Abstract
Dipole potential is the potential difference within the membrane bilayer, which originates due to the nonrandom arrangement of lipid dipoles and water molecules at the membrane interface. Cholesterol, an essential lipid in higher eukaryotic membranes, has previously been shown to increase membrane dipole potential. In this work, we explored the effect of stereoisomers of cholesterol, ent-cholesterol and epi-cholesterol, on membrane dipole potential, monitored by the dual wavelength ratiometric approach utilizing the probe di-8-ANEPPS. Our results show that cholesterol and ent-cholesterol share comparable ability in increasing membrane dipole potential. In contrast, epi-cholesterol displays a slight reduction in membrane dipole potential. Our results constitute the first report on the effect of stereoisomers of cholesterol on membrane dipole potential, and imply that an extremely subtle change in sterol structure can significantly alter the dipolar field at the membrane interface. These results assume relevance in the context of differential abilities of these stereoisomers of cholesterol in supporting the activity of the serotonin1A receptor, a representative G protein-coupled receptor. The close correlation between membrane dipole potential and receptor activity provides new insight in receptor-cholesterol interaction in terms of stereospecificity. We envision that membrane dipole potential could prove to be a sensitive indicator of lipid-protein interactions in biological membranes.
Keywords: Cholesterol, di-8-ANEPPS, dipole potential, ent-cholesterol, epi-cholesterol, serotonin1A receptor
1. Introduction
Dipole potential represents the potential difference within the membrane bilayer. The origin of membrane dipole potential is the nonrandom orientation of electric dipoles of lipid and water molecules at the membrane interface (Brockman, 1994; Clarke, 2001; O’Shea, 2005; Wang, 2012). The magnitude of dipole potential varies between 200–1000 mV, depending on membrane composition. Because dipole potential is operative over a relatively small distance in the membrane, the electric field generated due to dipole potential is enormous in magnitude and is in the range of 108–109 Vm−1 (Clarke, 2001; Wang, 2012). An important implication of membrane dipole potential is that it influences the function of membrane proteins and peptides such as Na+/K+-ATPase (Starke-Peterkovic et al., 2005) and the ion channel gramicidin (Duffin et al., 2003). We recently used membrane dipole potential as a useful parameter to monitor the binding of α-lactalbumin to membranes (Chaudhuri and Chattopadhyay, 2014). Importantly, it has been proposed that the dipole potential may play a crucial role in the structure and function of proteins associated with cholesterol-rich domains in the membrane (O’Shea, 2005).
Cholesterol is a crucial membrane lipid in higher eukaryotes and plays a vital role in membrane organization, dynamics, function, and sorting (Simons and Ikonen, 2000; Mouritsen and Zuckermann, 2004; Chaudhuri and Chattopadhyay, 2011). An important and emerging area is the role of cholesterol in the function and organization of membrane proteins and receptors (Burger et al., 2000; Pucadyil and Chattopadhyay, 2006; Paila and Chattopadhyay, 2010; Oates and Watts, 2011; Jafurulla and Chattopadhyay, 2013). The mechanism underlying the effect of membrane cholesterol on the structure and function of membrane proteins and receptors appears complex (Paila and Chattopadhyay, 2009, 2010; Paila et al., 2009; Lee, 2011). A possible mechanism by which membrane cholesterol has been proposed to influence the function of membrane receptors is by a direct (specific) interaction that induces subtle conformational changes in the receptor. An alternative mechanism envisages change in membrane physical properties in which the receptor is embedded. These mechanisms need not be mutually exclusive, i.e., another possibility could be a combination of both. Membrane cholesterol has been shown to modulate the function of a number of G protein-coupled receptors (GPCRs) in general (Burger et al., 2000; Pucadyil and Chattopadhyay, 2006; Paila and Chattopadhyay, 2010; Oates and Watts, 2011; Jafurulla and Chattopadhyay, 2013), and the serotonin1A receptor in particular (Pucadyil and Chattopadhyay, 2004, 2005; Paila et al., 2008; Shrivastava et al., 2010; Jafurulla et al., 2014).
