Abstract
The dopaminergic and renin angiotensin systems interact to regulate blood pressure. Disruption of the D4 dopamine receptor gene in mice produces hypertension that is associated with increased renal AT1 receptor expression. We hypothesize that the D4 receptor can inhibit AT1 receptor expression and function in renal proximal tubules (RPTs) cells from Wistar-Kyoto (WKY) rats but the D4 receptor regulation of AT1 receptor is aberrant in RPT cells from spontaneously hypertensive rats (SHRs). The D4 receptor agonist, PD168077, decreased AT1 receptor protein expression in a time and concentration-dependent manner in WKY cells. By contrast, in SHR cells, PD168077 increased AT1 receptor protein expression. The inhibitory effect of D4 receptor on AT1 receptor expression in WKY cells was blocked by a calcium channel blocker, nicardipine, or calcium-free medium, indicating that calcium is involved in the D4 receptor-mediated signaling pathway. Angiotensin II increased Na+-K+ ATPase activity in WKY cells. Pretreatment with PD168077 decreased the stimulatory effect of angiotensin II on Na+-K+ ATPase activity in WKY cells. In SHR cells, the inhibitory effect of D4 receptor on angiotensin II-mediated stimulation of Na+-K+ ATPase activity was aberrant; pretreatment with PD168077 augmented the stimulatory effect of AT1 receptor on Na+-K+ ATPase activity in SHR cells. This was confirmed in vivo; pre-treatment with PD128077 for one week augmented the anti-hypertensive and natriuretic effect of losartan in SHRs but not in WKY rats. We suggest that an aberrant interaction between D4 and AT1 receptors may play a role in the abnormal regulation of sodium excretion in hypertension.
Keywords: AT1 receptor, D3 receptor, renal proximal tubule cells, hypertension
Introduction
Essential hypertension is a major risk factor for stroke, myocardial infarction, heart failure, and kidney failure1. The kidney plays a major role in the long-term regulation of blood pressure, and abnormal sodium chloride metabolism is frequently encountered in hypertension2. Therefore, many studies have focused on the abnormal renal handling of sodium chloride in the pathogenesis of essential hypertension. Hypertensive subjects have increased sodium transport in several segments of the nephron, including the renal proximal tubule (RPT) and medullary thick ascending limb. The sodium retention in hypertension is due to enhanced sodium transport per se and/or a failure to respond appropriately to signals that decrease sodium transport.
Ion transport in the RPT and thick ascending limb of Henle, which is increased in essential hypertension, is regulated by numerous hormones and humoral factors, including angiotensin II and dopamine2, 3. Paracrine regulation of sodium reabsorption in the proximal tubule by the renin-angiotensin system occurs via several angiotensin receptor subtypes (AT1, and AT2)2, 3. The major effect of angiotensin II on sodium transport is stimulatory, via AT1 receptors. In the adult spontaneously hypertensive rat (SHR), renal AT1 receptor expression is similar to that found in normotensive rats but the AT1 receptor-mediated sodium reabsorption is increased in the RPT of SHRs4, 5. Proximal tubule fluid reabsorption/transport (NHE3 activity) is higher in SHRs than WKY rats at 5 weeks of age but may not be always increased at 12 weeks of age6-9. The ability of an angiotensin converting enzyme inhibitor to decrease proximal tubule fluid reabsorption has been reported to be greater in younger than older SHRs, indicating increased sensitivity to endogenous angiotensin II in the young SHR6, that may be related to increased renal AT1 receptors in the young5. However, the increased sensitivity of RPT transport to angiotensin II in the adult SHR4 is not due to increased renal expression of AT1R5.
The dopaminergic system also exerts a paracrine regulatory role on renal sodium transport in the RPT2, 3. Dopamine receptors, like AT1 receptor, are expressed in the brush border and basolateral membranes of the RPT3. In contrast to the stimulatory effect of the AT1 receptor on sodium transport in the RPT, the major consequence of the activation of dopamine receptors is inhibition of sodium transport2, 3. According to their structure and pharmacology, dopamine receptors are classified into D1-like (D1 and D5 receptors) and D2-like (D2, D3, and D4 receptors) subtypes. D1-like receptors stimulate, while D2-like receptors inhibit cAMP production3.
Increasing pieces of evidence show interaction between dopamine and angiotensin II receptors2. Our previous study also showed a negative interaction between the D3 and AT1 receptors, wherein activation of the D3 receptor inhibits AT1 receptor expression and function in RPT cells10. Disruption of the D4 dopamine receptor gene in mice produces hypertension that is associated with increased renal AT1 receptor expression11. The hypotensive effect of a bolus intravenous injection of the AT1 receptor antagonist losartan lasted longer in D4 receptor gene deficient mice than their wild-type littermates11. In the kidney, the D4 receptor is expressed in the proximal and distal convoluted tubules, collecting duct, and thick ascending limb of Henle in some species12. Because the RPT is responsible for about 70% of renal sodium reabsorption, we hypothesize that activation of the D4 receptor can inhibit AT1 receptor expression and function in the RPT from Wistar-Kyoto (WKY) rats, and their interaction may be aberrant in cells from SHRs. In order to test the above hypothesis, we studied D4 receptor and AT1 receptor interaction in immortalized RPT cells from WKY and SHRs. Meanwhile, the anti-hypertensive and natriuretic effect of AT1 receptor blocker with or without D4 receptor agonist in SHRs and WKY rats were also measured in vivo. These RPT cells behave similarly to freshly obtained RPT cells, at least with regard to dopamine receptors, the AT1 receptor, and responses to G protein stimulation12.
Methods
Cell Culture
Immortalized RPT cells from WKY and SHRs were cultured at 37°C in 95% air/5% CO2 atmosphere in DMEM/F-1210, 13. The cells (80% confluence) were extracted in ice-cold lysis buffer, sonicated, kept on ice for 1 hr, and centrifuged at 16,000 g for 30 min. All supernatant samples were stored at -70°C until use.
Preparation of kidney and RPT cells
The WKY and SHRs (Taconic, Germantown, NY) were anesthetized with pentobarbital (50 mg/kg, i.p.), after which the kidneys were removed and the rats sacrificed (intravenous pentobarbital, 100 mg/kg).
