioral studies of aged F344 rats was incorporated in the design of additional analyses to test specific complementary hypotheses. First, measures of inter- and intra-individual performance were tested to determine whether behavioral variability increases with age in F344×BN-F1 hybrids. Second, trial-to-trial and day-to-day differences in performance were compared to determine whether aged F344×BN-F1 rats are slower to acquire information or impaired at retaining information across days. Third, non-cognitive contributions to performance were considered to determine whether aged F344×BN-F1 rats exhibit sensorimotor deficits or impaired procedural learning.
2. Materials and Methods
2.1. Subjects
Male, Fisher 344 × Brown Norway F1 (F344×BN-F1) hybrid rats were obtained from the NIA Aging Rodent Colony (Harlan Laboratories, Indianapolis, IN, USA) and housed in a specific, pathogen-free vivarium at Wake Forest University for one month prior to and throughout behavioral testing. Rats were 6 (n=90), 18 (n=22), 24 (n=139) or 28 (n=27) months (mo) of age during the time of behavioral testing. Notably, the latter three time-points precede the ages of 95%, 90%, and 75% survival, respectively, for male rats of this strain (Sprott and Ramirez, 1997; Turturro et al., 1999; National Institute on Aging, 2011). Rats were tested in 10 independent cohorts from June 1 2008 to Jan 31 2012 (see Supplemental Materials S1 and Table S1). Throughout the course of the study, rats were regularly handled, weighed, and inspected by laboratory and animal care personnel to identify and address any possible health concerns that could adversely affect behavioral performance. All procedures were approved by the Institutional Animal Care and Use Committee of Wake Forest University. Data from a subset of these animals has been reported previously (McQuail et al., 2011, 2013).
2.2. Apparatus
The water maze was a 1.83 m diameter pool filled with water maintained at a temperature of 26±1 °C. The water was rendered opaque by the addition of white, non-toxic, tempera paint. The pool was encircled by black curtains affixed with various unique, white geometric cues. White noise from speakers within the testing room minimized incidental auditory cues during behavioral testing. Activity within the pool was recorded via a CCD camera mounted above the apparatus and connected to a DVD recorder and personal computer running EthoVision software (Noldus, Leesburg, VA, USA).
2.3. Place Learning Procedure
The behavioral training procedure used in this study is identical to that previously reported in Gallagher et al. (1993) and Bizon et al. (2009). Rats received 8 days of place training, learning to swim to a hidden, retractable platform submerged 2 cm below the water's surface in the center of one quadrant of the pool (SE). Rats were given 3 trials per day to swim to the hidden platform. On each trial, rats were released from one of four starting points (N, E, S, or W) in a pseudorandom, counterbalanced order. If the rat failed to locate the platform within 90 s, it was guided to the platform by an experimenter. The rat remained on the platform for 30 s followed by an additional 30 s in a holding chamber before beginning the next trial. Every sixth trial (i.e. the third trial on days 2, 4, 6 and 8) was a probe trial where the platform was lowered, requiring the rat to swim for a fixed 30 s duration. After the probe's conclusion, the platform was covertly raised to allow for escape, thus maintaining normal response-reinforcement task contingency. On training trials, path length, the total distance traveled during the trial, was used to evaluate performance. During probe trials, average proximity to the platform was calculated by sampling the distance between the rat and the platform location 10 times/s and averaging these values over the duration of the trial (Gallagher et al., 1993; Maei et al., 2009). The percentage of time spent searching in the training quadrant (“Percent Time in Quadrant”) was also recorded on each probe trial. Swim speed was measured in all training and probe trials. Latency and cumulative search error were not analyzed in the current study as they may be confounded by age-related differences in swimming speed.
2.4. Cue Training Procedure
After the completion of place training, rats were trained on a cue learning task requiring escape to a visible platform (raised ~2 cm above surface with black, high-contrast marking around the edge of the platform), the location of which varied from trial to trial. Rats were given one block of 6 consecutive cue trials (30 s maximum duration). On each trial rats were released from one of eight starting points (N, NE, E, SE, S, SW, W, or NW) in a pseudorandom, counterbalanced order. Path length and swim speed were recorded on each cue trial. All rats used in this study could locate the platform on at least 5 out of the 6 cue trials.
2.5. Behavioral Measures and Statistical Analyses
Data were analyzed using SPSS Statistics 21 (IBM, Armonk, NY, USA). Training, probe, and cue trial data were tested for statistical significance by two-way mixed analysis of variance (ANOVA) using age as a between-subject factor and training block (average of 5 training trials) or trial (probe and cue trials) as a repeated within-subject factor. Mauchly's test of sphericity was used to detect significant departures from sphericity and the Huyhn-Feldt correction was applied when ε≥0.75; when ε<0.75, the more conservative Greenhouse-Geisser correction was applied. Non-normally distributed data (Shapiro-Wilk test p<0.05) were analyzed using the non-parametric Kruskal-Wallis test. In all comparisons, p<0.05 was considered significant; nonsignificant (n.s.) trends (tr., 0.1>p≥0.05) are reported as well. Bonferroni (or Dunn-Bonferroni for non-parametric tests) post hoc tests were used to specify significant differences between age groups while controlling for multiple comparisons. Significant interactions are explored in the Supplementary Material S2 and Tables S2-S5.