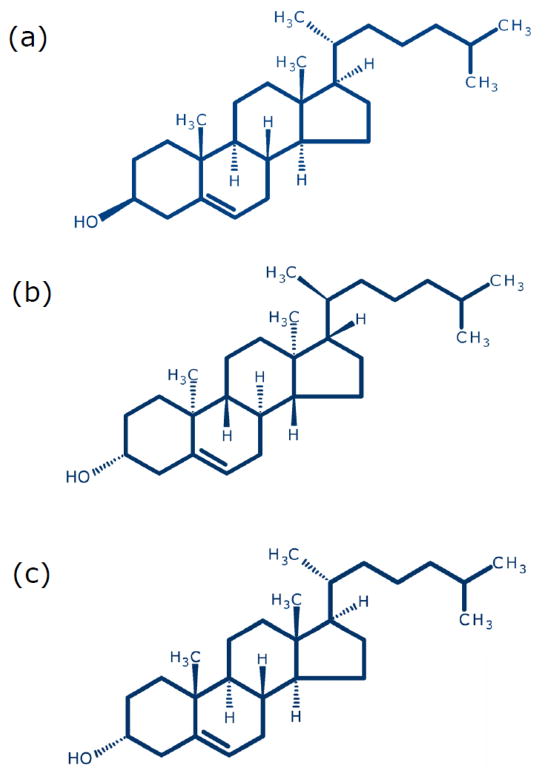
It has been reported earlier that membrane cholesterol increases dipole potential in model (Starke-Peterkovic et al., 2006; Haldar et al., 2012) and natural (Singh et al., 2013) membranes. However, the ability of a sterol to modulate membrane dipole potential is varied and was shown to depend on its exact molecular structure (Starke-Peterkovic et al., 2006; Haldar et al., 2012). For example, immediate biosynthetic precursors of cholesterol (7-dehydrocholesterol and desmosterol), differing with cholesterol merely in a double bond, lack the ability to increase membrane dipole potential. In other words, even a subtle difference in molecular structure (such as a double bond) can give rise to drastic difference in the ability to influence membrane dipole potential. With an overall goal to have a comprehensive understanding of finer structural details of the interaction of membrane cholesterol with membrane proteins and receptors, in this work, we explored the degree of structural (stereospecific) stringency in sterols in modulating membrane dipole potential. Toward this goal, we monitored the effect of two stereoisomers of cholesterol, ent-cholesterol and epi-cholesterol, on membrane dipole potential. The enantiomer of cholesterol (ent-cholesterol) is the non-superimposable mirror image of native (natural) cholesterol (see Fig. 1a,b). Enantiomers have identical physicochemical properties, except for the direction of rotation of plane-polarized light. As a result, membrane biophysical properties (such as compressibility and phase behavior) are same for native cholesterol and ent-cholesterol (Mannock et al., 2003; Westover et al., 2003; Westover and Covey, 2004; Covey 2009). In addition, both native cholesterol and ent-cholesterol support normal growth of a mutant mammalian cell line (Xu et al., 2005). An interesting use of ent-cholesterol is to distinguish specific interaction of cholesterol from nonspecific effects (Mickus et al., 1992; Covey, 2009; D’Avanzo et al., 2011; Kristiana et al., 2012). On the other hand, epi-cholesterol is a diastereomer of cholesterol in which only the orientation of the hydroxyl group at carbon-3 is inverted relative to native cholesterol and is not a mirror image of cholesterol (Fig. 1c). While ent-cholesterol shares identical physicochemical properties with cholesterol, previous studies have shown that the biophysical properties of epi-cholesterol and native cholesterol are different (Westover and Covey, 2004; Covey, 2009). epi-Cholesterol has been reported to differ in its tilt angles, condensing ability, and phase transition properties from cholesterol in membranes (Demel et al., 1972; Dufourc et al., 1984; Murari et al., 1986; Cheetham et al., 1989). We show here that cholesterol and ent-cholesterol share comparable ability in increasing membrane dipole potential. In contrast to this, epi-cholesterol does not exhibit any increase in membrane dipole potential. Rather, there is a slight decrease in membrane dipole potential with increasing concentration of epi-cholesterol. We further discuss the implications of these results in terms of relative abilities of these stereoisomers of cholesterol in supporting the activity of the serotonin1A receptor, previously reported by us (Jafurulla et al., 2014). These results provide novel insight into the subtle structural requirements of cholesterol in its interaction with membrane proteins.
Fig. 1. Chemical structures of sterols used: (a) cholesterol, (b) ent-cholesterol and (c) epi-cholesterol.
Both ent-cholesterol and epi-cholesterol are stereoisomers of cholesterol. ent-Cholesterol is the enantiomer of cholesterol. Enantiomers are non-superimposable mirror images of one another. epi-Cholesterol, on the other hand, is a diastereomer and is not a mirror image of cholesterol. ent-Cholesterol (but not epi-cholesterol) shares identical physicochemical properties with cholesterol. See text (section 1) for more details.