The renal cortices or cultured RPT cells were homogenized in ice-cold lysis buffer (PBS with 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L PMSF, 10 μg/ml aprotinin, and 10 μg /ml leupeptin), sonicated, kept on ice for 1 hr, and centrifuged at 16,000 g for 30 min. The supernatants were stored at -70°C until use for immunoblotting10, 11, 14-17. All experiments were approved by the Third Military Medical University Animal Use and Care Committee.
Immunoblotting
Polyclonal rabbit anti-AT1 receptor antibodies4 (1:500) and polyclonal goat anti-D4 receptor antibodies (1:300) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used.
Rat RPT cells were treated with vehicle (saline), D4 receptor agonist (PD168077)18, 19 (Tocris Cookson Ltd., Bristol, UK), or D4 receptor antagonist (L-745870)20 (Tocris Cookson Ltd., Bristol, UK), at the indicated concentrations and times. We designed our experiments so that a time control was not needed for each treatment period. Thirty-two hrs prior to cell lysis for immunoblotting, the cells were serum-starved. The cells were treated with PD168077 for 30hrs, 24hrs, 16hrs, 8hrs, 2hrs, or vehicle, as indicated. At 0 hr the drug-treated and vehicle-treated cells were prepared for immunoblotting. All cells were incubated for 32hrs including the control cells incubated with vehicle10.
Immunoblotting was performed as previously reported10, 11, 14-16 except that the transblots were probed with the AT1 receptor antibody (1:400). The amount of protein transferred onto the membranes was determined by immunoblotting for α-actin (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and used for the normalization of the receptor densities10.
Determination of the second messenger(s) involved in the D4 receptor-mediated regulation of AT1 receptor expression in WKY cells
To determine the second messenger(s) involved in the D4 receptor-mediated regulation of AT1 receptor expression in WKY cells, several inhibitors or agonists were used: protein kinase C (PKC) inhibitor (PKC inhibitor 19-31, 10-6M), protein kinase A (PKA) inhibitor (PKA inhibitor 14-22, 10-6M), and calcium channel blocker (nicardipine, 10-6M). Those reagents were added into the incubation medium 15 minutes prior to the addition of the D4 receptor agonist PD168077. The PKC inhibitor 19-31 and nicardipine were purchased from Sigma Co.; PKA inhibitor 14-22 was purchased from Calbiochem Company21-23 (Darmstadt, Germany).
Confocal microscopy of the double-stained kidney sections and RPT cells
Kidneys from WKY rats were fixed with 4% paraformaldehyde (30 min), embedded in paraffin, sectioned (4 μm), and mounted on slides. RPT cells, grown on coverslips, were fixed with 4% paraformaldehyde (30 min). The slides were incubated with rabbit anti-AT1 receptor and goat anti-D4 receptor antibodies (1: 100 dilution, Santa Cruz) overnight at 4°C, followed by FITC–conjugated mouse anti-goat IgG antibody (1:1000 dilution, green) and rhodamine–conjugated mouse anti-rabbit IgG antibody(1:1000 dilution, red; Jackson ImmunoResearch Laboratory, West Grove, Pa). Immunofluorescence images were acquired (Olympus AX70 laser confocal microscopy) at excitation wave-lengths of 350 nm and 507 nm; emission was detected at 450 and 529 nm. Cells or sections that were treated with only fluorescent-conjugated secondary antibodies revealed no immunofluorescence (data not shown).
Na+-K+-ATPase activity assay
Na+-K+-ATPase activity was determined as the rate of inorganic phosphate release in the presence or absence of ouabain24. Rat RPT cells were treated with vehicle (dH2O), and D4 receptor agonist (PD168077), at the indicated concentrations and durations of incubation. To prepare membranes for Na+-K+-ATPase activity assay, RPT cells cultured in 21 cm2 plastic culture dishes were collected and centrifuged at 3000g for 10 min. The cells were then placed on ice and lysed in 2 ml of lysis buffer (1 mM NaHCO3, 2 mM CaCl2 and 5 mM MgCl2). The cellular lysates were centrifuged at 3000g for 2 min to remove intact cells, debris, and nuclei. The resulting supernatant was suspended in an equal volume of 1 M sodium iodide, and the mixture was centrifuged at 48,000g for 25 min. The pellet (membrane fraction) was washed 2 times and suspended in 10 mM Tris containing 1 mM EDTA (pH 7.4). Protein concentrations were determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA, U.S.A.) and adjusted to 1 mg/ml. The membranes were stored at -70°C until further use.
Na+-K+-ATPase activity was measured by adding 100 μl of membrane fraction to an 800 μl reaction mixture consisting of 75 mM NaCl, 5 mM KCl, 5 mM MgCl2, 6 mM sodium azide, 1mM Na4EGTA, 37.5 mM imidazole, 75 mM Tris HCl, and 30 mM histidine; pH 7.4, in the presence or absence of 1 mM ouabain in a final volume of 1 ml and pre-incubated for 5 min in a water bath at 37°C. The reaction was initiated by adding 4 mM Tris ATP; after 15 min of incubation at 37°C the reaction was terminated by adding 50 μl of 50% trichloroacetic acid. Ouabain-insensitive ATPase activity was determined by omitting NaCl and KCl from the reaction mixtures with ouabain. The amount of phosphate produced was quantified by the addition of 1 ml of coloring reagent (10% ammonium molybdate in 10N sulfuric acid and ferrous sulfate mix buffer) to the reaction mixture. The mixture was then mixed thoroughly and centrifuged at 3000 g for 10 min. The resulting phosphomolybdate was quantified spectrophotometrically at 740 nm, using a standard curve prepared from K2HPO4. The difference between total and ouabain-insensitive ATPase activity was taken as Na+-K+-ATPase activity and expressed as nmol phosphate released per mg protein per min.
To eliminate the effect of proteases and phosphatases, protease inhibitors (1mM phenylmethylsulfonyl fluoride, 10 μg/ml each leupeptin and aprotinin) and a phosphatase inhibitor (50 μM sodium orthovanadate) were added in all solutions after drug/vehicle incubations25.
