Abstract
Mouse embryonic stem cells (ESCs) can be transfected by electroporation, liposomal reagents, and viral transduction methods. The cationic polymer polyethylenimine (PEI) has been shown to transfect a variety of differentiated mammalian cell types, including mouse ESCs, but existing methods require the use of additional equipment that is not readily accessible to most labs. Here we describe conditions that permit for the efficient transfection of mouse ESCs with low cytotoxicity and without the need for specialized equipment. Our goal was to devise a protocol for the PEI-mediated transfection of mouse ESCs that was comparable in ease to commercial transfection reagents. For these studies, we compared PEI transfection efficiency and cytotoxicity to a well-known liposomal transfection reagent, Lipofectamine2000™ (LF2K), using fluorescence microscopy, flow cytometry, cell viability assays, and western blotting. We provide evidence that PEI transfection of mouse ESCs compares favorably to LF2K. Our optimized protocol for efficient transfection of mouse ESCs with PEI is detailed in this report.
Keywords: Polyethylenimine, Mouse embryonic stem cells, Transfection
Introduction
Gene delivery systems are commonly used to transfect mammalian cells both transiently and stably for downstream analyses [1]. Transfection efficiency is celltype specific and generally depends on the reagent. The most common techniques utilize liposomes, viruses, chemicals, and electroporation. Regardless of the gene delivery system, two critical goals are achieving high transfection efficiency and minimizing cytotoxicity. For mouse embryonic stem cells (ESCs), lipid-based reagents, such as Lipofectamine2000™ (LF2K), are often used because they meet both of these criteria. Despite their effectiveness, the cost of commercial lipid-based reagents can be a limiting factor for labs that work with mouse ESCs.
Linear 25 kDa polyethylenimine (PEI) is a relatively inexpensive cationic polymer that has been shown to be a suitable transfection reagent for several types of differentiated mammalian cells used in many labs, including but not limited to human embryonic kidney cells (HEK293) [2–6], African green monkey kidney cells (Cos-7), human cervical epithelial carcinoma cells (HeLa) [7], and human embryonal carcinoma cells (NCCIT) [2]. Comparative studies have been performed to assay PEI transfection efficiency compared to liposomal gene delivery, electroporation, and other chemical reagents [2, 3, 7]. While PEI has been used in many cell types, its utility has only begun to be demonstrated for mouse ESCs [8, 9].
In this study, we set out to determine if PEI could be used for transfection of mouse ESCs in a manner similar to LF2K, and if so, to optimize the transfection conditions. By using LF2K transfection conditions, and then adjusting a number of the parameters, such as the amount of plasmid DNA, the concentration of PEI, the serum concentration, the final volume, and the order of component addition, we were able to determine the optimal transfection efficiency combined with low cytotoxicity. Flow cytometry was used to quantify the percentage of ESCs transfected with green fluorescent protein (GFP) using both LF2K and PEI. We also used Western blotting to show the efficient transfection and expression of unrelated plasmids. To ensure that the pluripotency of the ESCs remained unaffected by transfection, we measured the expression of both Oct4 (Pou5f1) and Nanog by RT-qPCR. While LF2K transfects ESCs with high efficiency and low cytotoxicity, PEI also transfects ESCs with high efficiency, maintains the expression of Oct4 and Nanog, while having the added benefit being slightly less toxic to ESCs. The protocol we developed for using cost-effective PEI results in approximately 34% of mouse ESCs being transfected, making PEI an alternative transfection reagent for mouse ESCs that performs comparably to commercial reagents such as LF2K.
Materials and Methods
Cell Culture
Feeder-free wild-type mouse ESCs (E14K) were grown on 0.1% gelatin-coated plates with high glucose DMEM (Invitrogen) supplemented with 15% fetal bovine serum (HyClone), 1% non-essential amino acids, 1% sodium pyruvate, 1% Lglutamine, 1% penicillin/streptomycin (Gibco) 55 µM 2-mercaptoethanol, and 1000 units/mL recombinant leukemia inhibitory factor (LIF) [10] or ESGRO (Millipore). Media was replenished every other day.