2. Materials and methods
2.1. Materials
1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC), cholesterol, EDTA, NaCl and Tris were obtained from Sigma Chemical Co. (St. Louis, MO). 3-Epicholesterol (5-cholesten-3α-ol), to be denoted as epi-cholesterol, was obtained from Steraloids (Newport, RI). The enantiomer of cholesterol (ent-cholesterol) was synthesized as previously described (Jiang and Covey, 2002; Westover and Covey, 2004). 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was purchased from Avanti Polar Lipids (Alabaster, AL). 4-(2-(6-(Dioctylamino)-2-naphthalenyl)ethenyl)-1-(3-sulfopropyl)-pyridinium inner salt (di-8-ANEPPS) was purchased from Molecular Probes (Eugene, OR). Pre-coated silica gel 60 thin layer chromatography plates were from Merck (Darmstadt, Germany). The purity of lipids was checked by thin layer chromatography on silica gel precoated plates in chloroform/methanol/water (65:35:5, v/v/v) and was found to give only one spot with a phosphate-sensitive spray and on subsequent charring (Baron and Coburn, 1984). Solvents used were of analytical grade. All other chemicals used were of the highest purity available. Water was purified through a Millipore (Bedford, MA) Milli-Q system and used throughout.
2.2. Methods
2.2.1. Estimation of phospholipids
Concentration of lipid phosphate was determined subsequent to total digestion by perchloric acid (McClare, 1971) using Na2HPO4 as standard. DMPC was used as an internal standard to assess lipid digestion. Samples without perchloric acid digestion produced negligible readings.
2.2.2. Sample preparation
Experiments were performed using large unilamellar vesicles (LUVs) of 100 nm diameter of POPC containing increasing concentrations (0–40 mol%) of a given sterol (any one of the following sterols: cholesterol/epi-cholesterol/ent-cholesterol). All samples contained 1 mol% di-8-ANEPPS. In general, 640 nmol of total lipid (phospholipid and sterol) and 6.4 nmol of di-8-ANEPPS were mixed well and dried under a stream of nitrogen while being warmed gently (~35 °C). After further drying under a high vacuum for at least 3 h, the lipid mixture was hydrated (swelled) by addition of 1.5 ml of 30 mM Tris, 1 mM EDTA, 150 mM NaCl, pH 7.2 buffer, and each sample was vortexed for 3 min to uniformly disperse the lipids and form homogeneous multilamellar vesicles. LUVs of 100 nm diameter were prepared by the extrusion technique using an Avestin Liposofast Extruder (Ottawa, Ontario, Canada) as previously described (MacDonald et al., 1991). Briefly, multilamellar vesicles were freeze-thawed five times using liquid nitrogen to ensure solute equilibration between trapped and bulk solutions and then extruded through polycarbonate filters (pore diameter of 100 nm) mounted in an extruder fitted with Hamilton syringes (Hamilton Company, Reno, NV). Samples were subjected to 11 passes through the polycarbonate filters to give the final LUV suspension. Background samples were prepared in the same way except that di-8-ANEPPS was not added to them. The optical density of the samples measured at 420 and 510 nm were less than 0.15 in all cases, which rules out any possibility of scattering artifacts. Samples were incubated in dark for 12 h at room temperature (~23 °C) for equilibration before measuring fluorescence. Experiments were performed with multiple sets of samples at room temperature (~23 °C).
2.2.3. Measurement of membrane dipole potential
Membrane dipole potential measurements were carried out by dual wavelength ratiometric approach using the voltage sensitive fluorescence probe di-8-ANEPPS (Gross et al., 1994; Clarke and Kane, 1997; Starke-Peterkovic et al., 2005, 2006; Haldar et al., 2012). Steady state fluorescence measurements were performed with a Hitachi F-7000 (Tokyo, Japan) spectrofluorometer using 1 cm path length quartz cuvettes at room temperature (~23 °C). Excitation and emission slits with a nominal bandpass of 3 nm were used for all measurements. Background intensities of samples were subtracted from each sample to cancel any contribution due to the solvent Raman peak and other scattering artifacts. Fluorescence intensities were recorded at two excitation wavelengths (420 and 510 nm). Emission wavelength was fixed at 670 nm. The fluorescence ratio (R), defined as the ratio of fluorescence intensities at an excitation wavelength of 420 nm to that at 510 nm (emission at 670 nm in both cases) was calculated (Starke-Peterkovic et al., 2006). The choice of the emission wavelength (670 nm) at the red edge of the fluorescence spectrum has previously been shown to rule out membrane fluidity effects (Clarke and Kane, 1997). Dipole potential (ψd) in mV was calculated from R using the linear relationship (Starke-Peterkovic et al., 2005, 2006):
R values remained invariant after dilution of membrane samples, indicating the absence of any scattering artifacts (Lentz et al., 1979).
