Abstract
Apart from control of circulating fluid, atrial natriuretic peptide (ANP) exhibits anti-inflammatory effects in the lung. However, molecular mechanisms of ANP anti-inflammatory effects are not well-understood. Peripheral microtubule (MT) dynamics is essential for agonist-induced regulation of vascular endothelial permeability. Here we studied the role of MT-dependent signaling in ANP protective effects against endothelial cell (EC) barrier dysfunction and acute lung injury induced by Staphylococcus aureus-derived peptidoglican-G (PepG). PepG-induced vascular endothelial dysfunction was accompanied by MT destabilization and disruption of MT network. ANP attenuated PepG-induced MT disassembly, NFkB signaling and activity of MT-associated Rho activator GEF-H1 leading to attenuation of EC inflammatory activation reflected by expression of adhesion molecules ICAM1 and VCAM1. ANP-induced EC barrier preservation and MT stabilization were linked to phosphorylation and inactivation of MT-depolymerizing protein stathmin. Expression of stathmin phosphorylation-deficient mutant abolished ANP protective effects against PepG-induced inflammation and EC permeability. In contrast, siRNA-mediated stathmin knockdown prevented PepG-induced peripheral MT disassembly and endothelial barrier dysfunction. ANP protective effects in a murine model of PepG-induced lung injury were associated with increased phosphorylation of stathmin, while exacerbated lung injury in the ANP knockout mice was accompanied by decreased pool of stable MT. Stathmin knockdown in vivo reversed exacerbation of lung injury in the ANP knockout mice. These results show a novel MT-mediated mechanism of endothelial barrier protection by ANP in pulmonary EC and animal model of PepG-induced lung injury via stathmin-dependent control of MT assembly.
Keywords: endothelium, permeability, cytoskeleton, vascular leak, lung injury
1. INTRODUCTION
Increased lung capillary leak and reduced alveolar liquid clearance capacity provoked by many pathologic factors including bacterial pathogens represent the major pathologic mechanisms of fluid accumulation in alveolar space. As a result, pulmonary edema combined with increased inflammatory cell infiltration in the lung leads to a life-threatening complication, the acute respiratory distress syndrome. Peptidoglycan (PepG) and lipoteichoic acid are two major cell wall components in gram-positive bacteria which stimulate inflammatory responses in vivo an in vitro via activation of toll-like receptors (TLRs) [1,2]. Of the ten TLRs known, only TLR2 has been clearly shown to be involved in the host defense against gram-positive bacteria [3,4]. Activation of TLR2 in endothelial cells leads to phosphorylation/activation of downstream targets including mitogen-activated protein kinases (MAPK) p42/p44, JNK1/2, and p38, nuclear factor kappa-B (NFkB) pathway [5]. Consistent with its key role in mediating inflammatory signaling from Gram-positive bacteria, siRNA-induced knockdown of TLR-2 decreased Raf phosphorylation and suppressed TLR2-mediated activation of Raf-MEK1/2-ERK1/2-IKK-NFkB cascade [6]. Increasing evidence suggests that, in addition to its role in body fluid control, atrial natriuretic peptide (ANP) exhibits direct anti-inflammatory and barrier effects on vascular endothelium which were demonstrated in the models of endothelial hyper-permeability induced by hypoxia, lysophospholipids and inflammatory mediators [7,8].
The two major ANP receptors, NPR-A and NPR-B act as membrane-associated guanylate cyclases [9], and elevation of cGMP levels is a primary response to ANP stimulation. ANP-induced elevation of cGMP decreased basal levels of lung EC permeability, attenuated pulmonary EC barrier dysfunction caused by hydrogen peroxide [10,11], and inhibited oxidant-induced pulmonary edema observed in perfused rabbit lungs [12]. However, ANP-mediated elevation of cGMP increased lung vascular permeability in the ischemia reperfusion model of lung injury [13], suggesting context-specific effects of cGMP and ANP in different models. Several reports also indicate the involvement of cAMP and cAMP-dependent protein kinase (PKA) in physiological responses elicited by ANP [14,15] including EC barrier protective effects mediated by Epac-Rap1-Rac1 signaling pathway [8]. The other report demonstrated PKA-independent activation of Rap1 by both cAMP and cGMP analogs and suggests activation of barrier protective Rap1 signaling through a cAMP/cGMP-regulated guanine nucleotide exchange factor [16]. ANP anti-inflammatory effects have been associated with attenuation of stress MAP kinase and NFkB cascade activities and Rho GTPase signaling [17,18], but precise molecular mechanisms of ANP-dependent attenuation of these pro-inflammatory pathways are not well-understood.
Regulation of vascular endothelial barrier is achieved via dynamic actin cytoskeletal remodeling in vascular endothelial cells (EC) coordinated with assembly and disassembly of cell-cell junctions [19]. Emerging evidence also indicates a critical role of crosstalk between actin networks and microtubules (MT) in precise regulation of EC permeability by chemical and mechanical factors [20,21]. MT-associated guanine nucleotide exchange factor H1 (GEF-H1) has been implicated in the MT-dependent regulation of Rho activity. In the MT-bound state, the nucleotide exchange activity of GEF-H1 is suppressed, whereas GEF-H1 release caused by MT disruption stimulates GEF-H1 [22].
MT dynamics controls many cellular processes including mitosis, locomotion, protein and organelle transport and permeability [23]. MT growth is regulated by a number of MT-associated proteins which control polymerization, depolymerization rates and MT stability. Stathmin is a regulator of MT dynamics which is expressed in endothelial cells and other cell types. In the unphosphorylated state, stathmin promotes MT destabilization by sequestration of soluble tubulin and by direct MT binding, which promotes MT shortening. Stathmin phosphorylation on one or more serine residues by PKA, Rac effector kinase PAK1 or other kinases reduces its MT-destabilizing activity [24]. This study elucidated the role of MT-dependent signaling in the EC barrier dysfunction and inflammatory activation induced by PepG in vitro and in vivo. We investigated the molecular mechanism of barrier-protective and anti-inflammatory effects of ANP via stathmin-mediated control of MT dynamics and MT-associated modulation of signaling pathways leading to EC permeability, inflammation and PepG-induced lung injury.
2. MATERIALS AND METHODS
2.1. Cell culture and reagents
Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza (East Rutherford, NJ) and used for experiments at passages 5–8. ANP and TRAP6 were purchased from Ana Spec (San Jose, CA). Reagents for immunofluorescence were purchased from Molecular Probes (Eugene, OR). HRP-linked anti-mouse and -rabbit IgG antibodies were obtained from Cell Signaling Inc. (Beverly, MA). Antibodies to phospho- myosin phosphatase targeting subunit1 (MYPT), GEF-H1, diphospho-myosin light chain (MLC), phospho-stathmin, and IκBα were from Cell Signaling Inc (Beverly, MA); stathmin and End-Binding protein-1 (EB1) were from BD Transduction Laboratories (San Diego, CA); ICAM-1 and VCAM-1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Peptidoglycan G (PepG) polymer covers cell surface of S. aureus Gram positive bacteria. PepG of 99% purity isolated from S. aureus, cat-77140, was obtained from Fluka (Buchs, Switzerland). Unless specified, other biochemical reagents including β-Tubulin and acetylated tubulin antibodies were obtained from Sigma (St. Louis, MO).
2.2. siRNA and DNA transfections
To deplete endogenous stathmin, pre-designed human or mouse Stealth™ Select siRNA sets of standard purity were ordered from Invitrogen (Carlsbad, CA). Transfection of pulmonary EC culture with siRNA was performed as previously described [20]. Nonspecific, non-targeting siRNA (nsRNA) was used as a control treatment. After 72 hrs of transfection, cells were used for experiments or harvested for western blot verification of specific protein depletion. For in vivo experiments, we used polymer-based administration of non-specific or specific siRNA conjugated with polycation polyethilenimine PEI-22 as described in our previous studies [20,25]. Plasmid encoding stathmin-S63A mutant bearing a His-tag was provided by G. Bokoch (Scripps, La Jolla, CA) and was used for transient transfections of human pulmonary EC cultures according to protocols described elsewhere [20]. Control transfections were performed with empty vectors.
2.3. Cell imaging
Endothelial monolayers plated on glass cover slips were subjected to immunofluorescence staining with Texas Red phalloidin to visualize F-actin as previously described [20]. Quantitative analysis of paracellular gap formation in EC monolayers treated with ANP and PepG was performed as previously described [26] .For microtubule quantification, cells were fixed with −20°C methanol and immunostaining was carried out with β-tubulin or EB1 antibodies as described previously [27]. Briefly, after the cell boundaries were outlined, the concentric outline shapes reduced to 70% were applied to the images to mark peripheral (outer 30% of diameter) and central (inner 70% of diameter) regions. The integrated fluorescence density in the peripheral area was measured using MetaMorph software and was calculated as a percentage of the integrated fluorescence density in the total cell area. The results were normalized in each experiment. Similar methods were applied to EB1 quantification in fixed cells except that EB1 immunoreactivity was manually counted and results were not normalized. Minimum 10 cells per condition, in three experimental repeats were analyzed.
2.4. Measurement of EC permeability
The Express permeability testing assay (XPerT) was recently developed in our group [28] and now available from Millipore (Vascular Permeability Imaging Assay, cat. #17–10398). This assay is based on high affinity binding of avidin-conjugated FITC-labeled tracer to the biotinylated extracellular matrix proteins immobilized on the bottom of culture dishes. Permeability assays were performed in 96-well plates, and the fluorescence of matrix-bound FITC-avidin was measured on Victor X5 Multilabel Plate Reader (Perkin Elmer, Waltham, MA). In permeability visualization experiments, cells were fixed with 3.7% formaldehyde. Images were acquired using Nikon video imaging system Eclipse TE 300 (Nikon, Tokyo, Japan) equipped with a digital camera (DKC 5000, Sony, Tokyo, Japan); 40× objective lenses were used. Images were processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). Measurements of transendothelial electrical resistance (TER) across confluent HPAEC monolayers were performed using the electrical cell-substrate impedance sensing system (ECIS) (Applied Biophysics, Troy, NY) as previously described [29].
2.5. GEF-H1 activation assay
Plasmid encoding GST-tagged RhoA(G17A) mutant for bacterial expression was a generous gift from K. Szaszi (St. Michael’s Hospital, Toronto, Canada). Active GEF-H1 was precipitated from cell lysates as previously described [30] using agarose beads with conjugated GST-tagged RhoA(G17A) mutant which cannot bind nucleotide and therefore has high affinity for activated GEFs [31]. Activated GEF-H1 in RhoA(G17A) pulldowns was detected by Western blotting and normalized to total GEF-H1 in cell lysates for each sample.