Microscopy
ESCs were visualized 24 hours post transfection using an Advanced Microscopy Group (AMG) Evos fluorescent microscope. Images presented in this manuscript are under 10-fold magnification using either transmitted light or a green fluorescent protein filter.
Flow Cytometry
Twenty-four hours post-transfection, ESCs were washed once with PBS, trypsinized, and centrifuged at 1500 rpm for 2 min. ESCs were resuspended in PBS supplemented with 0.1 mM EDTA to prevent clumping. A BD Accuri C6 flow cytometer was used to detect 2 µg pMaxGFP transfected in WT mESCs. The detection threshold was set using a ddH2O blank run to eliminate spurious events. 200,000 events were collected per sample. Gating was based on untransfected ESCs and percent transfected ESCs were calculated after the gating was set. Each sample was assayed in triplicate.
Transfection Reagents
Linear polyethylenimine was obtained from Polysciences, Inc. (cat. no. 23966). A stock solution of 40 µM PEI was prepared in 25 mM HEPES buffer [140 mM NaCl, 1.5 mM Na2HPO4, and pH adjusted to 7.05 using 5N NaOH]. The solution was sterilized through a 0.2 µM filter and stored at −20°C. Lipofectamine2000™ was purchased from Life Technologies (cat. no. 11668027).
Lipofectamine2000™ Transfection Protocol
The ESC transfection protocol, provided by Dr. Brad Doble (McMaster University), was adapted from [11]. 2 µg DNA was diluted in 50 µL Opti-MEM and 4 µL LF2K was diluted in 46 µL Opti-MEM per transfection reaction for a 6-well plate. LF2K:Opti-MEM was incubated at room temperature for 5 min. DNA:Opti- MEM was combined with LF2K:Opti-MEM and incubated at room temperature for 20 min. During the DNA:LF2K:Opti-MEM incubation, 10 cm confluent dishes of wild-type mouse ESCs were trypsinized and centrifuged at 1500 rpm for 2 min. Cells were resuspended in complete ESC growth media and live cells were counted using 0.4% Trypan Blue and a Countess Automated Cell Counter (Invitrogen). 1 × 106 cells per 6-well transfection reaction were aliquoted and centrifuged at 1500 rpm for 2 min. ESCs were resuspended in the DNA:LF2K:Opti-MEM mixture. The transfection reaction was plated in a 6-well plate with complete ESC media.
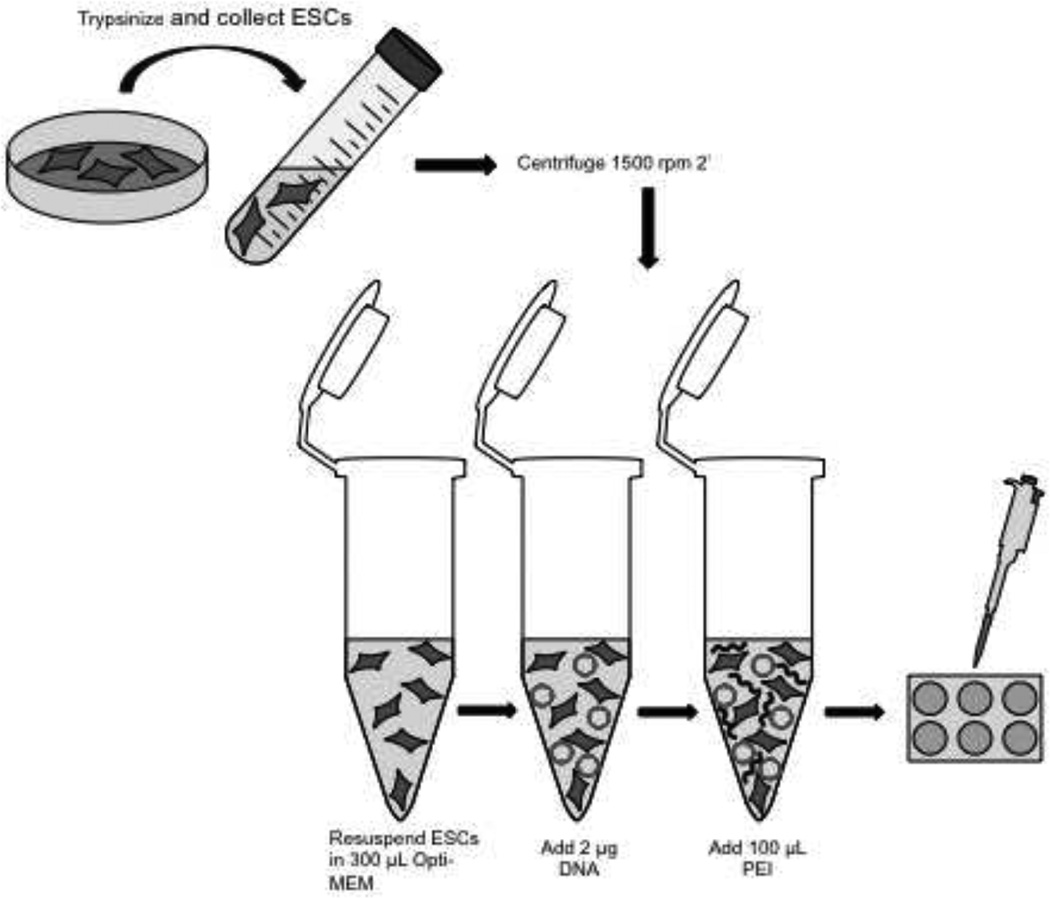
Modified PEI Transfection Protocol
1 × 106 cells per 6-well transfection reaction were aliquoted and centrifuged at 1500 rpm for 2 min. ESCs were immediately resuspended in 300 µL Opti-MEM and mixed by pipetting. 2 µg plasmid DNA then added to cells and gently mixed. 100 µL PEI added to ESC:DNA:Opti-MEM mixture and mixed by gentle pipetting, then incubated at room temperature for 30 min. ESC:DNA:Opti-MEM mixture was then added to single well of 6-well plate.