Determination of the effect of PD128077 and losartan on blood pressure, urine volume, and urine sodium of WKY and SHRs
The rats were pretreated with the D4 receptor agonist, PD168077 (10ng/kg/day, for one week), or vehicle (saline). After anesthesia with pentobarbital (50 mg/kg, i.p.) and cannulation of the left carotid artery, a thirty minute recovery was allowed before systolic blood pressure (SBP) from the carotid artery was measured, after which time losartan (0.3mg/kg) was given as a bolus intravenous injection via the carotid vein; SBP was then re-measured. Urine and sodium excretions were measured in rats kept in metabolic cages for 24hr before and after the injection of losartan via tail vein. Urine sodium was measured by an electrolyte analyzer.
Statistical analysis
The data are expressed as mean ± SEM. Comparison within groups was made by repeated measures ANOVA (or paired t-test when only 2 groups were compared), and comparison among groups (or t-test when only 2 groups were compared) was made by factorial ANOVA using the Holm-Sidak test. A value of P<0.05 was considered significant.
Results
D4 receptor localization in RPT cells and kidney RPTs
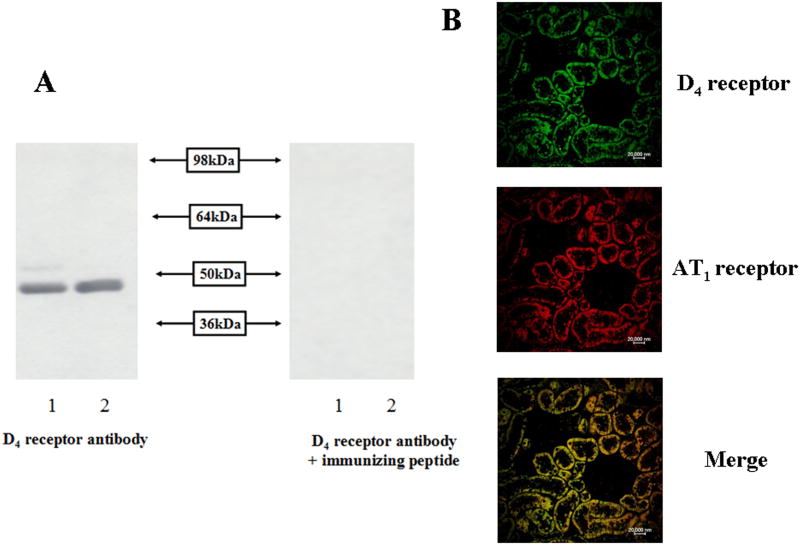
D4 receptor protein (49 kDa) in the RPT cells was detected by immunoblotting, using renal homogenates as positive control (Figure 1A). The 49 kDa band was D4 receptor protein specific because the 49 kDa band was no longer visible when the antibody was pre-adsorbed with the D4 receptor immunizing peptide. Immunofluorescence staining in the SD rat kidney also showed that the D4 and AT1 receptors colocalized in the RPT but neither D4 nor AT1 receptor was observed in the glomerulus (Figure 1B). The specificity of AT1 receptor antibody was verified in immotalized proximal tubule cells from AT1 receptor wild type (AT1R +/+) and knock-out (AT1R -/-) mouse by immunostaining (Figures S1A) and immunobloting (Figures S1B).
Figure 1.
D4 receptor expression in RPT cells and kidney RPTs.
A. D4 receptor protein expression in RPT cells determined by immunoblotting. RPT cell lysate proteins (lane 1) and renal homogenates (lane 2) (100 μg) from WKY rats were subjected to immunoblotting with anti-D4 receptor antibody (1:400). The 49 kDa band was no longer visible when the antibody was pre-adsorbed with the immunizing peptide (1:10 w/w incubation for 12 hrs).
B. Immunofluorescence staining of D4 and AT1 receptors in kidneys from SD rats. The kidney was washed, fixed, and immunostained for D4 and AT1 receptors, as described in the Methods. Colocalization appears as yellow after merging the images of D4 receptor (green) and AT1 receptor (red). These studies were repeated at least three times.
D4 receptor decreases AT1 receptor expression in WKY RPT cells
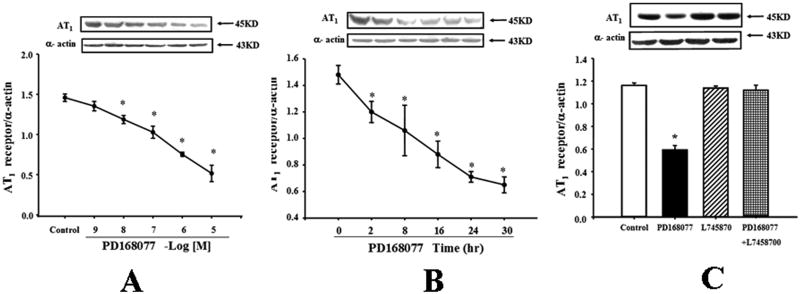
The D4 receptor agonist, PD168077, decreased AT1 receptor expression in a concentration- and time-dependent manner in WKY RPT cells. The inhibitory effect was evident at 10-8M (Figure 2A). The inhibitory effect of PD168077 (10-6 M) was noted as early as 2hrs and maintained for at least 30hrs (Figure 2B). The specificity of PD168077 as a D4 receptor agonist was also determined by studying the effect of the D4 receptor antagonist, L745870. Consistent with the study shown in Figures 2A and 2B, PD168077 (10-6M/24 hrs), decreased AT1 receptor expression. The D4 receptor antagonist, L745870 (10-6M), by itself, had no effect on AT1 receptor expression, but reversed the inhibitory effect of PD168077 on AT1 receptor expression (Figure 2C). To demonstrate that PD168077 and L745870 work as expected, we observed the effect of dopamine D4 receptor agonists and antagonists to cAMP accumulation in WKY RPT cells, and found PD168077 increased the cAMP accumulation and L-745870 inhibited the increased cAMP accumulation by PD168077, indicated the agonist and antagonist work as expected (Figures S2).
Figure 2.
Effect of a D4 receptor agonist, PD168077, on AT1 receptor expression in rat RPT cells.
A. Concentration-response of AT1 receptor protein expression in WKY cells treated with varying concentrations of a D4 receptor agonist, PD168077, for 24 hrs. Results are expressed as the ratio of AT1 receptor and α–actin densities (n = 5, *P<0.05 vs. control).