Aged rats show significant impairment relative to young adults during early phases of training, but may surmount this deficit with extended training. Consequently, analysis of a single probe trial administered at the very end of training may not yield evidence of impaired spatial search in aged rats (e.g. compare Markowska and Savonenko, 2002 and Adams et al. 2008). Therefore, spatial learning is more accurately characterized by a measure that reveals the rate of acquisition of a spatially directed search for the platform location. Accordingly, data from interpolated probe trials was used to compute a “Spatial Learning Index” for each individual rat (see Gallagher et al., 1993; Bizon et al., 2009). This measure is the weighted sum of average proximity on probe trials 2, 3, and 4. The weighting of probe trial performance is critical in the computation of the Index as it favors rapid acquisition of spatial information; better performance on earlier probe trials is weighted more favorably than similar performance on later probes. These weights were empirically determined by dividing mean average proximity of 6 mo rats on probe trial 1 by that on probe trials 2, 3, and 4; weights were 1.09 for probe 2, 1.30 for probe 3, and 1.41 for probe 4 (See Supplemental Materials S3 and Fig. S1-S2 for further description). As the Spatial Learning Index is derived from the average proximity of search to the training platform location on probes, a lower Index indicates search in closer proximity to the platform location (i.e. comparatively better), whereas higher values reflect search further from the platform location (i.e. comparatively worse). Spatial Learning Index was compared among age groups via one-way ANOVA while between-subject variability was tested using Levene's test of homogeneity of variance. Intra-individual, or probe-to-probe, variability was evaluated by calculating the standard deviation of the average proximity on probes 2, 3, and 4 (i.e. those used in the calculation of the Spatial Learning Index) for each rat (See Supplemental Materials S3 for further description). The Spatial Learning Index was also used to perform bivariate correlations with other behavioral parameters; in all instances, separate correlations were performed within each age group. Spearman's rho (ρ) was used in place of Pearson's r if a covariate was not normally distributed.
Training trial performance was parsed to separately evaluate changes in performance within daily training sessions and retention of performance between days of training. This was done by calculating the change in path length from one training trial to the next (i.e. Trial 1 – Trial 2, Trial 2 – Trial 3, etc). As start locations were not equidistant from the training platform (N and W were 122 cm from platform, S and E were 72 cm from platform), the start-to-platform distance was subtracted from path length to correct for these differences in start location; this “Corrected Path Length” reflects error in excess of direct swim to the platform and is suitable for direct comparison between trials, regardless of starting location. For those trials separated by a 30 s ITI (3 instances per block), the difference scores were averaged for each rat and block and termed “30 s Path Length Change.” For trials separated by a 24 h ITI (1 instance per block), the difference score was termed “24 h Path Length Change.” In follow-up comparisons, the average of each value across all blocks (“Mean 30 s Path Length Change” and “Mean 24 h Path Length Change”) was analyzed by one-sample T-tests to determine whether either parameter was significantly different from zero within each group. In addition to testing for correlation with Spatial Learning Index, Mean 30 s Path Length Change and Mean 24 h Path Length Change were also tested for correlation with each other.
To assess relative differences in the ability of each rat to learn procedural requirements, namely escaping onto the training platform, performance on each training trial was dichotomously characterized as either “Successful” (located the platform) or “Unsuccessful” (did not locate the platform; as in Ruediger et al., 2012), and “Total Successful Trials,” the sum of successful trials for each rat, was compared between age groups.
3. Results
3.1. Place Learning
On training trials, there was a main effect of age (F(3,274)=35.026, p<0.001), a main effect of block (F(3,822)=232.851, p<0.001) and an age×block interaction (F(9,822)= 2.148, p<0.05; Fig. 1A) on path length. Post hoc comparisons determined that 6 mo swam a shorter path length to the platform than 18 mo, 24 mo, and 28 mo (p<0.001 for each comparison vs. 6 mo). There was no main effect of age (F(3,274)= 1.895, p>0.1 n.s.) on swim speed, but there was an effect of block (F(3,822)=52.667 p<0.001) and an age×block interaction (F(9,822)=5.530, p<0.001; Fig 1D).
Fig. 1. Spatial reference memory and swim speed of aging F344×BN-F1 rats.
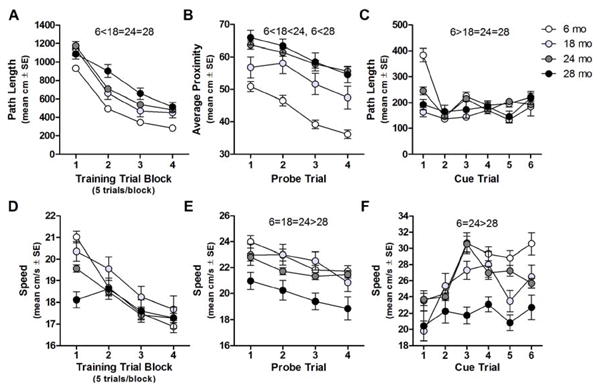
A: Path length (y-axis) decrearsed across all blocks of training trials (x-axis) but 18, 24, and 28 mo are impaired relative to 6 mo. B: Average distance from former the platform location (y-axis) was also greater in older rats across all probe trials (x-axis). C: Path length (y-axis) of 18, 24, and 28 mo was shorter than 6 mo on cue training trials (x-axis). D: Swim speed (y-axis) of 24 and 28 mo was slower than 6 mo only during the first block training trials (x-axis). E: Swim speed of 28 mo was slower than 6, 18, and 24 mo across all probe trials (x-axis). F: Swim speed of 28 mo was slower than 6 mo and 24 mo during cue training trials (x-axis).
On probe trials, there was an effect of age (F(3,272)=61.015, p<0.001) and trial (F(3,816)=26.267 p<0.001) on average proximity but no age×probe trial interaction (F(9,816)=1.365, p>0.2 n.s.; Fig. 1B). Post hoc comparisons determined that 6 mo searched closer than 18, 24, and 28 mo (p<0.001 for each comparison vs. 6 mo) and 18 mo searched closer than 24 mo (p<0.05). There was a main effect of age (F(3,272)=9.759, p<0.001) and probe trial (F(3,816)=12.404, p<0.001) on swim speed but no age×probe trial interaction (F(9,816)=1.030, p>0.4 n.s.; Fig 1E). Post hoc comparisons revealed that 28 mo swam significantly slower than 6 mo (p<0.001), 18 mo (p<0.01), and 24 mo (p<0.001).