Protein Isolation and Western Blot
Protein expression in LF2K and PEI-transfected ESCs was compared by Western blotting. ESCs were transfected with 200 ng pMaxGFP and 1800 ng Gsk-3β pDEST40. ESCs were resuspended in lysis buffer (137 mM NaCl, 10 mM Tris, pH 7.4, 1% Nonidet P-40) containing protease inhibitor cocktail (1:100 Sigma) and lysed by vigorous vortexing. Lysates were electrophoresed (25 µg/lane) through 7.5% Tris/Tricine gels and transferred onto nitrocellulose membranes at 100V for 1 h. Blots were blocked for 1 h with either 5% milk (V5 antibody)/TBST (150 mM NaCl, 50 mM Tris, pH 7.4, 0.1% Tween) or 5% BSA (tubulin)/TBST. Blots were incubated in primary antibody diluted in 5% milk/TBST or 5% BSA/TBST for 22 h at 4°C. Anti-V5 mouse mAb (Invitrogen cat. no. R960-25) was diluted 1:5000 and anti-tubulin rabbit mAb (Cell Signaling Technologies cat. no. 2125) was diluted 1:1000. Blots were incubated in anti-mouse or anti-rabbit IgG HRP secondary antibody (GE Healthcare) diluted 1:5000 in 5% milk/TBS or 5% BSA/TBST for 30 min. Proteins were visualized using ECL Plus detection reagents (GE Healthcare). Blots were stripped in a buffer consisting of 2% SDS, 62.5 mM Tris-HCl. pH 6.7, and 100 mM 2-mercaptoethanol at 55°C for 30 min followed by repeated rinsing with TBST prior to reprobing with antibody.
RNA Isolation, cDNA Synthesis and Quantitative RT-qPCR
To test mouse ESC pluripotency post-transfection, ESCs were transfected with 2 µg pMaxGFP using either PEI or LF2K. Transfected ESCs were pelleted 48- hours post transfection. Total RNA was isolated using the mirVana miRNA Isolation Kit (Ambion) following the manufacturer’s protocol. Complementary DNA was synthesized with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) following the manufacturer’s protocol. Quantitative RT-PCR was performed using a StepOne machine (Applied Biosystems). Reactions were prepared using TaqMan® assays for Pou5f1 (Mm03053917_g1) or Nanog (Mm02384862-g1) and TaqMan® Gene Expression master mix (Applied Biosystems). Three biological replicates and three technical replicates were used to compare PEI and LF2K transfected cells to untransfected cells. Threshold cycle values were normalized to a mouse GAPDH endogenous control (Mm99999915_g1). P-values were calculated using Data Assist software (Applied Biosystems).