B. Time-course of AT1 receptor protein expression in WKY cells treated with a D4 receptor agonist, PD168077 (10-6M) for varying durations of incubation. Results are expressed as the ratio of AT1 receptor and α–actin densities (n = 6, *P<0.05 vs. control [0 time-point]).
C. Effect of a D4 receptor agonist (PD168077, PD) and a D4 receptor antagonist (L745870) on AT1 receptor expression. The cells were incubated with the indicated reagents (PD168077, 10-6M; L745870, 10-6M) for 24 hrs. Results are expressed as the ratio of AT1 receptor and α–actin densities (n = 4, *P<0.05 vs. others).
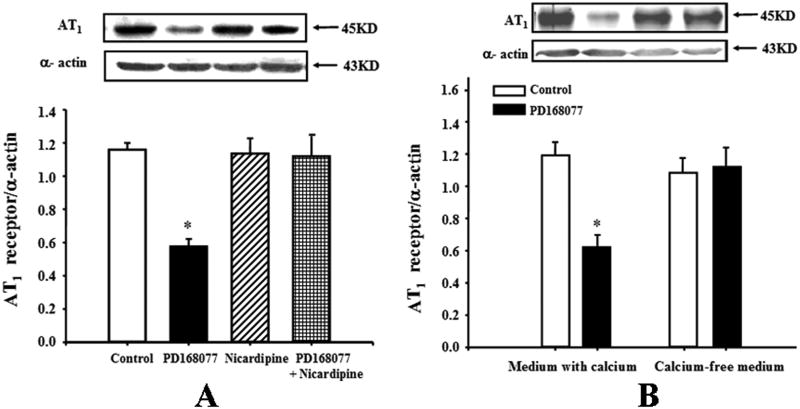
Calcium mediates the inhibitory effect of the D4 receptor on AT1 receptor expression in RPT cells
To investigate a mechanism of the D4 receptor down-regulation of AT1 receptor expression, RPT cells from WKY rats were treated with several agonists or antagonists. The calcium channel blocker nicardipine (10-6M), which had no effect on AT1 receptor expression by itself, blocked the inhibitory effect of D4 receptor on AT1 receptor expression in WKY cells (Figure 3A), indicating that calcium was involved as a signaling molecule in the D4 receptor-mediated signal transduction pathway. We also evaluated the involvement of other key cell signaling proteins with the use of a PKA inhibitor (PKA inhibitor 14-22, 10-6M) and PKC inhibitor (PKC inhibitor 19-31, 10-6M). None of these reagents was able to block the inhibitory effect of D4 receptor on AT1 receptor expression (data not shown).
Figure 3.
Calcium mediates the inhibitory effect of the D4 receptor on AT1 receptor expression in WKY RPT cells.
A. Effect of D4 receptor agonist, PD168077 (10-6M/24hrs) and calcium channel blocker, nicardipine (10-6M/24hrs), on AT1 receptor protein expression in WKY RPT cells. The cells were incubated with the indicated reagents. Results are expressed as the ratio of AT1 receptor and α–actin densities (n = 6, *P<0.05 vs. others).
B. Effect of a D4 receptor agonist, PD168077 (10-6M/24hrs), on AT1 receptor expression in WKY RPT cells incubated in medium with or without calcium. Results are expressed as the ratio of AT1 receptor and α–actin densities (n = 5, *P<0.05 vs. others).
To further determine, the importance of extracellular calcium entry in the D4 receptor-mediated down-regulation of AT1 receptor expression, we studied the effect of PD168077 on RPT cells grown in culture medium with or without calcium. The ability of PD168077 to down-regulate the expression of AT1 receptor protein was lost when the WKY RPT cells were maintained in calcium-free medium, indicating the requirement for cellular calcium entry in this action (Figure 3B).
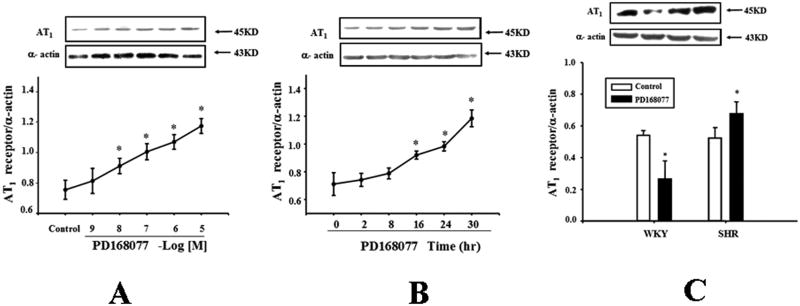
D4 receptor decreases AT1 receptor expression in WKY RPT cells but increases it in SHR RPT cells
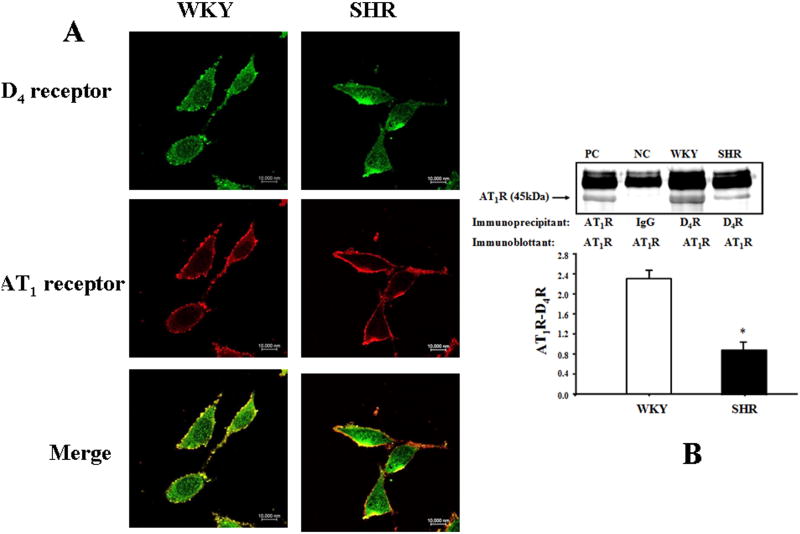
The D4 receptor differentially regulates AT1 receptor expression in WKY and SHRs, because PD168077 increased AT1 receptor expression in a concentration- and time-dependent manner in SHR RPT cells (Figures 4A and 4B), the opposite of that observed in WKY RPT cells (Figures 2 and 3). Additional studies confirmed that PD168077 (10-6M/24hrs) decreased AT1 receptor expression in WKY cells but increased AT1 receptor expression in SHR cells (Figure 4C). There was colocalization of D4 and AT1 receptors in RPT (Figure 1B) and RPT cells (Figure 5A). The co-immunoprecipitation of D4 receptor and AT1 receptor was lesser in SHR than WKY RPT cells (Figure 5B).