3.2. Cue Training
On cue trials, there was an effect of age (F(3,273)=6.529, p<0.001), trial (F(5,1365)=11.979, p<0.001), and an age×trial interaction (F(15,1365)=5.903, p<0.001) on path length (Fig. 1C). Post hoc comparisons determined that 6 mo swam longer path lengths than 18 (p<0.001), 24, and 28 mo (p<0.05 for both). There was a main effect of age (F(3,273)=10.386, p<0.001), trial (F(5,1365)=10.795, p<0.001), and an age×trial interaction (F(15,1365)=2.237, p<0.01; Fig. 1F) on swim speed. Post hoc comparisons determined that 28 mo swam slower than 6 and 24 mo (p<0.01 for both).
3.3. Individual Differences in Spatial Learning
Spatial Learning Index differed with age (F(3,277)=57.132, p<0.001; Fig. 2A); post hoc comparisons determined that Spatial Learning Index was significantly greater in 18, 24, and 28 mo relative to 6 mo (p<0.001 for all pair-wise comparisons vs. 6 mo; 18 vs. 24 mo p<0.08 n.s. tr.). Variance of Spatial Learning Index was not significantly different between age groups (F(2,274)=1.739, p>0.1 n.s.), nor was the standard deviation of performance of individual rats on probe trials 2, 3, and 4, a putative measure of within-subject variability, different between ages (H(3)=2.024, p>0.5 n.s.; Fig. 2B and also see Supplemental Materials S3 and Fig. S3).
Fig. 2. Individual differences in spatial learning in aging F344×BN-F1 rats.
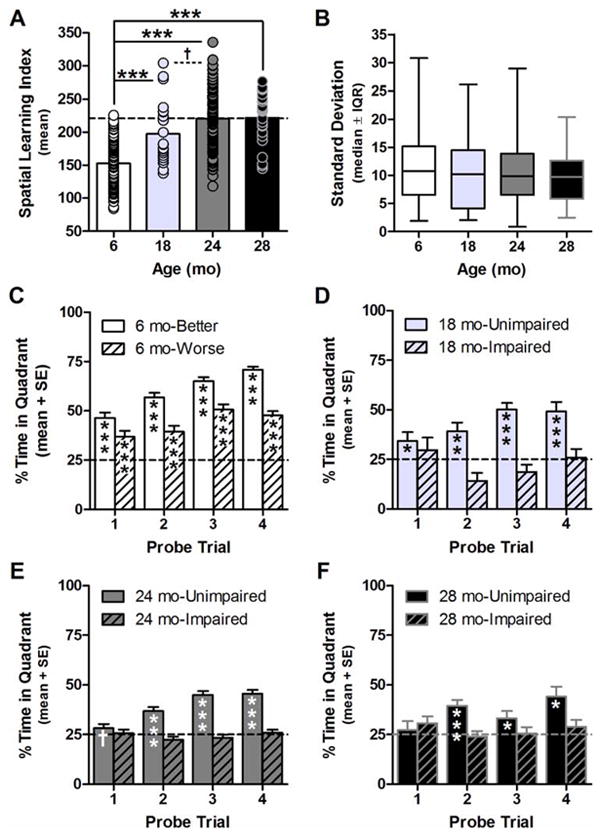
A: Spatial Learning Index was significantly greater in 18, 24, and 28 mo compared to 6 mo, but variance of this measure was not different among age groups (horizontal dashed line denotes criterion distinguishing impaired from unimpaired performance according to the 6 mo reference group). Solid lines and ***p<0.001; dashed line and †0.1>p>0.05 between ages indicated. B: The standard deviation of performance on probe trials 2-4 was not different among age groups. C: Percent Time in Quadrant (y-axis) was significantly greater than chance (dashed line) in 6 month-better performers and 6 month-worse performers on all probe trials (x-axis). D: Percent Time in Quadrant (y-axis) was significantly greater than chance in 18 mo-unimpaired on all probe trials (x-axis), while 18 mo-impaired never exceeded chance. E and F: Percent Time in Quadrant (y-axis) was significantly greater than chance in 24 mo- and 28 mo-unimpaired on probe trials 2-4 (but not probe trial 1; x-axis), while 24 mo- and 28 mo-impaired rats never exceeded chance. C-F *p<0.05, **p<0.01, ***p<0.001 and †0.1>p>0.05 vs. 25%.
As spatial learning performance was found to be reliable across age groups, a follow-up analysis was conducted to confirm whether behaviorally defined subgroups differed with respect to escape strategies during training. First, 18, 24, and 28 mo rats were sub-grouped relative to the normal range of 6 mo performance; Index scores within 2 standard deviations of the mean of the 6 mo group (Spatial Learning Index of less than or equal to 221) were categorized as “unimpaired” while greater scores were categorized as “impaired” (Gallagher et al., 1993; Bizon et al., 2009 and discussed in Baxter and Gallagher, 1996). Next, to equalize the consequences of reducing variance via subgrouping of aged rats across comparisons (as variance was not different between age groups), 6 mo rats were also sub-grouped according to whether their Spatial Learning Index was “better” or “worse” than average in this age group (an index score of 153). Then, to reveal whether or not each sub-group exhibited a spatial bias on any given probe trial, one-sample T-tests were used to determine whether Percent Time in Quadrant during each probe trial exceeded chance (i.e. 25%; α(one-tailed)=0.05). The 6 mo-better (n=44) and 6 mo-worse (n=46) subgroups both exhibited a significant bias to search in the training quadrant on all four probe trials, (ts=3.873-28.284, p<0.001 for all probes; Fig. 2C). The 18 mo-unimpaired (n=15; 68% of 18 mo group) exhibited a significant bias for the training quadrant on each probe (ts=2.109-7.663, ps<0.001-0.05; Fig. 2D). The 24 mo-unimpaired (n=65; 47% of 24 mo group) showed a trend towards spatial bias for the training quadrant on the first probe (t(64)=1.481, p<0.08 n.s. tr.) and a significant bias on all subsequent probes (ts=6.088-10.515, p<0.001 for all probes; Fig. 2E). While 28 mo-unimpaired (n=12; 44% of 28 mo group) did not show a significant bias for the training quadrant on the first probe (t(11)=0.514, p>0.3 n.s.), there was a significant bias on each subsequent probe (ts= 2.180-5.104, p<0.001-0.05; Fig. 2F). In contrast to the reliable expression of bias in aged-matched unimpaired groups, 18 mo-impaired (n=7; 32% of 18 mo group), 24 mo-impaired (n=74; 53% of 24 mo group), and 28 mo-impaired (n=15; 56% of 28 mo group) never exhibited a bias for the training quadrant on any probe (ts=0.152-1.155, ps>0.1-0.4 n.s. or ts<0, although t(12)=1.589, p<0.07 n.s. tr. for 28 mo-impaired on probe 1; Fig. 2D-F).