2.6. Microtubule reassembly assay and immunoblotting
Confluent HPAEC were stimulated with the agonist of interest for desired periods of time. MT disassembly was promoted by incubating of cells at +4°C for 15 min. To initiate MT reassembly, the dishes were transferred toa 37°C CO2 incubator for 10 min or 20 min. To analyze content of assembled MT in control and treated samples, the cells were washed with PBS and scraped into extraction buffer (100 mM Pipes, 1 mM EGTA, 1 mM MgSO4, 2 M glycerol, pH 6.75) with protease and phosphatase inhibitor cocktail and passed several times through a 26-G needle. The cytosolic fraction containing soluble tubulin and the pellet fraction containing insoluble tubulin (assembled MT) were separated by centrifugation at 12000 rpm for 15 min. Protein extracts were separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with specific antibodies as previously described [32]. Equal protein loading was verified by reprobing membranes with antibody to β-tubulin or the specific protein of interest.
2.7. In vivo model of acute lung injury and ANP knockout mice
All animal care and treatment procedures were approved by the University of Chicago Institutional Animal Care and Use Committee and were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice with targeted disruption of ANP gene (strain B6.129P2-Nppatm1Unc/J) were purchased from the Jackson Laboratories (Bar Harbor, ME). Genotyping of Nppa−/− mice was performed by PCR analysis of genomic DNA extracted from tail snips with assistance of the animal core facility. Adult male homozygous Nppa−/− and matched wild type controls, 8–10 week old, with average weight 20–25 grams (Jackson Laboratories, Bar Harbor, ME) were anesthetized, and PepG (2.5 mg/kg) was administered intratracheally with or without intravenous ANP (2 µg/kg) injection. At 24 hours, animals were sacrificed by exsanguination under anesthesia and cell count and protein concentration were measured in bronchoalveolar lavage fluid (BAL) [33]; myeloperoxidase (MPO) content was determined in lung tissue lysates [34]. Evans blue dye (30 ml/kg) was injected into the external jugular vein 2 hours before termination of ventilation to assess vascular leak as previously decribed [34]. Histological assessment of lung injury was performed as described elsewhere [33]. Protein levels of ICAM-1, phospho-stathmin, total and acetylated tubulin in lung tissue samples were analyzed by immunoblotting of lung tissue lysates.
2.8. Statistical analysis
Results are expressed as mean ± SD of three to five independent experiments. Stimulated samples were compared to controls by unpaired Student’s t-tests. For multiple-group comparisons, a one-way variance analysis (ANOVA), followed by Tukey’s post hoc multiple-comparison test, were used. P<0.05 was considered statistically significant.
3. RESULTS
3.1. ANP protects pulmonary EC monolayers against PepG-induced barrier disruption
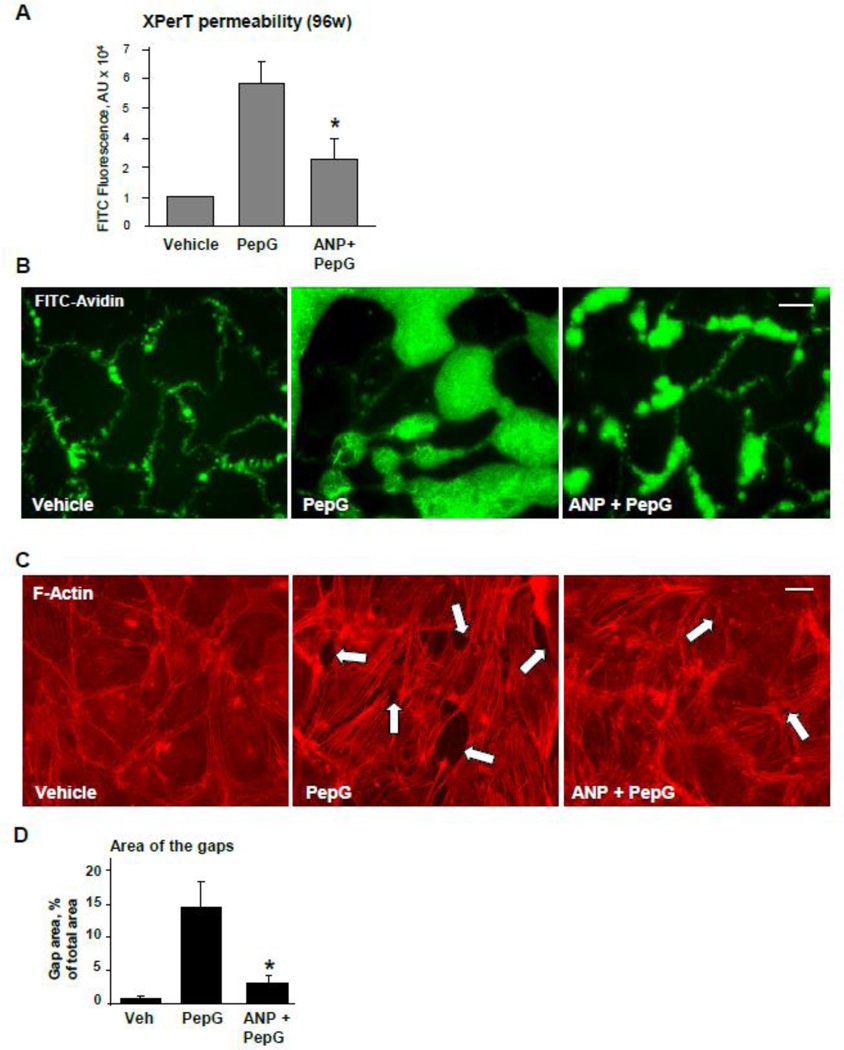
Effects of PepG and ANP on pulmonary EC permeability for macromolecules were tested in a novel permeability assay (XPerT) recently developed by our group [28]. PepG significantly increased EC monolayer permeability for FITC-labeled avidin, while ANP attenuated barrier disruptive effects of PepG (Figure 1A). Visualization of permeability sites in the PepG-challenged EC monolayers showed penetration of fluorescent probe via weakened cell-cell junctions and PepG-induced paracellular gaps. ANP pretreatment of EC monolayers prior to PepG challenge markedly decreased penetration of FITC-labeled avidin through intercellular junctions (Figure 1B). PepG-induced hyper-permeability was accompanied by formation of actin stress fibers and intercellular gaps observed after 5 hrs of PepG challenge. The barrier disruptive effects of PepG were attenuated by cell pretreatment with ANP (Figure 1C and D).
Figure 1. ANP attenuates PepG-induced EC permeability and actin remodeling.
HPAEC grown in 96-well plates (A) or on glass coverslips (B, bar=10 µm) with immobilized biotinylated gelatin (0.25 mg/ml) were pretreated with ANP (100 nM, 20 min) followed by challenge with PepG (0.25 µg/ml, 6 hrs), and addition of FITC-avidin (25 µg/ml, 3 min). Unbound FITC-avidin was removed, and FITC fluorescence was measured; normalized data are expressed as mean ± SD; *P <0.05 vs. PepG alone. C: EC grown on glass coverslips were pretreated with ANP (100 nM, 20 min) followed by challenge with PepG (0.25 µg/ml, 4 hrs) and immunofluorescence staining with Texas Red phalloidin to detect actin filaments. Intercellular gaps are shown by arrows; bar=10 µm. D: Bar graphs depict quantitative analysis of total gap area in control, PepG and ANP + PepG treated EC monolayers; data are expressed as mean ± SD; *P <0.05 vs. PepG alone.
3.2. ANP attenuates the PepG-induced activation of the inflammatory cascade and Rho-dependent signaling
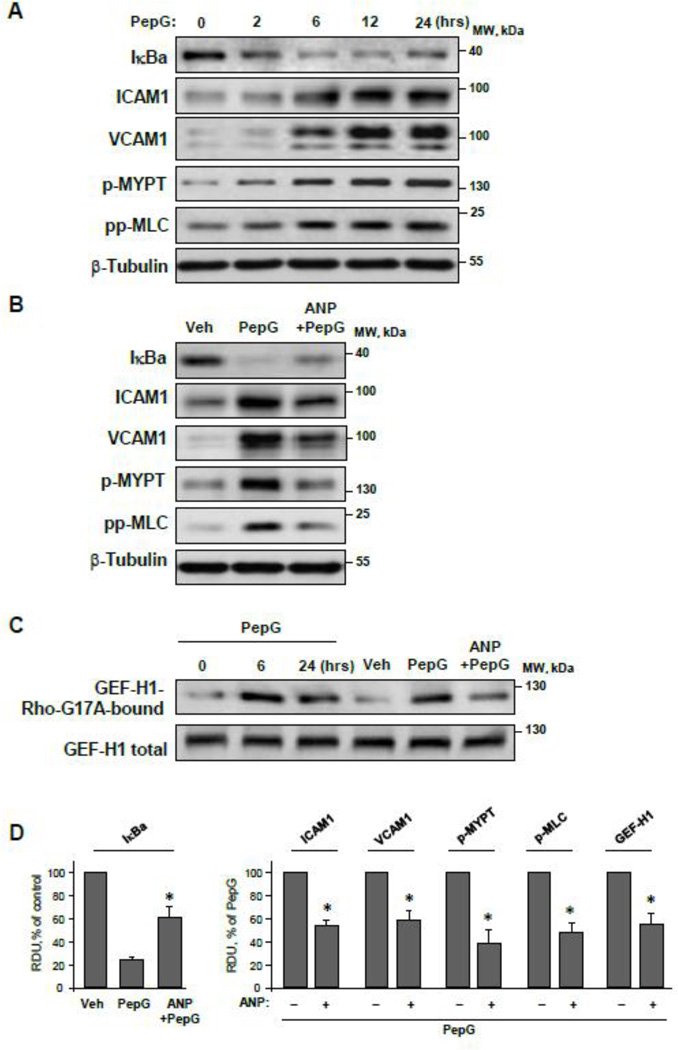
PepG stimulation caused time-dependent activation of inflammatory signaling in pulmonary EC reflected by a decreased level of inhibitory NFκB complex subunit, IκBα, and increased protein expression of EC adhesion molecules ICAM-1 and VCAM-1. Activation of inflammatory molecules 2–24 hours after PepG administration was also accompanied by increased phosphorylation of Rho GTPase targets, myosin phosphatase targeting subunit1 (MYPT) and myosin light chain (MLC). (Figure 2AD). Pretreatment with ANP abrogated PepG-induced activation of the NFκB cascade, ICAM-1 and VCAM-1 expression and stimulation of Rho signaling endpoints such as phosphorylation of MYPT and MLC (Figure 2BD).
Figure 2. Effects of PepG and ANP on EC inflammatory activation, Rho signaling and GEF-H1 activity.