Figure 4.
Differential effects of D4 receptor on AT1 receptor expression in RPT cells from WKY and SHRs.
A. Concentration-response of AT1 receptor protein expression in SHR RPT cells treated with varying concentrations of a D4 receptor agonist, PD168077, for 24 hrs. Results are expressed as the ratio of AT1 receptor and α–actin densities (n = 6, *P<0.05 vs. control).
B. Time-course of AT1 receptor protein expression in SHR cells treated with a D4 receptor agonist, PD168077 (10-6M) for varying durations of incubation. Results are expressed as the ratio of AT1 receptor and α–actin densities (n = 6, *P<0.05 vs. control (0 time-point)).
C. Effect of a D4 receptor agonist (PD168077; PD) and a D4 receptor antagonist (L745870) on AT1 receptor expression. WKY and RPT cells were incubated with PD168077 (10-6M) for 24hrs. Results are expressed as the ratio of AT1 receptor and α–actin densities (n = 8, *P<0.05 vs. control).
Figure 5.
Colocalization and co-immunoprecipitation of D4 and AT1 receptors in WKY and SHR RPT cells.
A. Colocalization of D4 and AT1 receptors in WKY and SHR RPT cells. The cells were washed, fixed, and immunostained for D4 receptor and AT1 receptor, as described in the Methods. Colocalization appears as yellow after merging the images of FITC-tagged D4 receptor (green) and rhodamine-tagged AT1 receptor (red).
B. Co-immunoprecipitation of D4 and AT1 receptors in WKY and SHR RPT cells. The cells were immunoprecipitated with D4 receptor antibodies and immunoblotted with AT1 receptor antibodies (*P<0.05 vs. WKY, n=3). One immunoblot (43 kDa) is depicted in the inset: (PC = positive control, NC = negative control). For the positive control, AT1 receptor antibody was used and for the negative control, IgG was used instead of D4 receptor antibody as the immunoprecipitant.
Pretreatment with D4 receptor agonist PD168077 decreases the stimulatory effect of AT1 receptor on Na+-K+ ATPase activity in WKY RPT but not in SHR RPT cells
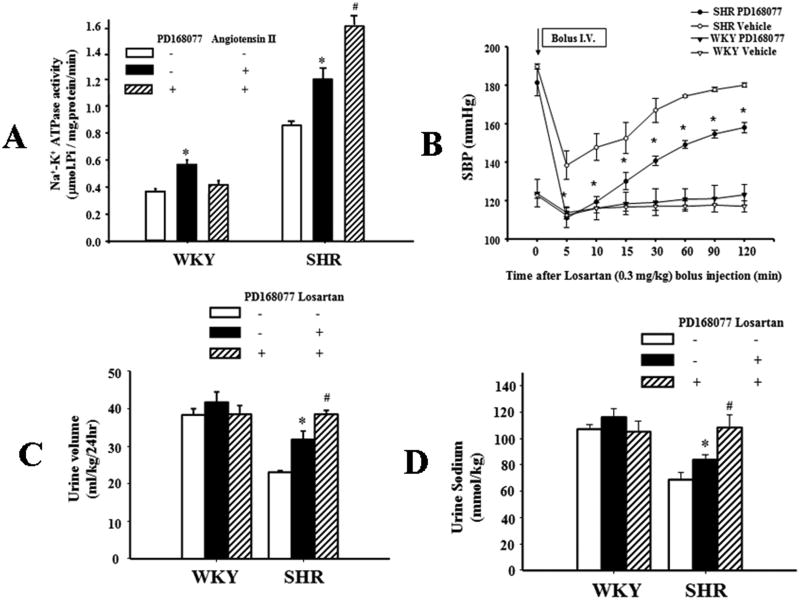
To investigate the physiological significance of D4/AT1 receptor interaction, the effects of D4 and/or AT1 receptor stimulation on Na+-K+ ATPase activity were determined in WKY and SHR RPT cells. Stimulation of AT1 receptors by angiotensin II (10-11M/15 min) increased Na+-K+ ATPase activities in WKY and SHR cells. However, pretreatment with PD168077 (10-6M) for 24hrs, decreased the stimulatory effect of angiotensin II (10-11M/15 min) on Na+-K+ ATPase activity in WKY cells, but increased it in SHR cells (Figure 6A), which could be accounted for by the disparate regulation of AT1 receptor expression by D4 receptor in WKY and SHR cells, as shown in Figures 2 and 4. Additional studies showed that the intravenous infusion of losartan (0.3mg/kg) significantly lowered SBP (Figure 6B) and increased urine volume and sodium excretion (Figures 6C and 6D) in PD168077 (10ng/kg/day, for one week)-treated SHRs but not similarly treated WKY rats. Because the D4 receptor expression in the kidney and RPT cells is increased in the SHR (Figures S3A and S3B), consistent with a previous report26, these data could be taken to indicate that the increase in renal D4 receptor expression in the SHR may be an attempt to compensate for the aberrant D4 receptor function, e.g., decreased diuretic and natriuretic effects of the D4 receptor agonist, PD168077 in the SHR (Figure S4).
Figure 6.
Effect of D4 receptor agonist on the function of the AT1 receptor.
A. Effect of pretreatment with D4 receptor agonist on the stimulatory effect of AT1 receptor on Na+-K+-ATPase activity in WKY and SHR RPT cells. The cells were pretreated with D4 receptor agonist, PD168077 (10-6M/24hrs), or vehicle (dH2O) for 24 hrs. After washing for 15 mins, the cells were treated with angiotensin II (10-11M) for 15 mins. Results are expressed as μmol phosphate released per mg protein per min (*P<0.05 vs. Control, #P<0.05 vs. angiotensin II alone, n = 8/group).