3.4. Changes in Performance after 30 s and 24 h Intertrial Intervals
There was an effect of age (F(3,274)=5.455, p<0.01), block (F(3,822)=10.059, p<0.001), and an age×block interaction (F(9,822)=3.228, p<0.05) on 30 s Path Length Change (Fig. 3B). Post hoc comparisons determined 30 s Path Length Change was lower in 28 mo compared to 6 mo (p<0.01). One-sample T-tests revealed that Mean 30 s Path Length Change was significantly greater than zero in 6 mo (t(89)=10.928, p<0.001), 18 mo (t(21)=4.232, p<0.001), and 24 mo (t(138)=7.866, p<0.001), but not 28 mo (t(26)=1.083, p>0.2 n.s.; Fig 3C). However, Mean 30 s Path Length Change was not correlated with spatial learning in any age group (rs=0.018-0.247, ps>0.2-0.8; Fig. 3D).
Fig 3. Changes in performance over 30 s and 24 h ITIs in aging F344×BN-F1 rats.
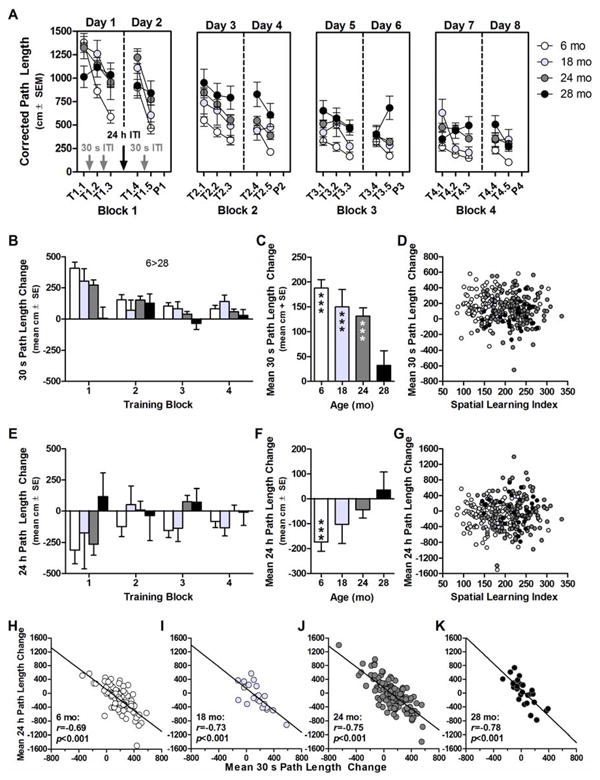
A: Corrected path length (y-axis) plotted as a function training trial (x-axis, “T” denotes training trial identified as “block#.trial#” and “P” denotes probe trial), day (separated by vertical solid and dashed lines) and block (separate panels). Training trials are separated by either 30 s intertrial interval (ITI; gray text/arrows) or 24 h ITI (black text/arrows). B: Relative to 6 mo, 30 s Path Length Change (y-axis) was lower in 28 mo (x-axis is training block). C: Mean 30 s Path Length Change was significantly greater than zero in 6, 18, and 24 mo (***p<0.001 vs zero), but not 28 mo. D: Mean acquisition (y-axis) was not reliably associated with Spatial Learning Index (x-axis) at any age. E: There was no effect of age on 24 h Path Length Change (y-axis) in any training block (x-axis). F: Mean 24 h Path Length Change was significantly less than zero in 6 mo (***p<0.001 vs zero) but not different from zero in 18, 24, or 28 mo. G: Mean 24 h Path Length Change (y-axis) was not reliably associated with Spatial Learning Index (x-axis) at any age. H-K: Mean 24 h Path Length Change (y-axis) and Mean 30 s Path Length Change (x-axis) were inversely correlated at all ages. Solid lines denote significant correlation. Inset: Pearson's r and p values.
There was a non-significant trend towards an effect of age (F(3,271)=2.593, p<0.06 n.s. tr.) on 24 h Path Length Change, but no effect of block (F(3,813)=0.551, p>0.6 n.s.) or age×block interaction (F(9,813)=0.902, p>0.5 n.s.; Fig. 3E). Mean 24 h Path Length Change was significantly less than zero in 6 mo (t(89)=-4.608, p<0.001) but not significantly different from zero in 18, 24, or 28 mo (ts=-1.355-0.490, ps>0.1-0.6; Fig. 3F). Lastly, Mean 24 h Path Length Change was not correlated with spatial learning in any age group (rs=-0.137-0.025, ps>0.1-0.7; Fig. 3G).
In a final set of correlations, Mean 30 s and Mean 24 h Path Length Change were tested for association, revealing that these two measures were inversely related in ages all groups (6 mo: r=-0.691, p<0.001; 18 mo: r=-0.734, p<0.05; 24 mo: r=-0.747, p<0.001; 28 mo: r=-0.779, p<0.001; Fig. 3H-K and see Supplemental Materials S4, Fig S4 and Table S6).
3.5. Procedural Learning
The fraction of successful trials was observed to increase with training until all age groups were eventually escaping onto the training platform with high rates of success; however, the performance slope of 18, 24, and 28 mo was not as steep as 6 mo (Fig 3A). Accordingly, Total Successful Trials was different between age groups (H(3)=84.496, p<0.001; Fig 4B). Post hoc comparisons determined that Total Successful Trials was lower in 18, 24, and 28 mo vs. 6 mo (p<0.001 for each pairwise comparison). Total Successful Trials was significantly correlated with Spatial Learning Index in 6 mo (ρ=-0.291, p<0.01) and 24 mo (ρ=-0.412, p<0.001), with similar trends in 18 mo (ρ=-0.397, p<0.07 n.s. tr.) and 28 mo (ρ=-0.338, p<0.09 n.s. tr); fewer successful trials was associated with worse performance (Fig 4C-F).