A: HPAEC were stimulated with PepG (0.25 µg/ml) for 2 – 24 hrs, and expression of IκBα, ICAM-1, VCAM-1 and phosphorylation levels of MYPT and MLC were evaluated by western blot analysis with appropriate antibody. B: HPAEC were stimulated with PepG (0.25 µg/ml, 6 hrs) with or without ANP pretreatment (100 nM, 20 min). Protein expression levels of IκBα, ICAM1, VCAM-1 and phosphorylation levels of MYPT and MLC were evaluated by western blot. Western blot detection of β-tubulin in corresponding total lysates was used as a normalization control. C: GEF-H1 activation in HPAEC stimulated with PepG, or PepG + ANP (6 hrs) was evaluated by GEF pulldown assay. Western blot detection of GEF-H1 in corresponding total lysates was used as a normalization control. F: Bar graphs depict quantitative densitometry analysis of western blot data; data are expressed as mean ± SD; *P <0.05 vs. PepG alone.
PepG-induced activation of Rho signaling was linked to time-dependent activation of Rho-specific guanine nucleotide exchange factor (GEF), GEF-H1 (Figure 2C), which was tested in pulldown assays using beads with immobilized Rho G17A mutant as described in Methods. PepG-induced GEF-H1 activation was observed after 6 and 24 hrs of PepG challenge and was suppressed by cell pretreatment with ANP (Figure 2CD). The next experiments tested effects of PepG and ANP on the MT cytoskeleton.
3.3. ANP prevents PepG-induced peripheral MT disassembly
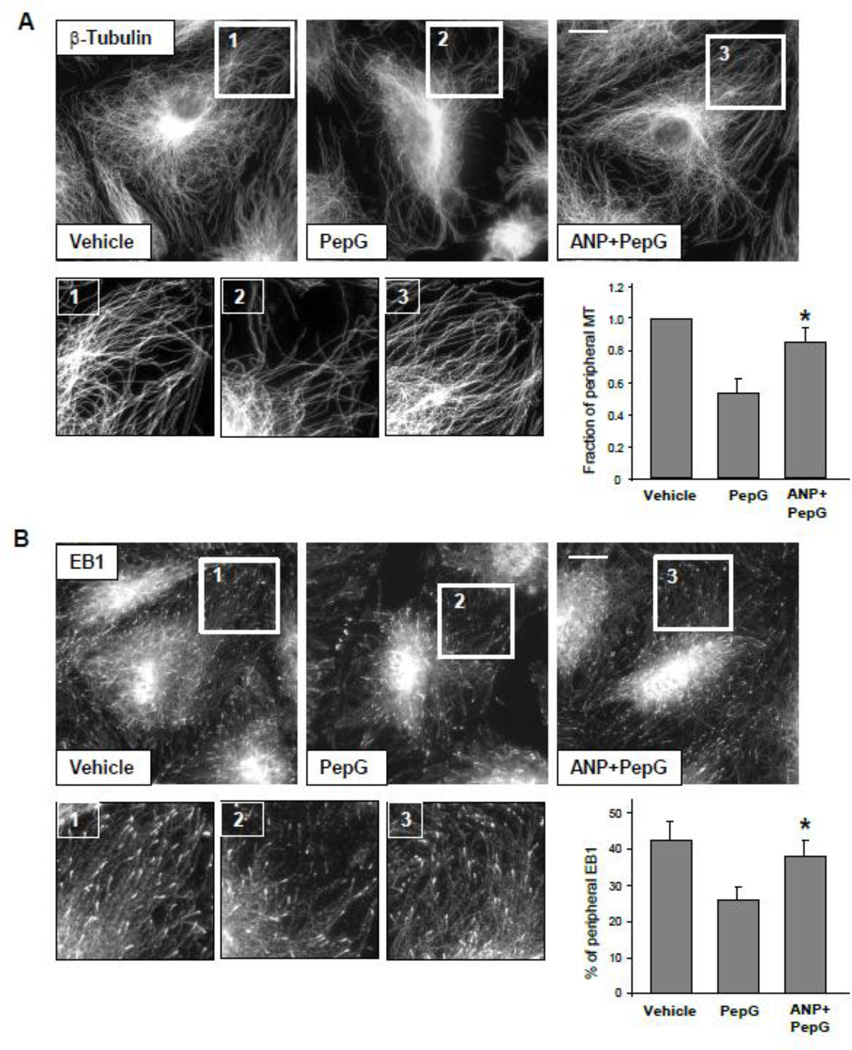
Effects of PepG and ANP on MT dynamics were examined by quantitative evaluation of β-tubulin and MT tip protein EB1 immunoreactivity at the peripheral areas of agonist-stimulated EC. Methanol-fixed EC were subjected to immunofluorescence staining with corresponding antibodies, and image analysis of peripheral β-tubulin and EB1 distribution in control cells and EC treated with PepG with or without ANP pretreatment was performed as described in Methods. The results show decreased fraction of peripheral MT (Figure 3A) and decreased EB1 immunoreactivity (Figure 3B) in PepG-challenged EC. Pretreatment with ANP significantly attenuated the PepG-induced decrease in peripheral MT content and EB1-positive MT growing ends, thus reflecting protection against PepG-induced peripheral MT disassembly (Figure 3, right panels). Insets in Figure 3A and B represent higher magnification images of microtubule network in the peripheral regions of control and stimulated cells.
Figure 3. ANP prevents PepG-induced disassembly of peripheral MT.
A: HPAEC grown on coverslips were stimulated with PepG (0.25 µg/ml, 6 hrs) with or without ANP pretreatment (100 nM, 20 min) followed by immunofluorescence staining with an antibody against β-tubulin; bar=5 µm. Bar graph depicts results of quantitative analysis of peripheral microtubules; data are expressed as mean ± SD; *P<0.05, ANP+PepG vs. PepG. B: Cells were treated with PepG or ANP + PepG. Endogenous EB1 was visualized by immunostaining with anti-EB1 antibody; bar=5 µm. Insets show high magnification images of cell periphery areas with EB1-positive microtubule tips. Bar graph depicts quantification of peripheral EB1 in methanol-fixed HPAEC monolayers; data are expressed as mean ± SD; *P<0.05, ANP+PepG vs. PepG.
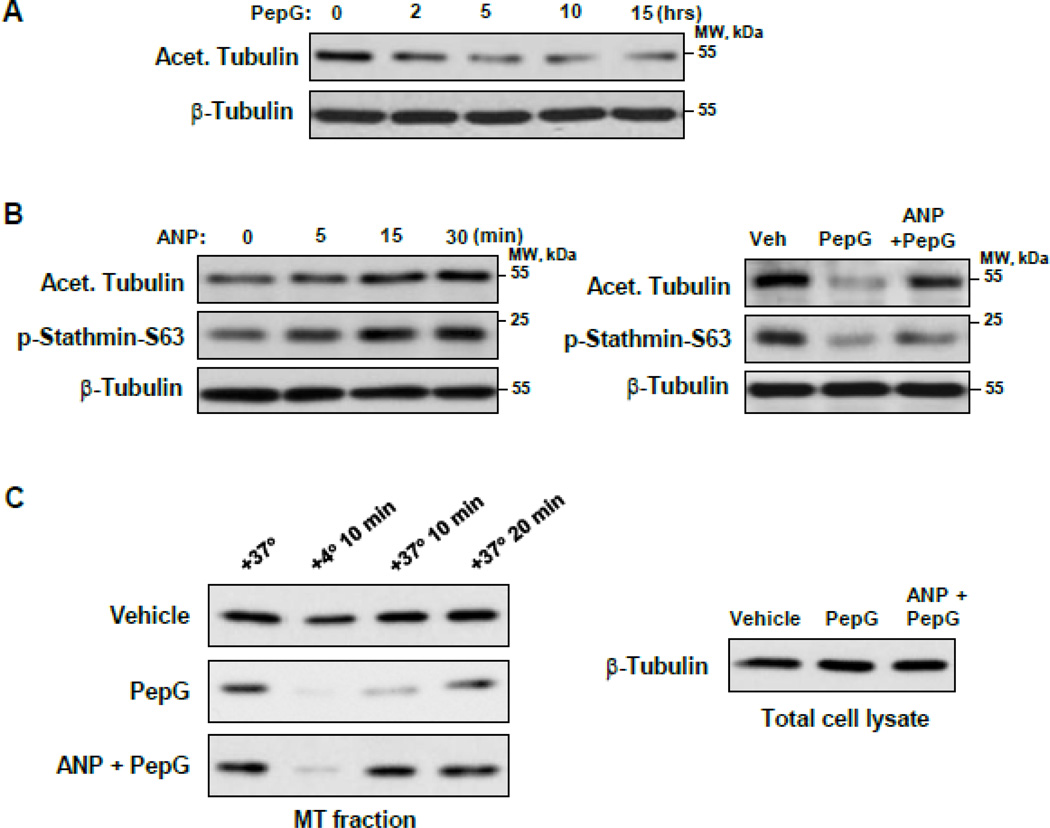
3.4. PepG and ANP exhibit opposite effects on MT stability, re-assembly and stathmin phosphorylation
Treatment of EC with PepG decreased the fraction of acetylated tubulin thus indicating a reduction of a pool of stable MT (Figure 4A). In contrast, ANP increased the levels of acetylated tubulin and the levels of stathmin phosphorylated at S63, the site known to inactivate its MT-destabilizing activity [35]. The pepG-induced decrease in acetylated tubulin and phosphorylated stathmin levels was reversed by ANP co-treatment (Figure 4B). The consequences of PepG and (ANP + PepG) treatment on MT dynamics were next tested in experiments with cold-induced MT disassembly and recovery after EC re-warming to 37°C (Figure 4C). In contrast to vehicle-treated cells, MT re-polymerization in EC exposed to PepG was significantly decreased. Cell pretreatment with ANP prior to PepG challenge and the cold-induced MT disassembly/reassembly step significantly increased the amount of polymerized β-tubulin which was monitored by western blot analysis. These results link sensitization of PepG-stimulated EC to cold-induced MT depolymerization and delayed MT recovery. Collectively, these results support the mechanism of ANP-induced protection of peripheral MT via stathmin phosphorylation.
Figure 4. ANP inhibits PepG-induced MT destabilization.
HPAEC were stimulated with (A) PepG (0.25 µg/ml) (A), ANP (100 nM) or combination of ANP added 20 min prior to PepG and PepG (B), for 6 hrs and levels of acetylated tubulin and phospho-S63-stathmin were evaluated by western blot. Probing of corresponding lysates for β-tubulin was used to verify equal protein loading. C: HPAEC treated with PepG (0.25 µg/ml, 6 hrs) or pretreated with ANP (100 nM, 20 min) prior to PepG, were incubated for 10 min at +4°C to depolymerize MT followed by re-warming at +37°C which stimulates MT re-polymerization. The microtubule-enriched fractions from cells exposed to cold and after 10 min and 20 min of re-warming were obtained as described in Methods. The amount of polymerized β-tubulin in MT pellets was determined by western blot.