3. Results and discussion
We carried out dipole potential measurements in POPC membranes in the presence of cholesterol and its stereoisomers by a dual wavelength ratiometric approach using the voltage-sensitive styrylpyridinium probe, di-8-ANEPPS (Gross et al., 1994; Clarke and Kane, 1997; Starke-Peterkovic et al., 2005, 2006). The dual wavelength ratiometric technique using di-8-ANEPPS represents a popular approach to monitor membrane dipole potential (Gross et al., 1994; Clarke and Kane, 1997; Starke-Peterkovic et al., 2006). Since membrane dipole potential has its origin in nonrandom orientation of dipolar residues and the majority of these residues are localized in the membrane interfacial region, the ideal location of any probe reporting dipole potential should be interfacial. We previously showed, using the parallax method (Chattopadhyay and London, 1987), that the fluorescent styrylpyridinium group in di-8-ANEPPS is localized at the membrane interface, at a distance of ~12 Å from the center of the bilayer (Haldar et al., 2012). The fluorescence ratio (R) of di-8-ANEPPS is sensitive to any change in the dipolar field at the membrane interface where the probe is localized. This is believed to be due to an electrochromic mechanism. According to this mechanism, the spectral shift displayed by di-8-ANEPPS is related to the electric field strength. It should be mentioned that the fluorescence ratio (R) of di-8-ANEPPS has been shown to be sensitive to only dipole potential and is independent of specific molecular interactions (Gross et al., 1994; Robinson et al., 2011).
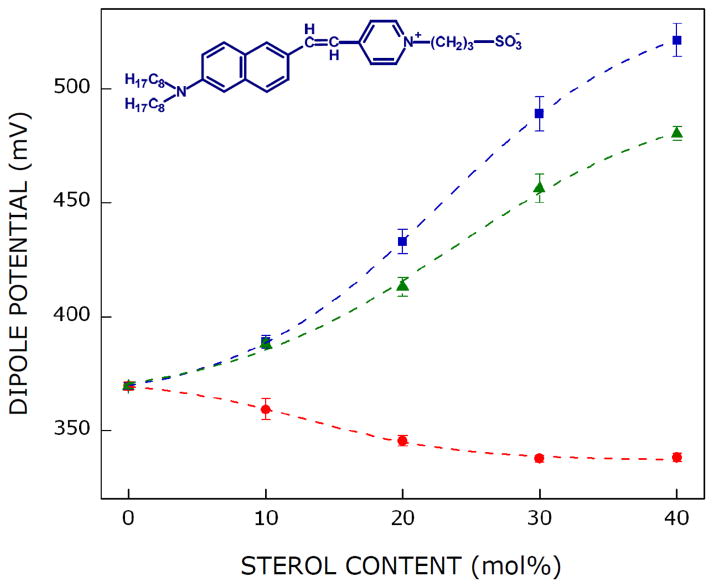
The effect of cholesterol and its stereoisomers on the dipole potential of POPC membranes is shown in Fig. 2. The figure shows that the dipole potential of POPC membranes is ~369 mV. The membrane dipole potential exhibits progressive increase with increasing concentration of cholesterol and reaches a value of ~521 mV (i.e., increases by ~41%) in presence of 40 mol% cholesterol. This is in agreement with previous work by us (Haldar et al., 2012; Singh et al., 2013) and others (Starke-Peterkovic et al., 2006) in which it was shown that cholesterol increases dipole potential in membranes. In order to explore the extent of structural stringency of cholesterol in its ability to modulate membrane dipole potential, we monitored the effect of stereoisomers of cholesterol, ent-cholesterol and epi-cholesterol, on membrane dipole potential. The change in membrane dipole potential is drastically different for ent-cholesterol and epi-cholesterol (see Fig. 2). The membrane dipole potential increased up to ~480 mV (~30% increase) when 40 mol% of ent-cholesterol was used. The increase in membrane dipole potential is therefore comparable in cases of cholesterol and ent-cholesterol, although not exactly same. This is in overall agreement with the fact that ent-cholesterol shares identical physicochemical properties with cholesterol. In contrast to this, the membrane dipole potential reduces to ~338 mV in presence of 40 mol% epi-cholesterol, thereby exhibiting a modest (~8%) decrease in dipole potential. This drastic difference in the pattern of change of membrane dipole potential in case of epi-cholesterol reinforces the different physicochemical properties of epi-cholesterol relative to cholesterol. Membrane dipole potential depends on a number of factors (Haldar et al., 2012). Although the molecular details underlying this difference in dipole potential (for cholesterol and epi-cholesterol) is not clear, it could be due to difference in sterol headgroup orientation (membrane tilt angle) along the bilayer normal.