Fig. 4. Procedural learning in aging F344×BN-F1 rats.
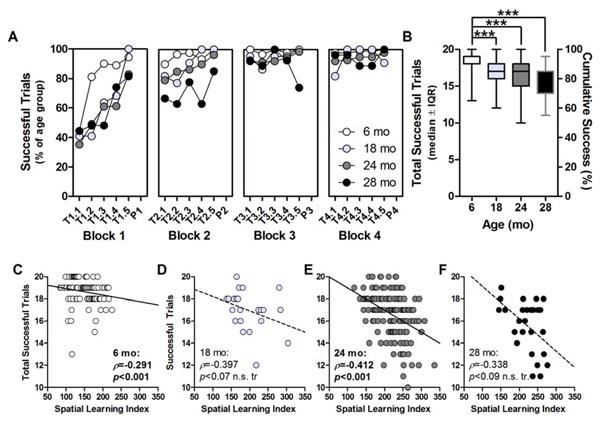
A: The fraction of successful trials (i.e. those rats where the rat escaped onto the training platform; y-axis) increased as a function of training trial (x-axis, “T” denotes training trial identified as “block#.trial#” and “P” denotes probe trial). B: Total number of successful trials per animal (y-axis-left; expressed as fraction of total trials on y-axis-right) was significantly lower in 18, 24, and 28 mo compared to 6 mo. ***p<0.001 for indicated comparisons. C-F: Total Successful Trials (y-axis) correlated with Spatial Learning Index (x-axis) in 6 mo and 24 mo with similar trends evident in 18 mo and 28 mo. Solid lines denote significant correlation and dashed lines indicated non-significant trend. Inset: Spearman's ρ and p values.
4. Discussion
F344×BN-F1 hybrid rats express a longer lifespan and improved health at advanced ages relative to other rat strains; therefore, these rats provide a robust model to evaluate age-related cognitive dysfunction without the confounds of neural pathology and environmental differences that complicate the interpretation of human cognitive aging data. The present study analyzed spatial reference memory in 6, 18, 24 and 28 month-old hybrid rats and revealed memory decrements between 6 and 18 months and 18 and 24 months of age. Using an individualized, graded summary of measure of spatial learning, it was determined that individual aged rats may be classified as unimpaired or impaired relative to young adults. Furthermore, aging was observed to impair learning over the short delays between trials within a day but retention between days was not significantly different between age groups. Finally, it was also determined that aging impairs procedural learning and is related to spatial learning.
4.1. Spatial reference memory in aging F344×BN-F1 rats and comparison to other strains
Only a handful of studies have examined spatial reference memory across the F344×BN-F1 lifespan. Prior studies variously depict the onset and trajectory of decline as delayed until greater than 30 months (Hebda-Bauer et al., 1999), progressively declining from 18 months until at least 30 months of age (Markowska and Savonenko, 2002), or declining until 18-20 months then plateauing through 29-32 months (Adams et al., 2008). The present findings are intermediate to the staging proposed in these latter studies (i.e. Markowska and Savonenko, 2002 and Adams et al., 2008); decrements in reference memory occur between 6 months and 18 months and between 18 months and 24 months, but not between 24 and 28 months. The distribution of individual differences in spatial learning at 24 and 28 months was such that rats are near-equally divided into unimpaired (within the range of young and showing evidence of a spatially guided search for the platform) and impaired subgroups (outside of the range of young and not showing significant spatial bias during the testing period). This distribution is similar to that reported for aged Long-Evans (LE; Gallagher et al., 1993) and F344 (Bizon et al., 2009) rats characterized using an identical training procedure. These aged subgroups are associated with reliable differences in performance across multiple probe trials, not inconsistent probe trial performance among older individuals (see Barnes et al., 1997 and Bizon et al., 2009).
It is notable that the prominent spatial learning deficit in 24 month-old F344×BN-F1 rats precedes the age of 90% survival of this strain (25 months; National Institute on Aging, 2011). In contrast, qualitatively similar impairments emerge at the age of 75% survival in male F344 rats (22 months; Bizon et al., 2009; National Institute on Aging, 2011) and 75-80% survival in male LE rats (24 months; Holloszy and Schechtman, 1991; Gallagher et al., 1993). While 24 and 28 month-old F344×BN-F1 rats were similarly impaired at spatial learning relative to 6 month-old controls, the average swim speed of 28 month-old rats was slower than 6 and 24 month-old rats during place and cue training. Slower swimming speed in this oldest group most likely reflects reduced physical function. Carter and colleagues reported age-related decrements in grip strength and open field locomotion of F344×BN-F1 rats; grip strength was significantly weaker by 12-15 months, and open field movement was significantly reduced by 25-27 months compared to 8 month-old controls (Carter et al., 2009). Furthermore, lower baseline physical function predicted reduced longevity of F344×BN-F1 rats in a longitudinal study between 24 and 30 months (Carter et al., 2002). Consistent with these observations, the mortality rate of male F344×BN-F1 rats sharply increases at 26 months of age while average body weight begins to decline precipitously (Sprott and Ramirez, 1997). Therefore, merely selecting performance measures that are less sensitive to slower swim speed of aged rats does not obviate the underlying fact that rats of advanced age are experiencing diverse and distributed effects of senescence that could confound the interpretation of either behavioral or biological data. Consequently, the manifestation of spatial learning impairments in 24 month-old F344×BN-F1 rats prior to a decrement in physical function suggests this rat is a robust model for the examination of mechanisms of cognitive change with age.