3.5. Stathmin phosphorylation is essential for barrier protective and antiinflammatory effects of ANP against PepG
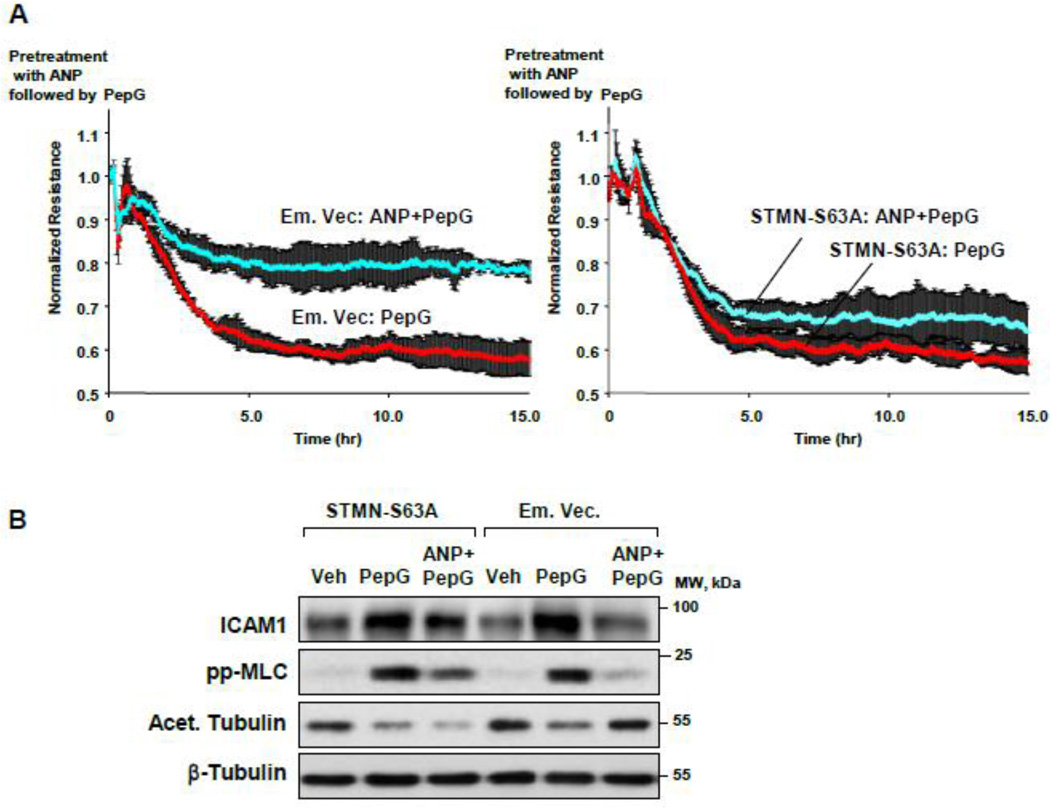
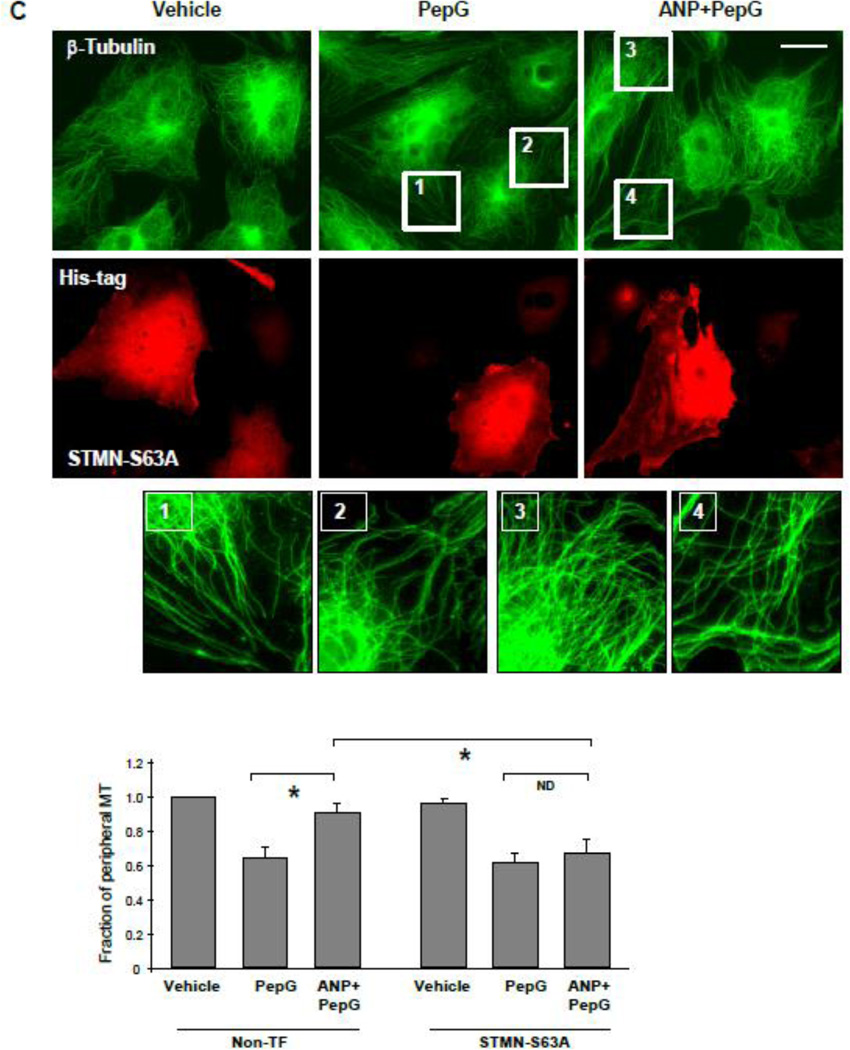
The role of stathmin phosphorylation in ANP-induced barrier protective and anti-inflammatory effects was directly tested using measurements of transendothelial electrical resistance (TER) and western blot analysis of inflammatory readout ICAM-1 and the Rho pathway monitored by phospho-MLC levels. Overexpression of phosphorylation-deficient stathmin-S63A mutant markedly attenuated the ANP protective effect on PepG-induced EC permeability (Figure 5A). Expression of stathmin-S63A blocked the ANP attenuating effects on ICAM-1 expression, MLC phosphorylation and MT stabilization monitored by the levels of acetylated tubulin (Figure 5B). In contrast to non-transfected cells or cells transfected with empty vector, immunofluorescence staining revealed lower peripheral microtubule density in pulmonary EC expressing stathmin-S63A and co-treated with PepG and ANP (Figure 5C). Taken together, these data suggest a critical role of stathmin phosphorylation in mediation of barrier protective and anti-inflammatory effects of ANP via control of MT dynamics.
Figure 5. ANP-dependent stathmin phosphorylation is critical for protection against PepG-induced EC barrier dysfunction.
Endothelial monolayers transfected with phosphorylation-deficient stathmin (STMN-S63A) or empty vector (Em. Vec.) were treated with PepG (0.25 µg/ml) or pretreated with ANP (100 nM, 20 min) prior to PepG stimulation. A: TER measurements were performed over 15 hrs. B: Expression of ICAM-1, phosphorylation of MLC and tubulin acetylation were detected by Western blot after 6 hrs of treatment. Western blot detection of β-tubulin in corresponding total lysates was used as a normalization control. C: Microtubule remodeling in EC treated with PepG or ANP+PepG (6 hrs) was examined by immunofluorescence staining with β-tubulin antibody. Staining with His-tag antibody was used to visualize STMN-S63A expressing cells; bar=10 µm. Insets depict high magnification images of MT network structure in the peripheral area of cells marked by rectangles. Bar graph depicts results of quantitative analysis of peripheral microtubules; data are expressed as mean ± SD; *P<0.05.
3.6. Stathmin knockdown attenuates PepG-induced EC permeability, inflammatory response and Rho signaling
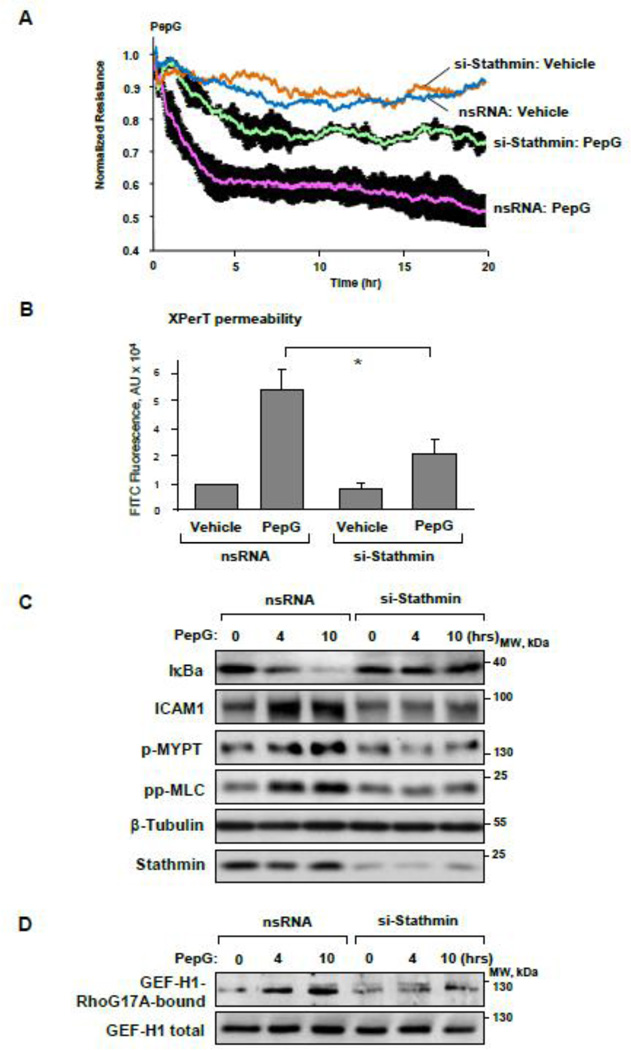
Stathmin depletion in pulmonary EC using gene-specific siRNA attenuated PepG-induced TER decline (Figure 6A). Attenuation of PepG-induced permeability by stathmin knockdown was further confirmed in experiments with measurement of EC monolayer permeability for fluorescently labeled avidin (Figure 6B). Stathmin knockdown inhibited PepG-induced IkBa degradation and expression of ICAM-1 observed after 4–10 hrs of PepG challenge (Figure 6C). Stathmin knockdown also attenuated PepG-induced GEF-H1 activation (Figure 6D) and phosphorylation of Rho-kinase effectors MYPT and MLC (Figure 6C).