Fig. 2. Effect of stereoisomers of cholesterol on dipole potential of membranes.
Dipole potential in POPC membranes plotted with increasing concentrations of cholesterol (■), ent-cholesterol (▲), and epi-cholesterol (●). Data points shown are means ± S.E. of at least three independent measurements. The ratio of di-8-ANEPPS to total lipid was 1:100 (mol/mol) and total lipid concentration was 0.43 mM. Measurements were carried out at room temperature (~23°C). Lines joining the data points are provided merely as viewing guides. The structure of voltage-sensitive probe di-8-ANEPPS is shown in the upper left side. See section 2 for details.
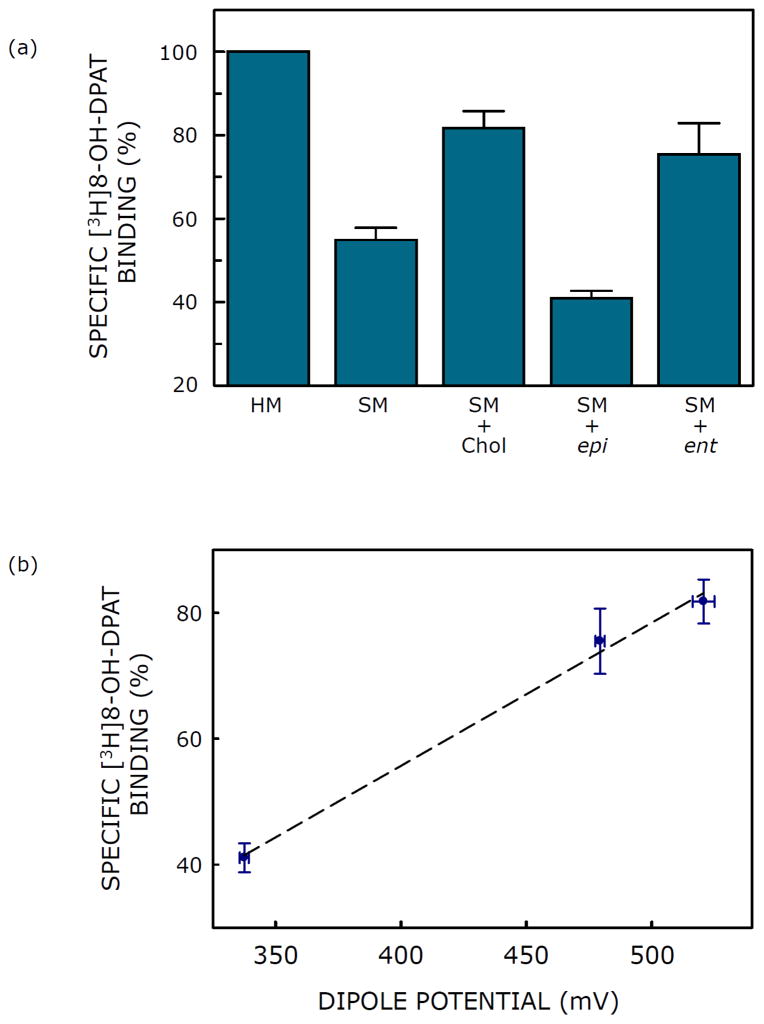
Our overall goal in the measurement of dipole potential in membranes containing cholesterol and its stereoisomers was to explore the role of dipole potential in the mechanism of receptor-cholesterol interaction, and to assess its functional implication. Fig. 3 brings out the relevance of membrane dipole potential in the context of the activity of the serotonin1A receptor, a representative GPCR (Pucadyil et al., 2005), as measured by specific agonist ([3H]8-OH-DPAT) binding. Fig. 3a shows that while ent-cholesterol could replace cholesterol in supporting the function of the serotonin1A receptor, epi-cholesterol could not (Jafurulla et al., 2014). These results imply that the requirement of membrane cholesterol for the serotonin1A receptor function is diastereospecific, yet not enantiospecific. Fig. 3b shows the correlation of membrane dipole potential with activity of serotonin1A receptors. A linear correlation was observed between these parameters with a correlation coefficient (r) ~0.99. The close correlation between membrane dipole potential and receptor activity is rather interesting. We conclude that membrane dipole potential could be a sensitive determinant of lipid-protein interactions in biological membranes.