Despite pronounced change in mean performance in each of the aged groups in this study, individual differences in spatial learning are not more variable among older F344×BN-F1 rats compared to young adults. This finding agrees with that of Frick and colleagues who reported similar variance of spatial reference memory between 4, 11, 17, and 24 month-old F344 rats (Frick et al., 1995). Likewise, Lindner reported that individual differences accounted for a larger proportion of variance in spatial reference memory than chronological age in a sample of F344 rats spanning 6 weeks to 27 months of age (Lindner, 1997). With this in mind, it is probable that inter-individual variability is less pronounced in aging inbred and hybrid rats relative to aged outbred rats where variance is reportedly greater (Gallagher et al., 1993). While genetically identical, F1 hybrids are heterozygous which in turn reduces biological variability compared to inbred, homozygous parental strains (Phelan and Austad, 1994). Thus, the F344×BN-F1 hybrid provides behavioral neuroscientists an opportunity to characterize the effects of normal, healthy aging on cognition independent of variable interactions with genetic or biological factors present in other commonly used outbred and inbred models of aging.
4.2. Aging impairs learning within daily training sessions but not retention between days of training
While learning and memory are presumed to integrate seamlessly in optimally performing young adults, it is important to consider whether these processes are differentially affected by age. Therefore, changes in performance between adjacent trials within a day (30 s inter-trial interval; ITI) or between days (24 h ITI) of training were parsed for discrete analysis. This approach revealed that older rats do not improve to the same extent as young adults within daily training sessions. In contrast, retention of information between days was comparable among all age groups. Although neither within-day nor between-day changes were reliably associated with Spatial Learning Index, these two parameters were inversely correlated in each age group. This latter observation was unexpected but provides novel evidence that different forms of learning and memory interact in a manner that varies between individual rats, regardless of age.
Although aging selectively impaired learning within daily training sessions, individual decrements to this parameter did not correlate with Spatial Learning Index, suggesting that learning within a day relates to a cognitive process other than reference memory. Working memory is differentiated from reference memory by emphasis on retention of trial-specific information generally on the order of seconds to minutes, but sometimes hours, in the rat (reviewed in Bizon et al., 2012). Spatial working memory is impaired in 12-14 and 20-24 month-old F344 rats but generally not correlated with spatial reference memory (Frick et al., 1995; Bizon et al., 2009; Guidi et al., 2014). While it remains to be definitively proven, it is plausible that the trial-to-trial changes in performance examined in the present study are supported, in part, by spatial working memory, particularly early in training when the age-effect was most prominent. Even if this is not the case, it is significant that the present study localized age-related impairments within, not between, daily training sessions. However, future studies are necessary to determine the relationship, if any, between short-term or working memory and other forms of cognition that decline with age or enable acquisition of spatial reference memory.
A recent review hypothesized that aged rats can acquire a spatial reference memory via an incremental, extrahippocampal learning process and, accordingly, this strategy would produce prominent deficits between days (i.e. >24 h) due to impaired retention (Foster, 2012). Indeed, when spatial training trials are massed into a single day, a subset of 18-24 mo F344 show impaired retention on a probe trial administered 24 hours post-training (Foster et al., 2001; Foster and Kumar, 2007). However, when training trials are distributed across several days, evidence for impaired retention in aging F344 rats is equivocal. Shukitt-Hale and colleagues found that when 22 mo F344 rats received 4 trials/day for 4 days, aged rats exhibited both within and between-day performance decrements relative to 6 mo controls (Shukitt-Hale et al., 1998). Conversely, in a 5-day, 2 trials/day reference memory protocol, Lindner reported 24 mo F344 rats exhibited a within-day, but no between-day, performance decrement relative to 2-2.5 mo controls (Lindner et al., 1992). Lastly, trial-specific data have been described for 30 mo F344×BN-F1 rats tested in a 5-day reference memory protocol (Markowska and Savonenko, 2002). Although within and between-day decrements were not discretely analyzed in that study, between-day changes in performance of ad libitum fed 30 mo rats (the experimental group was age matched, dietary restricted rats) are modest or absent, as in the current study. As they are, the present data counter the hypothesis that older rats incrementally acquire a reference memory using non-hippocampal-dependent mechanisms (Foster, 2012) as between-day retention was neither worse in aged rats nor associated with Spatial Learning Index. However, it is important to acknowledge that variations in training schedule may alter the propensity to observe specific decrements in aging rats as interpolated probe trials are a sensitive means to evaluate spatial learning compared to an analysis of a single probe trial (Markowska et al., 1993) and distributed training schedules enhance retention relative to massed training (Spreng et al., 2002).
4.3. Procedural learning is impaired in aged rats and related to spatial learning
Aged F344 rats are impaired in acquisition of cue learning (Lindner, 1997; Burke et al., 2010; Guidi et al., 2014 but see Bizon et al., 2009), a navigational task that does not require cognitive mapping. While decreased visual acuity could impair performance on such visible platform trials, this is an insufficient explanation as aged performance improves with continued training (Burke et al., 2010; Guidi et al., 2014) and visual acuity does not necessarily predict spatial learning in this strain (Lindner and Gribkoff, 1991). Therefore, it is plausible that impaired cue learning of aged rats reflects an inability to acquire navigational procedures, although this perspective is not well-investigated at present. In the current study, aged F344×BN-F1 rats were not impaired at swimming to the visible platform. In fact, 6 month-old rats were not as efficient as the older groups at swimming to the new, visible platform location as aged rats. This is likely due to the bias of young rats to utilize spatial strategies, whereas aged rats preferentially employ response strategies (Barnes et al., 1980). Consequently, it is possible that memory for procedures acquired during hidden platform training subsequently facilitated performance during cue training. In support of this view, the cue training deficit commonly observed in aged F344 prior to place learning is not observed when cue training is conducted after place training (Bizon et al., 2009). As the rats trained in the current study were experimentally naïve as of the start of hidden platform testing, it was hypothesized that impaired procedural learning would manifest as impaired acquisition of any effective (i.e. “successful”) escape strategy (Ruediger et al., 2012). Indeed, although non-spatial strategies could enable effective escape, older rats were less successful at escaping to the platform within the allotted trial duration during the first half of the training procedure (Blocks 1&2). However, during the second half of training (Blocks 3&4), the vast majority of aged rats were escaping as effectively as young. It is notable that the onset of stable, highly effective escape coincides with the first probe trial used in the computation of the Spatial Learning Index (Probe Trial 1 was excluded per (Gallagher et al., 1993; Bizon et al., 2009). This suggests that the summary measures of spatial and procedural learning used in this study (Spatial Learning Index and Total Successful Trials) are not grossly conflated for technical reasons. Therefore, the significant correlation between procedural learning and spatial learning in aged F344×BN-F1 rats may relate to genuine changes to information processing or neural circuitry common to both forms of memory.