Figure 6. Stathmin knockdown attenuates PepG-induced EC hyperpermeability and inflammatory activation.
HPAEC cells were transfected with stathmin-specific siRNA or with non-specific RNA for 72 hrs. A: TER measurements were performed over 20 hrs At the time indicated by the arrow, cells were stimulated with PepG (0.25 µg/ml). B: HPAEC grown in 96-well plates with immobilized biotinylated gelatin were treated with PepG (0.25 µg/ml, 6 hrs) and addition of FITC-avidin (25 µg/ml, 3 min). Unbound FITC-avidin was removed, and FITC fluorescence was measured; data are expressed as mean ± SD; *P <0.05 vs. nsRNA. C: After EC transfection with stathmin-specific siRNA or nonspecific RNA, the expression of IκBα and ICAM-1, phosphorylation of MYPT and MLC were detected by western blot after PepG treatment. Western blot detection of β-tubulin in corresponding total lysates was used as a normalization control. Stathmin knockdown was confirmed by western blot with stathmin antibody. D: PepG-induced GEF-H1 activation was evaluated by GEF pulldown assay. Testing of the GEF-H1 levels in total cell lysates was used as normalization control.
3.7. Stathmin knockdown confers protection against PepG-induced peripheral MT disassembly
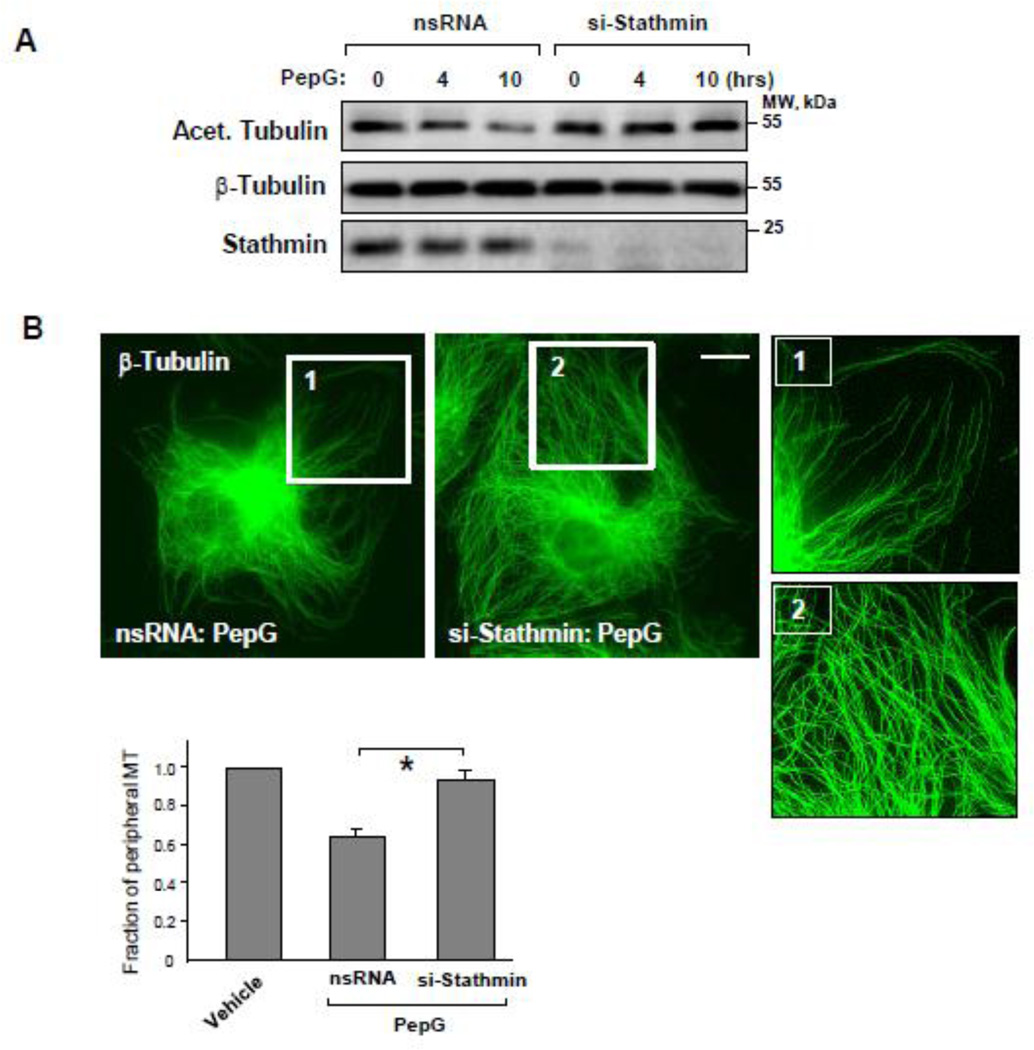
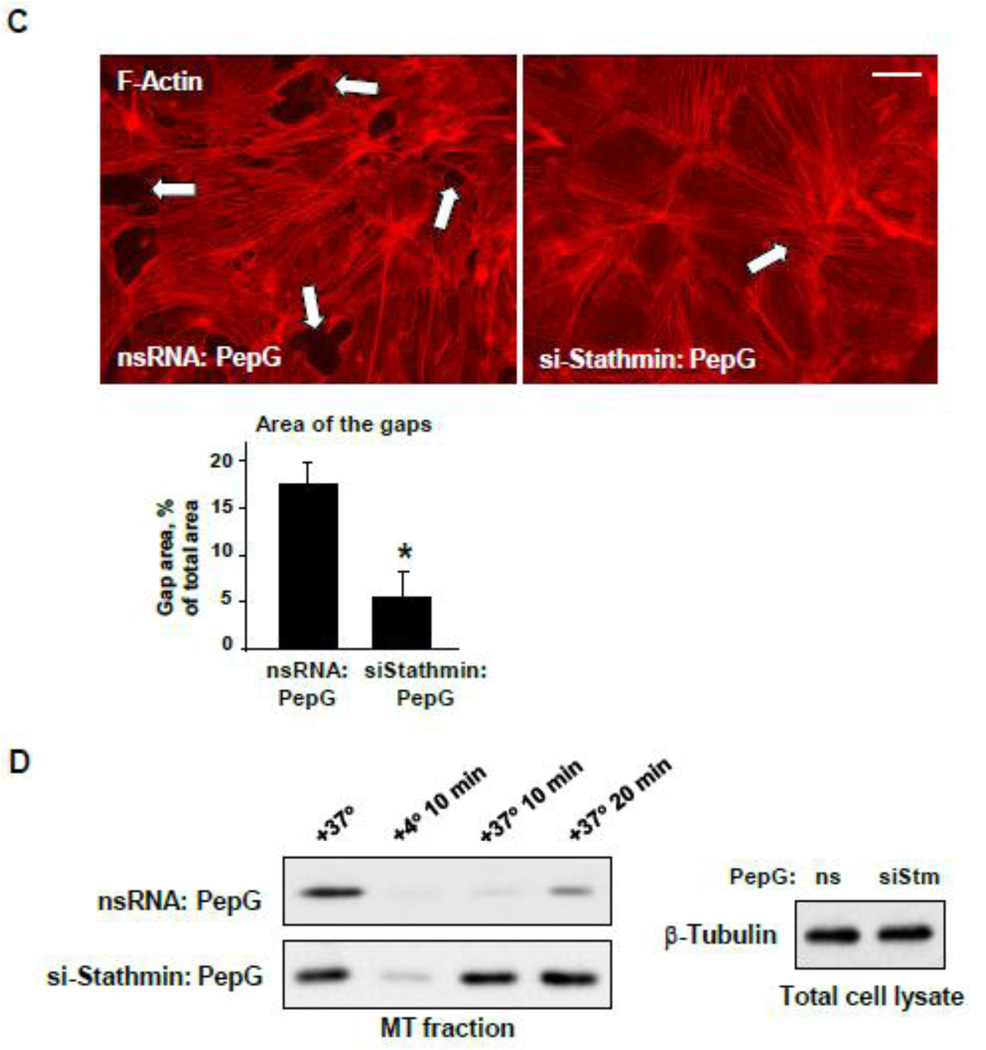
Using a stathmin knockown approach, we studied in more detail stathmin’s role in PepG-induced changes in MT dynamics and stability. Stathmin knockdown preserved the pool of acetylated tubulin in PepG-treated EC (Figure 7A). Stathmin knockdown also prevented PepG-induced disassembly of peripheral MT detected by immunofluorescence staining (Figure 7B) and attenuated PepG-induced EC barrier disruption reflected by formation of central actin stress fibers and intercellular gaps (Figure 7C). Depletion of stathmin promoted the MT reassembly after cold treatment of EC monolayers (Figure 7D). Functional role of stathmin and ANP-dependent stathmin phosphorylation was further evaluated in an animal model of PepG-induced lung injury.
Figure 7. Stathmin knockdown attenuates MT disassembly and EC monolayer disruption induced by PepG.
A: Levels of acetylated tubulin in total lysates of HPAEC transfected with stathmin-specific siRNA or with non-specific RNA (72 hrs) and stimulated with PepG (0.25 µg/ml) were detected by western blot. Probing of corresponding lysates for β-tubulin was used to verify equal protein loading. Stathmin knockdown was confirmed by western blot with stathmin antibody. Microtubule remodeling (B, bar=5 µm) and F-actin reorganization (C, bar=10 µm) in EC treated with stathmin-specific siRNA or with non-specific RNA in response to PepG challenge (6 hrs) was examined by immunofluorescence staining with β-tubulin antibody. Bar graphs depict results of quantitative analysis of peripheral microtubules (B) or paracellular gap formation (C); data are expressed as mean ± SD; *P<0.05, si-Stathmin vs. nsRNA. D: HPAEC transfected with stathmin-specific siRNA or with non-specific RNA were challenged with PepG (6 hrs) and incubated for 10 min at +4 °C to induce MT disassembly followed by re-warming at +37 °C to stimulate MT re-polymerization. The MT-enriched fractions from cells exposed to 10-min cooling to +4 °C followed by re-warming (10 min and 20 min) were obtained as described in Methods. The amount of polymerized β-tubulin in MT pellets and supernatants was determined by western blot.