B. Effect of AT1 receptor blockade on systolic blood pressure (SBP) in WKY and SHRs with long-term D4 receptor agonist treatment. The rats were pretreated with D4 receptor agonist, PD168077 (10ng/kg/day), or vehicle (saline) for one week. Losartan (0.3mg/kg) lowered SBP to a greater extent in PD168077- than vehicle-treated SHRs. The mice were anesthetized with pentobarbital and SBPs measured from the left carotid artery. SBPs were obtained after a 30 min stabilization period (n =3, *P<0.05 vs. vehicle treated SHR or WKY rats at the same time point).
C. Effect of a D4 receptor agonist, PD168077, and AT1 receptor antagonist on urine volume in WKY and SHRs. PD168077 and losartan were used as in Figure 6B. Twenty-four hour urine volume (normalized by body weight) was measured in rats kept in metabolic cages after losartan (0.3mg/kg) or vehicle injection (n =5, *P<0.05 vs. vehicle-treated SHRs; #P<0.05 vs. losartan treated SHRs).
D. Effect of PD168077 and AT1R antagonist on urine sodium in WKY and SHRs. Urine sodium excretion was measured in loasrtan (0.3mg/kg)- or vehicle- treated rats (n =5, *P<0.05 vs. vehicle-treated SHRs; #P<0.05 vs. losartan treated SHRs).
Discussion
Dopamine and angiotensin II are two important regulators of sodium and water transport in the kidney serving counteracting functions2, 3, 10, 11, 14, 16. Stimulation of the D1-like dopamine receptor inhibits renal renin secretion via inhibition of macula densa cyclo-oxygenase27. By contrast, in rats on low-salt diet, angiotensin II decreases renal dopamine production by increasing renal monoamine oxidase activity28. The inhibitory effects of dopamine receptor on the renin-angiotensin system (RAS) extend to the receptor level. D1-like or D2-like receptors inhibit AT1 receptor-mediated stimulation of sodium transport in the RPT.29, 30 D2-like receptors are comprised of three subtypes, D2, D3, and D4 receptors. It is not known which subtype(s) is involved in this action. Presynaptic D2 receptor is present in nerve cells, while post-synaptic D2, D3, and D4 receptors exist in RPT cells12. Our previous study found that activation of the D3 receptor decreases AT1 receptor expression in WKY RPT cells10. Whether or not the D4 receptor can inhibit AT1 receptor expression is not known. However, there is indirect evidence of a negative D4 and AT1 receptor interaction in D4 receptor null (D4-/-) mice. Renal AT1 receptor expression is higher in D4-/- mice than in the wild-type littermates and the extent and duration of the hypotensive effect of AT1 receptor blockade is greater and longer in these D4 receptor deficient mice11. We hypothesize that the D4 receptor may have an inhibitory effect on AT1 receptor expression and function in kidney and an aberrant interaction between D4 and AT1 receptor is involved into the pathogenesis of hypertension. This hypothesis was tested in this study; we found that stimulation of D4 receptor inhibits AT1 receptor expression in WKY RPT cells but increases it in SHR RPT cells. Pretreatment of WKY RPT cells with a D4 receptor agonist for 24 hrs inhibits AT1 receptor expression, thereby inhibiting the AT1-stimulated Na+-K+ ATPase activity. By contrast in SHR RPT cells, due to the stimulatory effect of the D4 receptor on AT1 receptor expression, pretreatment for 24 hrs with D4 receptor agonist augments the AT1 receptor-mediated stimulation of Na+-K+ ATPase activity, which would increase renal sodium reabsorption, and lead to increased blood pressure in vivo.
The regulation of AT1 receptor expression by the D4 receptor could be via direct or indirect mechanisms. It is known that angiotensin II can regulate AT1 receptor expression15 and the activation of a D2-like receptor, D3 receptor, inhibits the renin release16. The D4 receptor is also expressed in the RPT cells11 but whether or not the D4 receptor can affect angiotensin II synthesis in RPT cells is not known. Our previous study showed no difference in plasma renin concentration between D4-/- and D4+/+ mice11. Our present study did not find a difference in renin concentration in the culture medium of WKY RPT cells in the presence or absence of D4 receptor agonist (data not shown). We, therefore, suggest that the D4 receptor, independent of angiotensin II, can regulate AT1 receptor expression.
The mechanism for the decrease in AT1 receptor expression caused by the D4 receptor in WKY rats was also investigated in this study. Calcium plays an important role in the regulation of AT1 receptor expression; a high calcium diet decreases AT1 receptor expression in the rat kidney17. Elevation of [Ca2+]i in RPT cells leads to down-regulation of AT1 receptors in kidney of diabetic rats, and normalization of the [Ca2+]i by treatment of the diabetic rats with calcium channel blocker, amlodipine, prevents the elevation of [Ca2+]i and down-regulation of AT1 receptor protein and mRNA expressions31. Our present study found that a decrease in intracellular calcium, caused by a calcium channel blocker or calcium-free medium, blocks the inhibitory effect of the D4 receptor on AT1 receptor expression in WKY cells. This indicates that calcium is involved as a signaling molecule in the D4 receptor-mediated down-regulation of AT1 receptor expression in WKY cells.
In summary, we have demonstrated that the D4 receptor down-regulates AT1 receptor expression in WKY RPT cells via the activation of the calcium channel. The regulation of the AT1 receptor by the D4 receptor has physiological significance. Pretreatment of WKY RPT cells with a D4 receptor agonist for 24hrs reduces the stimulatory effect of AT1 receptor on Na+-K+ ATPase activity. However, in SHR RPT cells, the regulation of D4 receptor of AT1 receptor expression and function is aberrant; D4 receptor stimulation (PD128077) increases AT1 receptor expression and augments the stimulatory effect of angiotensin II on Na+-K+ ATPase activity. Pre-treatment with PD128077 for one week augments the anti-hypertensive, diuretic, and natriuretic effects of losartan in SHRs. An aberrant interaction between D4 and AT1 receptors may be involved in the pathogenesis of genetic hypertension.