Fig. 3. Correlation of receptor activity with membrane dipole potential.
(a) Effect of replenishment of cholesterol, epi-cholesterol (epi) and ent-cholesterol (ent) into solubilized membranes (SM) on specific binding of the agonist [3H]8-OH-DPAT to the serotonin1A receptor. Solubilized hippocampal membranes were replenished with cholesterol, epi-cholesterol or ent-cholesterol using sterol:MβCD complex. Values are expressed as percentages of specific binding obtained in native hippocampal membranes (HM). Data shown are means ± S.E. of at least four independent experiments (taken from Jafurulla et al., 2014). (b) Correlation of membrane dipole potential with activity of serotonin1A receptors. Specific [3H]8-OH-DPAT binding to serotonin1A receptors (values taken from Fig. 3a) and corresponding values of membrane dipole potential containing 40 mol% sterol (from Fig. 2) are shown. Linear regression analysis yielded a correlation coefficient (r) ~0.99. The tight correlation between membrane dipole potential and receptor activity is noteworthy. See sections 2 and 3 for more details.
Highlights.
Cholesterol and ent-cholesterol increase dipole potential to comparable extent.
In contrast, epi-cholesterol reduces membrane dipole potential.
Membrane dipole potential and serotonin1A receptor activity are closely correlated.
Acknowledgments
This work was supported by the Council of Scientific and Industrial Research (Govt. of India) Network project BSC0115 to A.C., and NIH grant GM47969 to D.F.C. H.C. thanks the Council of Scientific and Industrial Research for the award of a Senior Research Associateship. A.C. is an Adjunct Professor of Jawaharlal Nehru University (New Delhi), Indian Institute of Science Education and Research (Mohali), Indian Institute of Technology (Kanpur) and Honorary Professor of the Jawaharlal Nehru Centre for Advanced Scientific Research (Bangalore). A.C. gratefully acknowledges J.C. Bose Fellowship (Department of Science and Technology, Govt. of India). We thank Md. Jafurulla for help during the preparation of the manuscript and useful discussion, and members of the Chattopadhyay laboratory for their comments and discussions.
Abbreviations
- di-8-ANEPPS
4-(2-(6-(dioctylamino)-2-naphthalenyl)ethenyl)-1-(3-sulfopropyl)-pyridinium inner salt
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- GPCR
G protein-coupled receptor
- HM
hippocampal membranes
- LUV
large unilamellar vesicle
- MβCD
methyl-β-cyclodextrin
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- SM
solubilized membranes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron CB, Coburn RF. Comparison of two copper reagents for detection of saturated and unsaturated neutral lipids by charring densitometry. J Liq Chromatogr. 1984;7:2793–2801. [Google Scholar]
- Brockman H. Dipole potential of lipid membranes. Chem Phys Lipids. 1994;73:57–79. doi: 10.1016/0009-3084(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Burger K, Gimpl G, Fahrenholz F. Regulation of receptor function by cholesterol. Cell Mol Life Sci. 2000;57:1577–1592. doi: 10.1007/PL00000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A, London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 1987;26:39–45. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Chattopadhyay A. Transbilayer organization of membrane cholesterol at low concentrations: implications in health and disease. Biochim Biophys Acta. 2011;1808:19–25. doi: 10.1016/j.bbamem.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Chattopadhyay A. Lipid binding specificity of bovine α-lactalbumin: A multidimensional approach. Biochim Biophys Acta. 2014;1838:2078–2086. doi: 10.1016/j.bbamem.2014.04.027. [DOI] [PubMed] [Google Scholar]
- Cheetham JJ, Wachtel E, Bach D, Epand RM. Role of the stereochemistry of the hydroxyl group of cholesterol and the formation of nonbilayer structures in phosphatidylethanolamines. Biochemistry. 1989;28:8928–8934. doi: 10.1021/bi00448a036. [DOI] [PubMed] [Google Scholar]