3.8. ANP protective effects against PepG-induced lung injury are associated with increased levels of phospho-stathmin and acetylated tubulin
In the lungs, PepG induces acute pulmonary inflammation in a dose-dependent way, characterized by neutrophilic influx and IL-6 production in the bronchoalveolar lavage fluid [36]. For in vivo studies, the PepG was administered intratracheally at 2.5 mg/kg, the dose relevant to previous reports [36,37]. This dose causes sub-lethal lung injury and represents conditions associated with bacterial pneumonia. The following experiments examined ANP effects in the murine model of PepG-induced lung injury. One group was challenged with PepG for 24 hours, while another PepG-stimulated group received concurrent ANP administration. Control mice were treated with vehicle (saline solution). At the end of the experiment, lung injury was evaluated by measurements of bronchoalveolar lavage (BAL) protein concentration, cell count, measurements of Evans blue accumulation in the lung tissue and histological analysis of lung sections. PepG instillation caused pronounced lung inflammation with prominent increase in BAL protein concentration cell counts. These effects were significantly attenuated by ANP (Figure 8A).
Figure 8. Effects of ANP on PepG-induced lung injury and activation of microtubule proteins.
A: C57BL/6J mice were treated with PepG (2.5 mg/kg) with or without concurrent treatment with ANP (2 µg/kg) or sterile saline solution for 24 hr. Control animals were treated with sterile saline solution. Protein concentration and cell count were measured in bronchoalveolar lavage (BAL) fluid, n=9 per condition; data are expressed as mean ± SD; *p<0.05 vs. PepG alone. B: Lung vascular permeability was assessed by Evans blue dye (30 ml/kg, i/v) accumulation in the lung tissue; n=4 per condition; data are expressed as mean ± SD; *p<0.05 vs. PepG alone. C: Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded lung sections. Original magnification: x20. Images are representative of 5 to 8 lung specimens for each condition. D: ICAM-1 expression, stathmin phosphorylation and amount of acetylated tubulin after mice exposure to PepG with or without ANP were determined by western blot analysis of lung tissue homogenates. Western blot detection of β-tubulin in corresponding tissue lysates was used as a normalization control. Bar graphs depict quantitative densitometry analysis of western blot data; data are expressed as mean + SD; N=5; *P <0.05 vs. PepG alone.
The protective effects of ANP against PepG-induced lung vascular leak were further assessed by measurement of Evans blue accumulation in the lung tissue. PepG induced noticeable Evans blue accumulation in the lung parenchyma, which was suppressed by ANP co-treatment (Figure 8B). Quantitative analysis of Evans blue-labeled albumin extravasation further confirmed these results. Histological analysis of lung tissue showed alveolar wall thickening, increased leukocyte infiltration into the lung interstitium and alveolar space of PepG-challenged lungs. These effects were attenuated by ANP cotreatment (Figure 8C). Western blot analysis of lung tissue samples showed that ANP also attenuated the PepG-induced ICAM-1 expression. Anti-inflammatory and lung protective effects of ANP were associated with increased levels of phospho-stathmin and preservation of the acetylated tubulin pool, the parameters reflecting increased stability of MT cytoskeleton (Figure 8D).
3.9. PepG-induced lung injury is exacerbated in ANP knockout mice
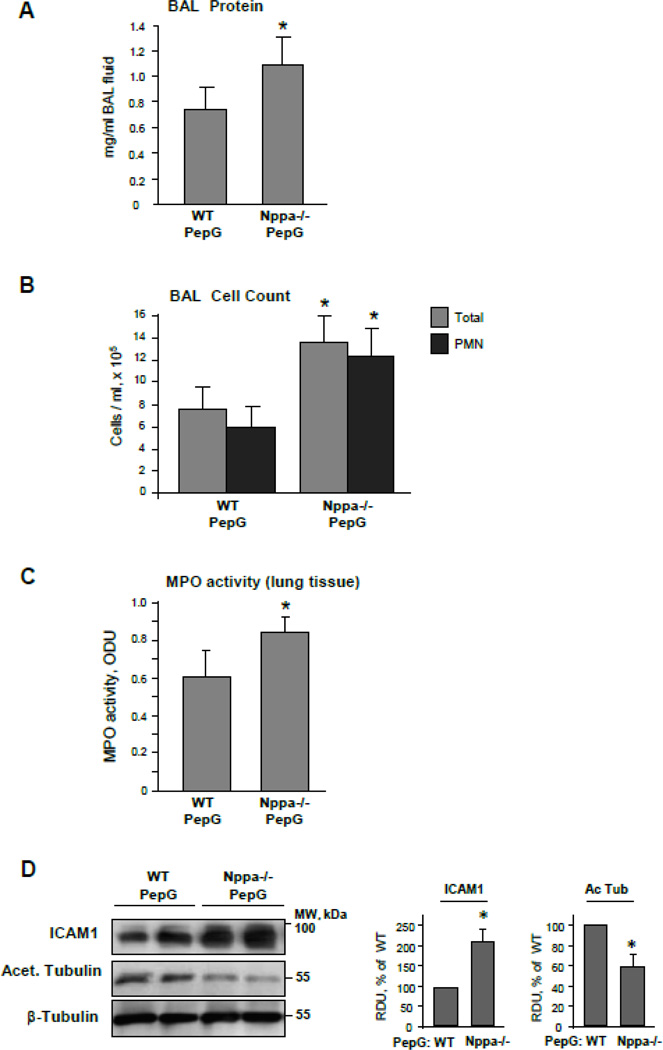
In comparison to wild type mice, the mice with ANP knockout developed more prominent lung inflammation and vascular leak in response to PepG challenge characterized by a 1.5-fold increase in BAL protein content and a nearly 2-fold increase in total cell and neutrophil counts in BAL (Figure 9AB). Measurement of myeloperoxidase (MPO) activity in the lung tissue was used as an additional parameter of neutrophilic activation and inflammation. PepG induced a more pronounced increase in MPO activity in ANP knockout mice, as compared to wild type controls (Figure 9C). Interestingly, western blot analysis demonstrated increased ICAM-1 expression but decreased levels of acetylated tubulin in lung tissue samples from PepG-stimulated ANP knockout mice in comparison to PepG-stimulated wild type controls (Figure 9D). All tests described above showed no significant difference between untreated wild type and ANP knockout mice (data not shown). These data strongly suggest that ANP plays a key role in modulation of ALI induced by bacterial pathogens, and ANP protective effects involve stabilization of the MT cytoskeleton. The role of stathmin in ANP-mediated protective effects in vivo was investigated in further experiments.
Figure 9. Assessment of PepG-induced lung injury in ANP-deficient mice.
Wild type or ANP knockout mice (strain B6.129P2-Nppatm1Unc/J) were treated with PepG (2.5 mg/kg) for 24 hours. Control animals were treated with sterile saline solution. A and B: Protein concentration (A) and total cell and neutrophil count (B) were performed in BAL samples; n=6 per condition; data are expressed as mean ± SD; *p<0.05 vs. wild type. C: MPO activity was measured in lung homogenates. D: ICAM1 expression and acetylated tubulin levels in lung tissue lysates were measured by western blot. Western blot detection of β-tubulin in corresponding tissue lysates was used as a normalization control. Bar graphs depict quantitative densitometry analysis of western blot data; data are expressed as mean ± SD; *P <0.05 vs. wild type.
3.10. Stathmin exhibits protective effects against PepG-induced lung injury in ANP knockout mice
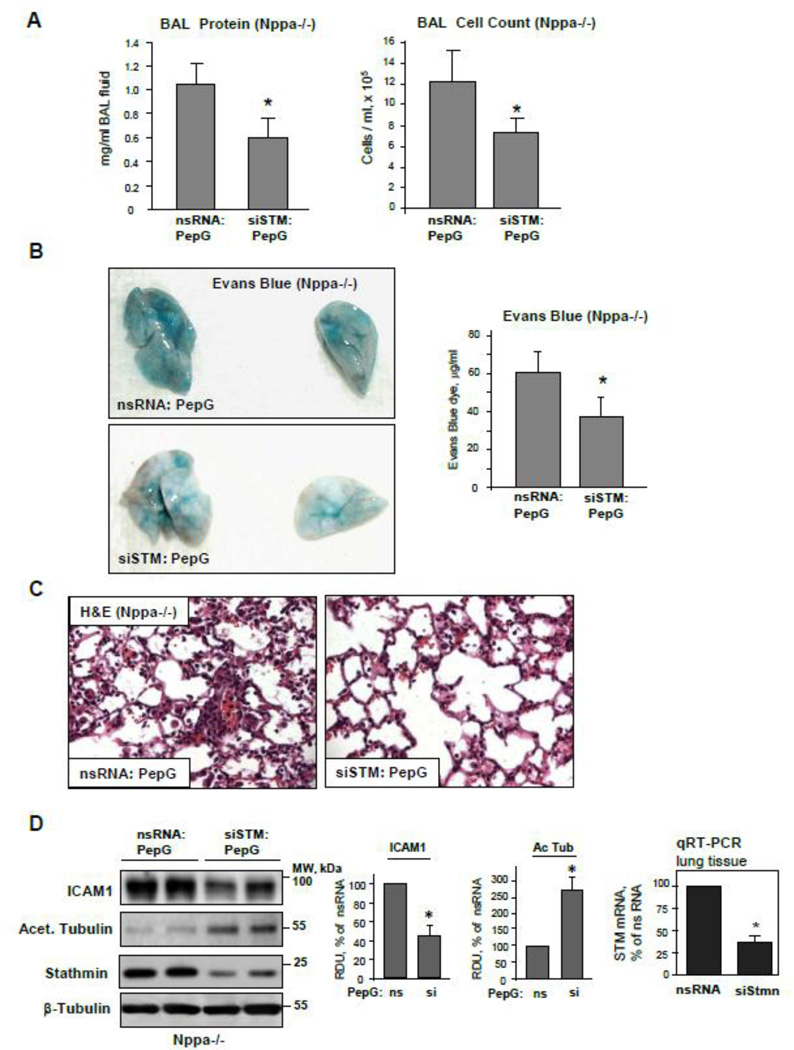
A role for stathmin in the mechanisms of ANP-mediated lung protection against PepG-induced injury was directly tested by in vivo knockdown of stathmin using a PEI-based siRNA delivery protocol [25]. Control injections were performed with nonspecific RNA duplexes. Stathmin knockdown decreased the protein and total cell accumulation in BAL samples of PepG-challenged ANP knockout mice (Figure 10A) and reduced the vascular leak detected by Evans Blue extravasation (Figure 10B). Histochemical evaluation of inflammatory cell infiltration in lung parenchyma confirmed protective effects of stathmin knockdown against PepG-induced lung injury (Figure 10C). Stathmin knockdown reduced ICAM-1 expression, but increased the tubulin acetylation levels in PepG-challenged lungs (Figure 10D, left panel). No difference in morphological lung structure or basal BAL parameters was found between untreated wild type or ANP knockout mice (data not shown). Stathmin depletion in the lungs after 72 hrs of transfection was confirmed by western blot (Figure 10D, left panel) and qRT-PCR analysis of lung tissue samples (Figure 10D, right panel).