Perspectives
Dopamine, produced in neural and non-neural tissues, is now recognized to serve an important role in the regulation of sodium balance and blood pressure. According to their structure and pharmacology, dopamine receptors are classified into D1-like (D1 and D5) and D2-like (D2, D3, and D4) receptors. D1-like receptors stimulate, while D2-like receptors inhibit cAMP production3, 12. Previous studies have found that the activation of D1-like or D2-like receptor inhibits AT1 receptor-mediated stimulation of sodium transport in RPTs and RPT brush border membranes29, 30. Other studies have also shown that stimulation of the D1, D3, and Ds receptors inhibits AT1 receptor expression and function10, 12, 14, 15. Our present study found that the D4 receptor, similar to D1, D3 and D5 receptors, is also involved in this process. Because D1-like and D2-like receptors synergistically increase sodium excretion32, it is possible that the D4 receptor, together with the D1, D3, and Ds receptors, synergistically inhibits AT1 receptor expression and function in WKY RPT cells. However, this conjecture needs to be confirmed in future studies.
Supplementary Material
Novelty and Significance.
What Is New?
The dopaminergic and renin angiotensin systems interact to regulate blood pressure. Disruption of the D4 dopamine receptor gene in mice produces hypertension that is associated with increased renal AT1 receptor expression. In these studies, we found that the D4 receptor down-regulates AT1 receptor expression in WKY RPT cells via the activation of the calcium channel. The regulation of the AT1 receptor by the D4 receptor has physiological significance. Pretreatment of WKY RPT cells with a D4 receptor agonist for 24hrs reduces the stimulatory effect of AT1 receptor on Na+-K+ ATPase activity. However, in SHR RPT cells, the regulation of D4 receptor of AT1 receptor expression and function is aberrant; D4 receptor stimulation (PD128077) increases AT1 receptor expression and augments the stimulatory effect of angiotensin II on Na+-K+ ATPase activity. Pre-treatment with PD128077 for one week augments the anti-hypertensive, diuretic, and natriuretic effects of losartan in SHRs. We suggest that an aberrant interaction between D4 and AT1 receptors may play a role in the abnormal regulation of sodium excretion in hypertension.
What Is Relevant?
The present study reinforces the role of dopamine D4 receptor in hypertension and shows that the different regulation of D4 receptor on AT1 receptor expression and function in WKY rats and SHRs. The aberrant interaction between D4 and AT1 receptors may be involved in the pathogenesis of genetic hypertension. The results imply that the regulation of the AT1 receptor by the D4 receptor may be an effective therapeutic approach for essential hypertension.
Summary.
The present study reinforces the role of renal dopamine receptor in hypertension and shows the D4 receptor down-regulates AT1 receptor expression in WKY RPT cells via the activation of the calcium channel. However, the regulation of D4 receptor of AT1 receptor expression and function is aberrant in hypertension. The aberrant interaction between D4 and AT1 receptors may be involved in the pathogenesis of genetic hypertension.
Acknowledgments
Sources of Funding: These studies were supported in part by grants from the National Natural Science Foundation of China (31130029, 30925018), the National Basic Research Program of China (2012CB517801), and grant from the US National Institutes of Health, 5P01HL074940.
Footnotes
Disclosures: No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CV, Cohen DL, Cadet JC, Jean-Charles RR, Taler S, Kountz D, Townsend R, Chalmers J, Ramirez AJ, Bakris GL, Wang J, Schutte AE, Bisognano JD, Touyz RM, Sica D, Harrap SB. Clinical practice guidelines for the management of hypertension in the community a statement by the american society of hypertension and the international society of hypertension. J Hypertens. 2014;32:3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz PA, Garvin JL. Intrarenal transport and vasoactive substances in hypertension. Hypertension. 2001;38:621–624. doi: 10.1161/hy09t1.093361. [DOI] [PubMed] [Google Scholar]
- 3.Asghar M, Tayebati SK, Lokhandwala MF, Hussain T. Potential dopamine-1 receptor stimulation in hypertension management. Curr Hypertens Rep. 2011;13:294–302. doi: 10.1007/s11906-011-0211-1. [DOI] [PubMed] [Google Scholar]
- 4.Thomas D, Harris PJ, Morgan TO. Altered responsiveness of proximal tubule fluid reabsorption of peritubular angiotensin II in spontaneously hypertensive rats. J Hypertens. 1990;8:407–410. doi: 10.1097/00004872-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Cheng HF, Wang JL, Vinson GP, Harris RC. Young SHR express increased type 1 angiotensin II receptors in renal proximal tubule. Am J Physiol. 1998;274:F10–17. doi: 10.1152/ajprenal.1998.274.1.F10. [DOI] [PubMed] [Google Scholar]
- 6.Thomas D, Harris PJ, Morgan TO. Age-related changes in angiotensin II-stimulated proximal tubule fluid reabsorption in the spontaneously hypertensive rat. J Hypertens Suppl. 1988;6:S449–451. doi: 10.1097/00004872-198812040-00141. [DOI] [PubMed] [Google Scholar]
- 7.LaPointe MS, Sodhi C, Sahai A, Batlle D. Na+/H+ exchange activity and NHE-3 expression in renal tubules from the spontaneously hypertensive rat. Kidney Int. 2002;62:157–165. doi: 10.1046/j.1523-1755.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 8.Crajoinas RO, Lessa LM, Carraro-Lacroix LR, Davel AP, Pacheco BP, Rossoni LV, Malnic G, Girardi AC. Posttranslational mechanisms associated with reduced NHE3 activity in adult vs. Young prehypertensive shr. Am J Physiol Renal Physiol. 2010;299:F872–881. doi: 10.1152/ajprenal.00654.2009. [DOI] [PubMed] [Google Scholar]