- Clarke RJ. The dipole potential of phospholipid membranes and methods for its detection. Adv Colloid Interface Sci. 2001;89–90:263–281. doi: 10.1016/s0001-8686(00)00061-0. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Kane DJ. Optical detection of membrane dipole potential: avoidance of fluidity and dye-induced effects. Biochim Biophys Acta. 1997;1323:223–239. doi: 10.1016/s0005-2736(96)00188-5. [DOI] [PubMed] [Google Scholar]
- Covey DF. ent-Steroids: novel tools for studies of signaling pathways. Steroids. 2009;74:577–585. doi: 10.1016/j.steroids.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avanzo N, Hyrc K, Enkvetchakul D, Covey DF, Nichols CG. Enantioselective protein-sterol interactions mediate regulation of both prokaryotic and eukaryotic inward rectifier K+ channels by cholesterol. PLoS One. 2011;6:e19393. doi: 10.1371/journal.pone.0019393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel RA, Bruckdorfer KR, van Deenen LLM. Structural requirements of sterols for the interaction with lecithin at the air-water interface. Biochim Biophys Acta. 1972;255:311–320. doi: 10.1016/0005-2736(72)90030-2. [DOI] [PubMed] [Google Scholar]
- Duffin RL, Garrett MP, Flake KB, Durrant JD, Busath DD. Modulation of lipid bilayer interfacial dipole potential by phloretin, RH421, and 6-ketocholestanol as probed by gramicidin channel conductance. Langmuir. 2003;19:1439–1442. [Google Scholar]
- Dufourc EJ, Parish EJ, Chitrakorn S, Smith ICP. Structural and dynamical details of cholesterol-lipid interaction as revealed by deuterium NMR. Biochemistry. 1984;23:6062–6071. [Google Scholar]
- Gross E, Bedlack RS, Jr, Loew LM. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophys J. 1994;67:208–216. doi: 10.1016/S0006-3495(94)80471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar S, Kanaparthi RK, Samanta A, Chattopadhyay A. Differential effect of cholesterol and its biosynthetic precursors on membrane dipole potential. Biophys J. 2012;102:1561–1569. doi: 10.1016/j.bpj.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafurulla M, Chattopadhyay A. Membrane lipids in the function of serotonin and adrenergic receptors. Curr Med Chem. 2013;20:47–55. [PubMed] [Google Scholar]
- Jafurulla M, Rao BD, Sreedevi S, Ruysschaert JM, Covey DF, Chattopadhyay A. Stereospecific requirement of cholesterol in the function of the serotonin1A receptor. Biochim Biophys Acta. 2014;1838:158–163. doi: 10.1016/j.bbamem.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Covey DF. Total synthesis of ent-cholesterol via a steroid C,D-ring side-chain synthon. J Org Chem. 2002;67:4893–4900. doi: 10.1021/jo025535k. [DOI] [PubMed] [Google Scholar]
- Kristiana I, Luu W, Stevenson J, Cartland S, Jessup W, Belani JD, Rychnovsky SD, Brown AJ. Cholesterol through the looking glass: ability of its enantiomer also to elicit homeostatic responses. J Biol Chem. 2012;287:33897–33904. doi: 10.1074/jbc.M112.360537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG. Biological membranes: the importance of molecular detail. Trends Biochem Sci. 2011;36:493–500. doi: 10.1016/j.tibs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Lentz BR, Moore BM, Barrow DA. Light-scattering effects in the measurement of membrane microviscosity with diphenylhexatriene. Biophys J. 1979;25:489–494. doi: 10.1016/S0006-3495(79)85318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald RC, MacDonald RI, Menco BP, Takeshita K, Subbarao NK, Hu LR. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- Mannock DA, McIntosh TJ, Jiang X, Covey DF, McElhaney RN. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophys J. 2003;84:1038–1046. doi: 10.1016/S0006-3495(03)74920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClare CWF. An accurate and convenient organic phosphorous assay. Anal Biochem. 1971;39:527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- Mickus DE, Levitt DG, Rychnovsky SD. Enantiomeric cholesterol as a probe of ion-channel structure. J Am Chem Soc. 1992;114:359–360. [Google Scholar]
- Mouritsen OG, Zuckermann MJ. What’s so special about cholesterol? Lipids. 2004;39:1101–1113. doi: 10.1007/s11745-004-1336-x. [DOI] [PubMed] [Google Scholar]
- Murari R, Murari MP, Baumann WJ. Sterol orientations in phosphatidylcholine liposomes as determined by deuterium NMR. Biochemistry. 1986;25:1062–1067. doi: 10.1021/bi00353a017. [DOI] [PubMed] [Google Scholar]