Procedural memory encompasses non-cognitive, or implicit, acquisition of skills and habits according to principles of stimulus-response learning (reviewed in Mishkin et al., 1997; Squire and Zola, 1996; Milner et al., 1998; Devan et al., 2011). In contrast to cognitive-spatial learning mediated by the hippocampus, the procedural memory system is centered on the striatum. Indeed, rats with striatal lesions are impaired in both place learning and cue learning, consistent with the hypothesis that these are rats slower to acquire procedural information that enables effective escape even when spatial strategies are not necessary (Whishaw et al., 1987; Devan and White, 1999). Qualitatively, rats that are impaired at procedural learning fail to explore the expanse of the maze in a manner that is necessary for spatial learning and less frequently encounter the training platform (Sutherland et al., 1987; Devan et al., 1992; Devan and White, 1999; Leggio et al., 2006). On hidden platform trials, the cognitive-spatial strategy will optimize escape, but rodents progress to this tactic after trial-and-error with less efficient strategies (Ruediger et al., 2012). Alternatively, young rats may utilize purely procedural memory to escape the maze when spatial information is unreliable (Devan et al., 1992).
The role of procedural learning in the Morris water maze is understudied relative to cognitive-spatial learning, although logically the two should be interlinked under normal circumstances. A parsimonious explanation of the relationship between spatial learning and procedural learning in aged rats could be that cognitive-spatial impaired rats revert to less efficient, striatal-dependent processes that require more trials for learning relative to the hippocampus (Barnes et al., 1980). Similarly, rats with cognitive-spatial impairment may lack even modest localization capabilities necessary to escape to the platform within the allotted trial duration (90 s). However, the actual data are not consistent with this simplistic explanation. Within the 24 month-old group, individual rats that escaped as effectively as young adults (i.e. >17 successful escapes from 20 training trials) span the full range of unimpaired and impaired cognitive-spatial performance. This pattern reveals that even aged rats with cognitive-spatial learning impairment can show acquisition of task procedures and escape just as effectively as young adults and age-matched cognitive-spatial unimpaired individuals. Furthermore, the data indicate more than 50% of 24 month-old rats were impaired in using a spatial strategy as late as the final probe trial, whereas 96% of rats in this age group were able to escape onto the platform on at least 4 out of 5 trials in the last block of training (versus 99% for 6 month-old). Therefore, nearly all rats exhibited evidence of procedural learning during the training paradigm, but acquisition of procedural knowledge did not necessarily facilitate a cognitive-spatial strategy in an identical proportion of aged rats.
4.4. Conclusions
The present data reveal that reference memory declines in F344×BN-F1 hybrid rats as early as 18 months with maximal impairment evident by 24 months, an earlier time-point than typically examined in aging studies that utilize this strain. Analysis of performance at 28 months did not reveal evidence for more pervasive reference memory impairment, although physical function had deteriorated relative to other age groups. These cross-sectional differences in spatial learning are not associated with greater variability. Consequently, reliable individual differences may be leveraged to identify factors that covary with spatial learning. Specifically, older rats were impaired at improving performance within daily training sessions, not at retaining information between days of training. Although neither parameter predicted individual differences in spatial learning in any age group, within-day and between-day changes in performance were inversely related in every age group. However, spatial learning was related to procedural learning in aged rats. These latter two observations are original and interesting, but future work is necessary to clarify these novel inter-relationships. Collectively, these data describe normative parameters of cognitive and non-cognitive function in this important, healthful aging rat model that may be used to better elucidate behavioral and neural correlates of normal brain aging.
Supplementary Material
Highlights.
Spatial reference memory was analyzed in 6, 18, 24 and 28 month-old hybrid rats
Memory decrements were observed between 6 and 18 months and 18 and 24 months
Individual aged rats may be classified as unimpaired or impaired relative to young
Aging impairs learning over short delays not retention between days
Aging impairs procedural learning and is related to spatial learning
Acknowledgments
This work was supported by NIH Grants F31-AG038266 to JAM and R01-AG020572 to MMN. The authors thank Adam Wilson, Ashley Donahue, Dennis Rookwood, Kate Davis, Amie Severino, Brian Horman and Dr. Erasmo Nieves-Martinez for technical assistance in the behavioral testing of animals used in this study. We also thank Drs. Jennifer Bizon and Barry Setlow for advice on statistical tests and manuscript preparation.