Figure 10. Stathmin knockdown attenuates the exacerbation of PepG-induced lung injury in ANP-deficient mice.
Control and ANP knockout mice were transfected with non-specific or stathmin-specific siRNA for 72 hrs followed by treatment with PepG for 24 hrs. A: Protein concentration and total cell count were performed in BAL samples. B: Lung vascular permeability was assessed by Evans blue accumulation in the lung tissue; n=4 per condition; data are expressed as mean ± SD; *p<0.05 vs. nsRNA. B: Morphological changes in lungs from PepG-challenged control and ANP-deficient mice were evaluated by hematoxilin and eosin staining of lung tissue sections. D: ICAM-1 expression and acetylated tubulin levels in lung tissue lysates were measured by western blot (left panel). Western blot detection of β-tubulin in corresponding tissue lysates was used as a normalization control. Bar graphs depict quantitative densitometry analysis of western blot data; data are expressed as mean ± SD; *P <0.05 vs. nsRNA. Stathmin knockdown in lung tissue was confirmed by qRT-PCR (right panel).
4. DISCUSSION
Previous studies by our group have shown that stabilization of MT mediated by PKA-induced stathmin phosphorylation attenuates thrombin-induced rapid release of GEF-H1 from MT and thrombin receptor-induced Rho activation of EC barrier disruptive Rho signaling [38]. On the other hand, ANP-induced phosphorylation of GEF-H1 at serine-885 mediated by Rac GTPase-activated serine/threonine kinase PAK1 increased GEF-H1 binding to MT and attenuated GEF-H1-dependent activation of Rho signaling in thrombin-stimulated cells [39]. This mechanism has been further tested in the model of thrombin-induced EC permeability and aseptic mouse two-hit model of acute lung injury caused by high tidal volume mechanical ventilation combined with injection of thrombin-receptor activating peptide [39].
The current study demonstrates for the first time the MT-dependent mechanism of ANP protection against inflammation caused by bacterial pathogen. This is also the first report of stathmin role in the inhibition of inflammation via stathmin phosphorylation-dependent MT stabilization. EC stimulation with the Gram-positive bacterial compound PepG caused MT destabilization and partial disassembly reflected by the decreased pool of acetylated tubulin and diminished MT peripheral growth. ANP treatment preserved the MT network and attenuated inflammatory signaling activated by PepG. Interestingly, this study showed the PepG-induced decrease in stathmin phosphorylation. In contrast, EC barrier preservation and anti-inflammatory ANP effects were associated with increased stathmin phosphorylation. Expression of phosphorylation-deficient stathmin-S63A mutant destabilized MT in PepG-treated EC (as detected by decreased acetylated tubulin content), which led to exacerbation of PepG EC barrier dysfunction and further activation of inflammatory signaling. Taken together, these results suggest that inhibition of PepG-induced Rho, p38 and NFkB inflammatory cascades is dependent on stabilization of MT and involves ANP-induced stathmin phosphorylation.
MT-dependent modulation of Rho signaling has a high impact on the PepG-induced inflammatory cascade. In addition to direct effects on EC permeability, PepG-induced activation of the GEF-H1 – Rho pathway may stimulate transcription of pro-inflammatory genes, while inhibition of Rho has been shown to reduce expression of TNFα, CXC chemokines, leukocyte infiltration, and endotoxin-induced lung edema [40,41]. Pharmacological inhibition of Rho attenuates lung injury caused by both Gram-negative and Gram-positive pathogens [18,37,40,41]. Altogether, our data suggest that disruptive effects of inflammatory agents on MT stability are counteracted by ANP-induced stathmin phosphorylation which plays an essential role in ANP anti-inflammatory and barrier protective effects by stimulating the peripheral MT growth, stabilizing existing MT and attenuating the activity of MT-associated GEF-H1 leading to downregulation of Rho signaling. However, stathmin-mediated MT stabilization may also downregulate Rho-independent inflammatory mechanisms by sequestering other MT-binding molecules participating in inflammatory pathways. This possibility requires further investigation.
How is endogenous ANP regulated and how does it function in inflammatory ALI? Plasma levels of ANP and brain natriuretic peptide in normal patients are approximately 10 fmol/ml and become elevated 10- to 30-fold in patients with septic shock, congestive heart or acute renal failure [42–44]. Elevated plasma natriuretic peptides levels are associated with higher disease severity and mortality rates [43,45–47]. However, the precise causal relationship between ANP elevation and the severity of acute lung injury (ALI) remain unclear. For example, introduction of ANP reduced mortality rate and had a beneficial effect in patients with ALI [48]. Transient elevation of ANP mRNA levels was observed in the lungs of LPS-challenged mice [25,49] and is consistent with the hypothesis of increased endogenous ANP levels as a compensatory mechanism to reduce the lung’s inflammatory response to bacterial agents.
The current study shows exacerbation of PepG-induced lung injury, inflammation and destabilization of MT cytoskeleton in ANP knockout mice. Our data suggest that basal ANP levels or ANP elevation in ALI conditions moderate the extent of lung injury, which may represent a negative feedback control of inflammation by endogenous ANP. Interestingly, knockdown of stathmin significantly improved lung vascular barrier function and parameters of inflammation in ANP knockout mice. These effects were associated with stabilization of the MT cytoskeleton (detected by the increased pool of acetylated tubulin). These data support the stathmin-microtubule-dependent mechanism of ANP-induced anti-inflammatory effects and lung protection and suggest a new protein target for drug design.
Host response to bacterial pathogens during ALI is accompanied by increased production of inflammatory cytokines. Among them, TNFα induces capillary leak [50] and inhibition of alveolar fluid clearance [51]. Because ANP inhibits production of inflammatory cytokines TNFα and IL-6 [52] and directly blocks TNFα-induced inflammatory signaling [53], these effects may act in synergy with the MT-dependent mechanism described in this study and further contribute to the ANP-induced protection in ALI.
Stathmin is constitutively expressed in pulmonary vascular EC, but its expression levels may be developmentally regulated in other tissues. For example, stathmin expression is repressed during the post-natal liver development, but required for hepatocyte regeneration [54] and proliferation as opposed to differentiation [55]. These data suggest additional functions of stathmin beyond control of MT stability and have to be taken into consideration for treatments using agents neutralizing stathmin activity. It is also important to note that, in addition to stathmin-dependent mechanisms of lung vascular barrier protection and anti-inflammatory activities exhibited by ANP, ANP-induced elevation of cGMP in alveolar epithelium may reduce opening probability of alveolar epithelial ENaC sodium channels [56] and exhibit negative effects on alveolar fluid clearance capacity during ARDS [57]. Whether stathmin-mediated MT dynamics is involved in these adverse effects of ANP remains to be investigated.
In summary, the current study presents a novel paradigm of ANP-induced attenuation of inflammation caused by Gram-positive pathogens via stathmin-dependent stabilization of microtubules. We demonstrate a new mechanism of lung vascular endothelial barrier dysfunction and lung inflammation enhanced by PepG-induced destabilization of MT dynamics, which can be rescued by ANP acting to block the stathmin - GEF-H1 axis of Rho signaling. We also show that exacerbation of ALI and lung vascular endothelial permeability caused by Gram-positive pathogens in Nppa−/− mice can be rescued by stabilization of MT structure and stimulation of MT peripheral growth through molecular inhibition of stathmin. These studies advance our understanding of protective mechanisms induced by ANP in ALI conditions and emphasize the importance of MT-associated signaling in regulation of lung barrier and inflammation as a unifying mechanism playing a role in a variety of pathological conditions.
Highlights.
-
-
The state of peripheral microtubule network modulates cell inflammatory responses
-
-
ANP attenuates PepG-induced endothelial dysfunction via microtubule stabilization
-
-
Stathmin controls lung inflammation via destabilization of microtubules
-
-
Modulation of stathmin activity may be a new approach in prevention of vascular leak
AKNOWLEDGEMENTS
This work was supported by Public Health Service grants HL89257 and HL107920 from the National Heart, Lung, and Blood Institute. The authors wish to thank K. Szaszi (St. Michael’s Hospital, Toronto, Canada) for sharing the RhoG17A construct.