Results
Since our lab routinely uses PEI to efficiently transfect standard cell lines, such as HEK-293T [12], HeLa and Neuro2A (unpublished data), we wanted to see if PEI could also be used to transfect mouse ESCs. A protocol for efficiently transfecting mouse ESCs, our lab has been using a LF2K protocol adapted from Bugeon et. al. To determine if PEI could replace LF2K for transfecting mouse ESCs, we began by simply replacing LF2K with PEI in the modified Bugeon et. al protocol. To assess mouse ESC transfection efficiency, our assay initially consisted of visualizing GFP expression in live cells via microscopy 24-hours post-transfection. We included LF2K-transfected ESCs as a positive control.
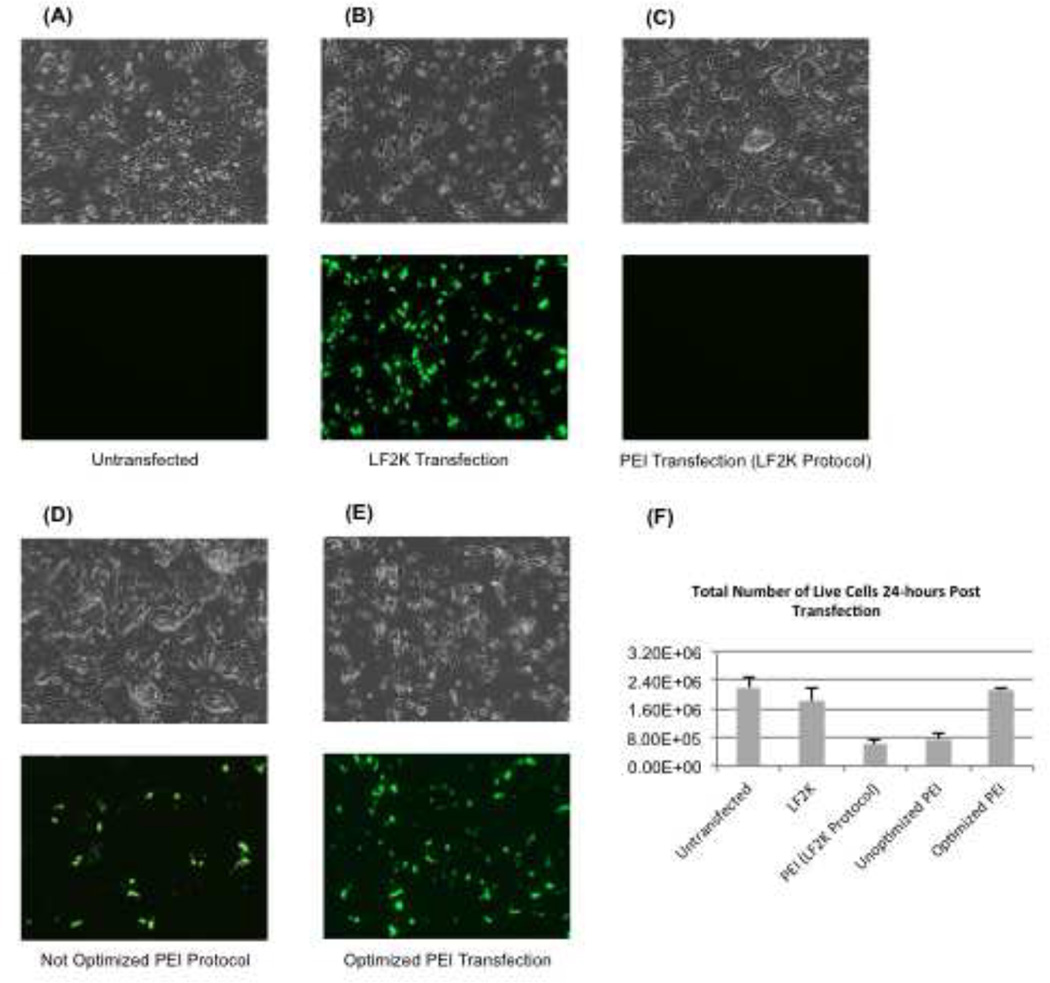
We incubated 2 µg pMaxGFP plasmid DNA with either PEI or LF2K. Compared to untransfected ESCs (Fig. 1A), LF2K transfections yielded a high percentage of GFP-positive cells (Fig. 1B). Substitution of PEI for LF2K did not result in any GFP-positive cells (Fig. 1C). However, when we increased the amount of PEI by 4-fold, some ESCs fluoresced green, indicating that it was indeed possible to transfect mouse ESCs using PEI (Fig. 1D). This result suggested that, with optimization, we might be able to achieve increased transfection efficiency with PEI in mouse ESCs. After empirically determining the optimal conditions for PEI transfections, we visualized roughly the same number of transfected cells as seen with LF2K (Fig. 1E).