- 9.Li XX, Xu J, Zheng S, Albrecht FE, Robillard JE, Eisner GM, Jose PA. D1 dopamine receptor regulation of NHE3 during development in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1650–1656. doi: 10.1152/ajpregu.2001.280.6.R1650. [DOI] [PubMed] [Google Scholar]
- 10.Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- 11.Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, Jose PA. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension. 2006;47:288–295. doi: 10.1161/01.HYP.0000198427.96225.36. [DOI] [PubMed] [Google Scholar]
- 12.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: Studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–569. doi: 10.1152/ajpheart.01036.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parenti A, Cui XL, Hopfer U, Ziche M, Douglas JG. Activation of MAPKS in proximal tubule cells from spontaneously hypertensive and control Wistar-Kyoto rats. Hypertension. 2000;35:1160–1166. doi: 10.1161/01.hyp.35.5.1160. [DOI] [PubMed] [Google Scholar]
- 14.Gildea JJ, Wang X, Jose PA, Felder RA. Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension. 2008;51:360–366. doi: 10.1161/HYPERTENSIONAHA.107.100099. [DOI] [PubMed] [Google Scholar]
- 15.Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, Jose PA. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45:804–810. doi: 10.1161/01.HYP.0000155212.33212.99. [DOI] [PubMed] [Google Scholar]
- 16.Sanada H, Yao L, Jose PA, Carey RM, Felder RA. Dopamine D3 receptors in rat juxtaglomerular cells. Clin Exp Hypertens. 1997;19:93–105. doi: 10.3109/10641969709080807. [DOI] [PubMed] [Google Scholar]
- 17.Porsti I, Fan M, Koobi P, Jolma P, Kalliovalkama J, Vehmas TI, Helin H, Holthofer H, Mervaala E, Nyman T, Tikkanen I. High calcium diet down-regulates kidney angiotensin-converting enzyme in experimental renal failure. Kidney Int. 2004;66:2155–2166. doi: 10.1111/j.1523-1755.2004.66006.x. [DOI] [PubMed] [Google Scholar]
- 18.Polakowski JS, Segreti JA, Cox BF, Hsieh GC, Kolasa T, Moreland RB, Brioni JD. Effects of selective dopamine receptor subtype agonists on cardiac contractility and regional haemodynamics in rats. Clin Exp Pharmacol Physiol. 2004;31:837–841. doi: 10.1111/j.1440-1681.2004.04095.x. [DOI] [PubMed] [Google Scholar]
- 19.Gu Z, Yan Z. Bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II activity by dopamine D4 receptors in prefrontal cortex. Mol Pharmacol. 2004;66:948–955. doi: 10.1124/mol.104.001404. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Okada Y, Kanno T, Otomo A, Yanagisawa Y, Shouguchi-Miyata J, Suga E, Kohiki E, Onoe K, Osuga H, Aoki M, Hadano S, Itoyama Y, Ikeda JE. A dopamine receptor antagonist l-745,870 suppresses microglia activation in spinal cord and mitigates the progression in ALS model mice. Exp Neurol. 2008;211:378–386. doi: 10.1016/j.expneurol.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Cui Z, He D, Ren H, Han Y, Yu C, Fu C, Wang Z, Yang C, Wang X, Zhou L, Asico LD, Villar VA, Hopfer U, Mi M, Zeng C, Jose PA. Insulin increases D5 dopamine receptor expression and function in renal proximal tubule cells from Wistar-Kyoto rats. Am J Hypertens. 2009;22:770–776. doi: 10.1038/ajh.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YJ, Park SH, Han HJ. ATP stimulates Na+-glucose cotransporter activity via cAMP and p38 MAPK in renal proximal tubule cells. Am J Physiol Cell Physiol. 2005;289:C1268–1276. doi: 10.1152/ajpcell.00002.2005. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Yang J, Ren H, He D, Pascua A, Armando MI, Yang C, Zhou L, Felder RA, Jose PA, Zeng C. Inhibitory effect of ETNreceptor on Na+-K+ ATPase activity by extracellular Ca2+ entry and Ca2+ release from the endoplasmic reticulum in renal proximal tubule cells. Hypertens Res. 2009;32:846–852. doi: 10.1038/hr.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotlo K, Shukla S, Tawar U, Skidgel RA, Danziger RS. Aminopeptidase n reduces basolateral Na+ -K+ -ATPase in proximal tubule cells. Am J Physiol Renal Physiol. 2007;293:F1047–1053. doi: 10.1152/ajprenal.00074.2007. [DOI] [PubMed] [Google Scholar]
- 25.Sorbel JD, Brooks DM, Lurie DI. Shp-1 expression in avian mixed neural/glial cultures. J Neurosci Res. 2002;68:703–715. doi: 10.1002/jnr.10262. [DOI] [PubMed] [Google Scholar]
- 26.Shin Y, Kumar U, Patel Y, Patel SC, Sidhu A. Differential expression of D2-like dopamine receptors in the kidney of the spontaneously hypertensive rat. J Hypertens. 2003;21:199–207. doi: 10.1097/00004872-200301000-00030. [DOI] [PubMed] [Google Scholar]
- 27.Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53:564–570. doi: 10.1161/HYPERTENSIONAHA.108.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Luca Sarobe V, Nowicki S, Carranza A, Levin G, Barontini M, Arrizurieta E, Ibarra FR. Low sodium intake induces an increase in renal monoamine oxidase activity in the rat. Involvement of an angiotensin ii dependent mechanism. Acta Physiol Scand. 2005;185:161–167. doi: 10.1111/j.1365-201X.2005.01473.x. [DOI] [PubMed] [Google Scholar]
- 29.Hussain T, Abdul-Wahab R, Kotak DK, Lokhandwala MF. Bromocriptine regulates angiotensin II response on sodium pump in proximal tubules. Hypertension. 1998;32:1054–1059. doi: 10.1161/01.hyp.32.6.1054. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh-Hamad D, Wang YP, Jo OD, Yanagawa N. Dopamine antagonizes the actions of angiotensin II in renal brush-border membrane. Am J Physiol. 1993;264:F737–743. doi: 10.1152/ajprenal.1993.264.4.F737. [DOI] [PubMed] [Google Scholar]
- 31.Marcinkowski W, Zhang G, Smogorzewski M, Massry SG. Elevation of [Ca2+]i of renal proximal tubular cells and down-regulation of mRnA of PTH-PTHRP, V1a and AT1 receptors in kidney of diabetic rats. Kidney Int. 1997;51:1950–1955. doi: 10.1038/ki.1997.266. [DOI] [PubMed] [Google Scholar]
- 32.Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1071–1078. doi: 10.1152/ajpregu.2001.281.4.R1071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.