Supported by NIH NHLBI grants HL089257 and HL107920
Non-standard Abbreviations
- ANP
atrial natriuretic peptide
- BAL
bronchoalveolar lavage fluid
- cAMP
cyclic adenosine monophoshapte
- cGMP
cyclic guanosine monophosphate
- EC
endothelial cells
- ECIS
electrical cell-substrate impedance sensing system
- GEF
guanine nucleotide exchange factor
- HPAEC
human pulmonary artery endothelial cells
- MAPK
mitogen activated protein kinase
- MT
microtubules
- MPO
myeloperoxidase
- MLC
myosin light chain
- MYPT
myosin phosphatase targeting subunit1
- nsRNA
non-specific RNA
- PepG
peptidoglican-G
- PKA
cAMP-dependent protein kinase
- TER
transendothelial electrical resistance
- TLR2
Toll-like receptor 2
- XPerT
express permeability testing assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wang JE, Dahle MK, McDonald M, et al. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock. 2003;20:402–414. doi: 10.1097/01.shk.0000092268.01859.0d. [DOI] [PubMed] [Google Scholar]
- 2.Kielian T, Haney A, Mayes PM, et al. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect Immun. 2005;73:7428–7435. doi: 10.1128/IAI.73.11.7428-7435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Netea MG, van der Graaf C, Van der Meer JW, et al. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J Leukoc Biol. 2004;75:749–755. doi: 10.1189/jlb.1103543. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Arbibe L, Mira JP, Teusch N, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 6.Chiu WT, Lin YL, Chou CW, et al. Propofol inhibits lipoteichoic acid-induced iNOS gene expression in macrophages possibly through downregulation of tolllike receptor 2-mediated activation of Raf-MEK1/2-ERK1/2-IKK-NFkappaB. Chem Biol Interact. 2009;181:430–439. doi: 10.1016/j.cbi.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Irwin DC, Tissot van Patot MC, Tucker A, et al. Direct ANP inhibition of hypoxia-induced inflammatory pathways in pulmonary microvascular and macrovascular endothelial monolayers. Am J Physiol Lung Cell Mol Physiol. 2005;288:L849–L859. doi: 10.1152/ajplung.00294.2004. [DOI] [PubMed] [Google Scholar]
- 8.Birukova AA, Zagranichnaya T, Alekseeva E, et al. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008;215:715–724. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahluwalia A, MacAllister RJ, Hobbs AJ. Vascular actions of natriuretic peptides Cyclic GMP-dependent and -independent mechanisms. Basic Res Cardiol. 2004;99:83–89. doi: 10.1007/s00395-004-0459-6. [DOI] [PubMed] [Google Scholar]
- 10.Rentsendorj O, Mirzapoiazova T, Adyshev D, et al. Role of vasodilator-stimulated phosphoprotein in cGMP-mediated protection of human pulmonary artery endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2008;294:L686–L697. doi: 10.1152/ajplung.00417.2007. [DOI] [PubMed] [Google Scholar]
- 11.Moldobaeva A, Welsh-Servinsky LE, Shimoda LA, et al. Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L919–L930. doi: 10.1152/ajplung.00434.2005. [DOI] [PubMed] [Google Scholar]
- 12.Lofton CE, Baron DA, Heffner JE, et al. Atrial natriuretic peptide inhibits oxidant-induced increases in endothelial permeability. J Mol Cell Cardiol. 1991;23:919–927. doi: 10.1016/0022-2828(91)90134-8. [DOI] [PubMed] [Google Scholar]
- 13.Dodd-o JM, Hristopoulos ML, Kibler K, et al. The role of natriuretic peptide receptor-A signaling in unilateral lung ischemia-reperfusion injury in the intact mouse. Am J Physiol Lung Cell Mol Physiol. 2008;294:L714–L723. doi: 10.1152/ajplung.00185.2007. [DOI] [PubMed] [Google Scholar]
- 14.Sanghi S, Kumar R, Smith M, et al. Activation of protein kinase A by atrial natriuretic peptide in neonatal rat cardiac fibroblasts: role in regulation of the local renin-angiotensin system. Regul Pept. 2005;132:1–8. doi: 10.1016/j.regpep.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Kulhanek-Heinze S, Gerbes AL, Gerwig T, et al. Protein kinase A dependent signalling mediates anti-apoptotic effects of the atrial natriuretic peptide in ischemic livers. J Hepatol. 2004;41:414–420. doi: 10.1016/j.jhep.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 16.von Lintig FC, Pilz RB, Boss GR. Quantitative determination of Rap 1 activation in cyclic nucleotide-treated HL-60 leukemic cells: lack of Rap 1 activation in variant cells. Oncogene. 2000;19:4029–4034. doi: 10.1038/sj.onc.1203741. [DOI] [PubMed] [Google Scholar]
- 17.Ladetzki-Baehs K, Keller M, Kiemer AK, et al. Atrial natriuretic peptide, a regulator of nuclear factor-kappaB activation in vivo. Endocrinology. 2007;148:332–336. doi: 10.1210/en.2006-0935. [DOI] [PubMed] [Google Scholar]
- 18.Xing J, Birukova AA. ANP attenuates inflammatory signaling and Rho pathway of lung endothelial permeability induced by LPS and TNFalpha. Microvasc Res. 2010;79:26–62. doi: 10.1016/j.mvr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 20.Birukova AA, Fu P, Xing J, et al. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2010;298:L837–L848. doi: 10.1152/ajplung.00263.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivanna M, Srinivas SP. Microtubule stabilization opposes the (TNF-alpha)-induced loss in the barrier integrity of corneal endothelium. Experimental eye research. 2009;89:950–959. doi: 10.1016/j.exer.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 23.Waterman-Storer CM, Salmon E. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol. 1999;11:61–67. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
- 24.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of microtubule destabilizing activity of Op18/stathmin downstream of Rac1. J Biol Chem. 2004;279:6196–6203. doi: 10.1074/jbc.M307261200. [DOI] [PubMed] [Google Scholar]
- 25.Birukova AA, Xing J, Fu P, et al. Atrial natriuretic peptide attenuates LPS-induced lung vascular leak: role of PAK1. Am J Physiol Lung Cell Mol Physiol. 2010;299:L652–L663. doi: 10.1152/ajplung.00202.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birukova AA, Smurova K, Birukov KG, et al. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: Role of Rho-dependent mechanisms. J Cell Physiol. 2004;201:55–70. doi: 10.1002/jcp.20055. [DOI] [PubMed] [Google Scholar]
- 27.Komarova Y, De Groot CO, Grigoriev I, et al. Mammalian end binding proteins control persistent microtubule growth. J Cell Biol. 2009;184:691–706. doi: 10.1083/jcb.200807179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest. 2013;93:254–263. doi: 10.1038/labinvest.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birukov KG, Bochkov VN, Birukova AA, et al. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95:892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 30.Kakiashvili E, Speight P, Waheed F, et al. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem. 2009;284:11454–11466. doi: 10.1074/jbc.M805933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Mata R, Wennerberg K, Arthur WT, et al. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 2006;406:425–437. doi: 10.1016/S0076-6879(06)06031-9. [DOI] [PubMed] [Google Scholar]
- 32.Birukova AA, Birukov KG, Gorshkov B, et al. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol. 2005;289:L75–L84. doi: 10.1152/ajplung.00447.2004. [DOI] [PubMed] [Google Scholar]
- 33.Fu P, Birukova AA, Xing J, et al. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J. 2009;33:612–624. doi: 10.1183/09031936.00014808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meliton AY, Munoz NM, Meliton LN, et al. Mechanical induction of group V phospholipase A(2) causes lung inflammation and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;304:L689–L700. doi: 10.1152/ajplung.00047.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gradin HM, Larsson N, Marklund U, et al. Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J Cell Biol. 1998;140:131–141. doi: 10.1083/jcb.140.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leemans JC, Vervoordeldonk MJ, Florquin S, et al. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am J Respir Crit Care Med. 2002;165:1445–1450. doi: 10.1164/rccm.2106045. [DOI] [PubMed] [Google Scholar]
- 37.Xing J, Moldobaeva N, Birukova AA. Atrial natriuretic peptide protects against Staphylococcus aureus-induced lung injury and endothelial barrier dysfunction. J Appl Physiol. 2011;110:213–224. doi: 10.1152/japplphysiol.00284.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian X, Tian Y, Sarich N, et al. Novel role of stathmin in microtubule-dependent control of endothelial permeability. Faseb J. 2012;26:3862–3874. doi: 10.1096/fj.12-207746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian X, Tian Y, Gawlak G, et al. Control of vascular permeability by atrial natriuretic peptide via GEF-H1-dependent mechanism. J Biol Chem. 2014;289:5168–5183. doi: 10.1074/jbc.M113.493924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tasaka S, Koh H, Yamada W, et al. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol. 2005;32:504–510. doi: 10.1165/rcmb.2004-0009OC. [DOI] [PubMed] [Google Scholar]
- 41.Slotta JE, Braun OO, Menger MD, et al. Fasudil, a Rho-kinase inhibitor, inhibits leukocyte adhesion in inflamed large blood vessels in vivo. Inflamm Res. 2006;55:364–367. doi: 10.1007/s00011-006-6013-2. [DOI] [PubMed] [Google Scholar]
- 42.Potter LR. Domain analysis of human transmembrane guanylyl cyclase receptors: implications for regulation. Front Biosci. 2005;10:1205–1220. doi: 10.2741/1613. [DOI] [PubMed] [Google Scholar]
- 43.Ueda S, Nishio K, Akai Y, et al. Prognostic value of increased plasma levels of brain natriuretic peptide in patients with septic shock. Shock. 2006;26:134–139. doi: 10.1097/01.shk.0000226266.99960.d0. [DOI] [PubMed] [Google Scholar]
- 44.Kokot F, Klimek D, Wiecek A, et al. Atrial natriuretic peptide and arginine-vasopressin secretion in patients with active renal stone disease. Int Urol Nephrol. 1998;30:357–365. doi: 10.1007/BF02550323. [DOI] [PubMed] [Google Scholar]
- 45.Karmpaliotis D, Kirtane AJ, Ruisi CP, et al. Diagnostic and prognostic utility of brain natriuretic Peptide in subjects admitted to the ICU with hypoxic respiratory failure due to noncardiogenic and cardiogenic pulmonary edema. Chest. 2007;131:964–971. doi: 10.1378/chest.06-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doust JA, Pietrzak E, Dobson A, et al. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. Bmj. 2005;330:625. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rana R, Vlahakis NE, Daniels CE, et al. B-type natriuretic peptide in the assessment of acute lung injury and cardiogenic pulmonary edema. Crit Care Med. 2006;34:1941–1946. doi: 10.1097/01.CCM.0000220492.15645.47. [DOI] [PubMed] [Google Scholar]
- 48.Mitaka C, Hirata Y, Nagura T, et al. Beneficial effect of atrial natriuretic peptide on pulmonary gas exchange in patients with acute lung injury. Chest. 1998;114:223–228. doi: 10.1378/chest.114.1.223. [DOI] [PubMed] [Google Scholar]
- 49.Xing J, Yakubov B, Poroyko V, et al. Opposite effects of ANP receptors in attenuation of LPS-induced endothelial permeability and lung injury. Microvasc Res. 2012;83:194–199. doi: 10.1016/j.mvr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrache I, Birukova A, Ramirez SI, et al. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol. 2003;28:574–581. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- 51.Yamagata T, Yamagata Y, Nishimoto T, et al. The regulation of amiloride-sensitive epithelial sodium channels by tumor necrosis factor-alpha in injured lungs and alveolar type II cells. Respir Physiol Neurobiol. 2009;166:16–23. doi: 10.1016/j.resp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Moro C, Klimcakova E, Lolmede K, et al. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia. 2007;50:1038–1047. doi: 10.1007/s00125-007-0614-3. [DOI] [PubMed] [Google Scholar]
- 53.Weber NC, Blumenthal SB, Hartung T, et al. ANP inhibits TNF-alpha-induced endothelial MCP-1 expression--involvement of p38 MAPK and MKP-1. J Leukoc Biol. 2003;74:932–941. doi: 10.1189/jlb.0603254. [DOI] [PubMed] [Google Scholar]
- 54.Okazaki T, Himi T, Peterson C, et al. Induction of stathmin mRNA during liver regeneration. FEBS Lett. 1993;336:8–12. doi: 10.1016/0014-5793(93)81598-t. [DOI] [PubMed] [Google Scholar]
- 55.Rowlands DC, Harrison RF, Jones NA, et al. Stathmin is expressed by the proliferating hepatocytes during liver regeneration. Clinical molecular pathology. 1995;48:M88–M92. doi: 10.1136/mp.48.2.m88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo LJ, Alli AA, Eaton DC, et al. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am J Physiol Renal Physiol. 2013;304:F930–F937. doi: 10.1152/ajprenal.00638.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]