Footnotes
Disclosure statement: Neither author has any actual or potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Nadel L, Honig WK. Spatial memory deficit in senescent rats. Can J Psychol. 1980;34:29–39. doi: 10.1037/h0081022. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Neurobiological substrates of behavioral decline: models and data analytic strategies for individual differences in aging. Neurobiol Aging. 1996;17:491–495. doi: 10.1016/0197-4580(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Koffeman A, Scheltens P. Diabetes and cognitive impairment. Clinical diagnosis and brain imaging in patients attending a memory clinic. J Neurol. 2006;253:477–482. doi: 10.1007/s00415-005-0036-4. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Foster TC, Alexander GE, Glisky EL. Characterizing cognitive aging of working memory and executive function in animal models. Front Aging Neurosci. 2012;4:19. doi: 10.3389/fnagi.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci. 2009;64:850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci. 2002;57:B193–197. doi: 10.1093/gerona/57.5.b193. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau WE, Kesner RP. A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behav Neurosci. 2000;114:1096–1108. doi: 10.1037//0735-7044.114.6.1096. [DOI] [PubMed] [Google Scholar]
- Devan BD, Blank GS, Petri HL. Place navigation in the Morris water task: Effects of reduced platform interval lighting and pseudorandom platform positioning. Psychobiology. 1992;20:120–126. [Google Scholar]
- Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol Learn Mem. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- Devan BD, Hong NS, McDonald RJ. Parallel associative processing in the dorsal striatum: segregation of stimulus-response and cognitive control subregions. Neurobiol Learn Mem. 2011;96:95–120. doi: 10.1016/j.nlm.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96:283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem. 2007;87:522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci Off J Soc Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Guidi M, Kumar A, Rani A, Foster TC. Assessing the emergence and reliability of cognitive decline over the life span in Fisher 344 rats using the spatial water maze. Front Aging Neurosci. 2014;6:2. doi: 10.3389/fnagi.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebda-Bauer EK, Morano MI, Therrien B. Aging and corticosterone injections affect spatial learning in Fischer-344 X Brown norway rats. Brain Res. 1999;827:93–103. doi: 10.1016/s0006-8993(99)01310-4. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Schechtman KB. Interaction between exercise and food restriction: effects on longevity of male rats. J Appl Physiol Bethesda Md 1985. 1991;70:1529–1535. doi: 10.1152/jappl.1991.70.4.1529. [DOI] [PubMed] [Google Scholar]
- Kidd PM. Alzheimer's disease, amnestic mild cognitive impairment, and age-associated memory impairment: current understanding and progress toward integrative prevention. Altern Med Rev J Clin Ther. 2008;13:85–115. [PubMed] [Google Scholar]
- Leggio MG, Federico F, Neri P, Graziano A, Mandolesi L, Petrosini L. NMDA receptor activity in learning spatial procedural strategies I. The influence of hippocampal lesions Brain Res Bull. 2006;70:347–355. doi: 10.1016/j.brainresbull.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Lindner MD. Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol Learn Mem. 1997;68:203–220. doi: 10.1006/nlme.1997.3782. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Balch AH, VanderMaelen CP. Short forms of the “reference-” and “working-memory” Morris water maze for assessing age-related deficits. Behav Neural Biol. 1992;58:94–102. doi: 10.1016/0163-1047(92)90303-l. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Gribkoff VK. Relationship between performance in the Morris water task, visual acuity, and thermoregulatory function in aged F-344 rats. Behav Brain Res. 1991;45:45–55. doi: 10.1016/s0166-4328(05)80179-2. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the Most Sensitive Measure of Water Maze Probe Test Performance? Front. Integr Neurosci. 2009;3:4. doi: 10.3389/neuro.07.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Long JM, Johnson CT, Olton DS. Variable-interval probe test as a tool for repeated measurements of spatial memory in the water maze. Behav Neurosci. 1993;107:627–632. doi: 10.1037//0735-7044.107.4.627. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol Aging. 2002;23:75–86. doi: 10.1016/s0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Davis KN, Miller F, Hampson RE, Deadwyler SA, Howlett AC, Nicolle MM. Hippocampal Gαq/11 but not Gαo-coupled receptors are altered in aging. Neuropharmacology. 2013;70:63–73. doi: 10.1016/j.neuropharm.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Riddle DR, Nicolle MM. Neuroinflammation not associated with cholinergic degeneration in aged-impaired brain. Neurobiol Aging. 2011;32(2322):e1–4. doi: 10.1016/j.neurobiolaging.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Suzuki WA, Gadian DG, Vargha-Khadem F. Hierarchical organization of cognitive memory. Philos Trans R Soc Lond B Biol Sci. 1997;352:1461–1467. doi: 10.1098/rstb.1997.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Aging. Strain Survival Information. [accessed 6.24.14];2011 [WWW Document]. http://www.nia.nih.gov/research/dab/aged-rodent-colonies-handbook/strain-survival-information.
- Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JP, Austad SN. Selecting animal models of human aging: inbred strains often exhibit less biological uniformity than F1 hybrids. J Gerontol. 1994;49:B1–11. doi: 10.1093/geronj/49.1.b1. [DOI] [PubMed] [Google Scholar]
- Ruediger S, Spirig D, Donato F, Caroni P. Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning. Nat Neurosci. 2012;15:1563–1571. doi: 10.1038/nn.3224. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol. 1998;33:615–624. doi: 10.1016/s0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- Siervo M, Arnold R, Wells JCK, Tagliabue A, Colantuoni A, Albanese E, Brayne C, Stephan BCM. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev Off J Int Assoc Study Obes. 2011;12:968–983. doi: 10.1111/j.1467-789X.2011.00903.x. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol Aging. 1994;15:319–328. doi: 10.1016/0197-4580(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Spreng M, Rossier J, Schenk F. Spaced training facilitates long-term retention of place navigation in adult but not in adolescent rats. Behav Brain Res. 2002;128:103–108. doi: 10.1016/s0166-4328(01)00266-2. [DOI] [PubMed] [Google Scholar]
- Sprott RL. Development of animal models of aging at the National Institute of Aging. Neurobiol Aging. 1991;12:635–638. doi: 10.1016/0197-4580(91)90113-x. [DOI] [PubMed] [Google Scholar]
- Sprott RL, Ramirez I. Current Inbred and Hybrid Rat and Mouse Models for Gereontological Research. ILAR J Natl Res Counc Inst Lab Anim Resour. 1997;38:104–109. doi: 10.1093/ilar.38.3.104. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci U S A. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Chew GL, Baker JC, Linggard RC. Some limitations on the use of distal cues in place navigation by rats. Psychobiology. 1987;15:48–57. doi:10.3758/BF03327263. [Google Scholar]
- Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neurosci. 2002;22:9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Van der Staay FJ, Blokland A. Behavioral differences between outbred Wistar, inbred Fischer 344, brown Norway, and hybrid Fischer 344 x brown Norway rats. Physiol Behav. 1996;60:97–109. doi: 10.1016/0031-9384(95)02274-0. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate-putamen lesions in rats. Behav Brain Res. 1987;24:125–138. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