- O’Shea P. Physical landscapes in biological membranes: physico-chemical terrains for spatio-temporal control of biomolecular interactions and behavior. Philos Trans A Math Phys Eng Sci. 2005;363:575–588. doi: 10.1098/rsta.2004.1509. [DOI] [PubMed] [Google Scholar]
- Oates J, Watts A. Uncovering the intimate relationship between lipids, cholesterol and GPCR activation. Curr Opin Struct Biol. 2011;21:802–807. doi: 10.1016/j.sbi.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Paila YD, Chattopadhyay A. The function of G-protein coupled receptors and membrane cholesterol: specific or general interaction? Glycoconj J. 2009;26:711–720. doi: 10.1007/s10719-008-9218-5. [DOI] [PubMed] [Google Scholar]
- Paila YD, Chattopadhyay A. Membrane cholesterol in the function and organization of G-protein coupled receptors. Subcell Biochem. 2010;51:439–466. doi: 10.1007/978-90-481-8622-8_16. [DOI] [PubMed] [Google Scholar]
- Paila YD, Murty MRVS, Vairamani M, Chattopadhyay A. Signaling by the human serotonin1A receptor is impaired in cellular model of Smith-Lemli-Opitz Syndrome. Biochim Biophys Acta. 2008;1778:1508–1516. doi: 10.1016/j.bbamem.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Paila YD, Tiwari S, Chattopadhyay A. Are specific nonannular cholesterol binding sites present in G-protein coupled receptors? Biochim Biophys Acta. 2009;1788:295–302. doi: 10.1016/j.bbamem.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Chattopadhyay A. Cholesterol modulates ligand binding and G-protein coupling to serotonin1A receptors from bovine hippocampus. Biochim Biophys Acta. 2004;1663:188–200. doi: 10.1016/j.bbamem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Chattopadhyay A. Cholesterol modulates the antagonist-binding function of hippocampal serotonin1A receptors. Biochim Biophys Acta. 2005;1714:35–42. doi: 10.1016/j.bbamem.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Chattopadhyay A. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog Lipid Res. 2006;45:295–333. doi: 10.1016/j.plipres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Kalipatnapu S, Chattopadhyay A. The serotonin1A receptor: A representative member of the serotonin receptor family. Cell Mol Neurobiol. 2005;25:553–580. doi: 10.1007/s10571-005-3969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Besley NA, O’Shea P, Hirst JD. Di-8-ANEPPS emission spectra in phospholipid/cholesterol membranes: a theoretical study. J Phys Chem B. 2011;115:4160–4167. doi: 10.1021/jp1111372. [DOI] [PubMed] [Google Scholar]
- Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin1A receptors. Biochemistry. 2010;49:5426–5435. doi: 10.1021/bi100276b. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- Singh P, Haldar S, Chattopadhyay A. Differential effect of sterols on dipole potential in hippocampal membranes: implications for receptor function. Biochim Biophys Acta. 2013;1828:917–923. doi: 10.1016/j.bbamem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Starke-Peterkovic T, Turner N, Else PL, Clarke RJ. Electric field strength of membrane lipids from vertebrate species: membrane lipid composition and Na+-K+-ATPase molecular activity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R663–R670. doi: 10.1152/ajpregu.00434.2004. [DOI] [PubMed] [Google Scholar]
- Starke-Peterkovic T, Turner N, Vitha MF, Waller MP, Hibbs DE, Clarke RJ. Cholesterol effect on the dipole potential of lipid membranes. Biophys J. 2006;90:4060–4070. doi: 10.1529/biophysj.105.074666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Measurements and implications of the membrane dipole potential. Annu Rev Biochem. 2012;81:615–635. doi: 10.1146/annurev-biochem-070110-123033. [DOI] [PubMed] [Google Scholar]
- Westover EJ, Covey DF. The enantiomer of cholesterol. J Membr Biol. 2004;202:61–72. doi: 10.1007/s00232-004-0714-7. [DOI] [PubMed] [Google Scholar]
- Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278:51125–51133. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Rychnovsky SD, Belani JD, Hobbs HH, Cohen JC, Rawson RB. Dual roles for cholesterol in mammalian cells. Proc Natl Acad Sci USA. 2005;102:14551–14556. doi: 10.1073/pnas.0503590102. [DOI] [PMC free article] [PubMed] [Google Scholar]