Figure 1.
Initial optimization of PEI transfection conditions. Mouse ESCs were visualized by fluorescence microscopy 24-hours after transfection with either LF2K or PEI. All images are shown at 10× magnification under either transmitted (top) or GFP filter (bottom). All cells were transfected with 2 µg pMaxGFP for visual analysis of transfection efficiency. (A) Untransfected WT mouse ESCs were used as a reference for ESC morphology. (B) LF2K-transfected ESCs were less in number than the untransfected ESCs, but displayed high transfection efficiency. (C) Substitution of PEI for LF2K with the modified Bugeon et. al protocol did not result in any GFP-positive cells. (D) Increasing the amount of PEI 4-fold resulted in some GFP-positive cells. (E) Optimized PEI transfection resulted in many GFP-positive cells, and a few more adhered cells than LF2K post transfection. (F) This graph represents the percent of live cells 24-hours after reagent exposure (n=3 independent transfections for each condition). The xaxis lists the conditions compared in this manuscript and the y-axis represents the percentage of total live cells. Error bars represent standard error of the mean (SEM).
Microscopy confirmed that transfections with PEI were feasible, but in order to quantify and compare the cytotoxicity between PEI and LF2K, we counted the number of live cells 24-hours post transfection by performing trypan blue dye exclusion assays using a Countess Automated Cell Counter. Transfection of 1 × 106 ESCs with either LF2K or PEI results in small but significant effects on cell viability. Our data indicated that 24-hours post-transfection, 72% of cells that were exposed to LF2K were alive and 84% of cells exposed to PEI were alive (Fig. 1F). These data indicate that the PEI reagent does not affect mouse ESC viability, and is actually slightly less toxic than LF2K.
Our goal was to increase transfection efficiency, while keeping cytotoxicity low. As such, we adjusted the conditions as follows:
-
◦
The amount of DNA was increased from 2 µg to 4 µg
-
◦
Serum starvation was applied (3.5 hours prior to transfection and/or plating ESCs in 0% serum, reduced serum, or complete media)
-
◦
PEI levels were varied further (1:20 – 1:2 in Opti-MEM)
-
◦
The final volume of transfection mixture was varied (50 µL – 400 µL)
-
◦
The order of combining DNA, PEI, and ESCs was varied
We found that using more than 2 µg of DNA was toxic to ESCs. Therefore, the optimized protocol included no more than 2 µg of DNA total. We also found that serum starvation prior to transfection was not healthy for the cells, even when plated in complete medium overnight. Therefore, ESCs were grown in Opti-MEM for no more than 30 minutes prior to the application of the PEI:DNA complexes, and plated in complete medium overnight to maintain cell viability. It was also not healthy for mouse ESCs to be exposed to more than 1:4 of PEI in Opti-MEM, as cytotoxicity greatly increased. We found that the optimal procedure was to resuspend the ESCs in Opti-MEM first, add the DNA next, and lastly add PEI. Most importantly, we discovered that preparing PEI in a larger final volume, while keeping the cell number constant (1 × 106 per well of a 6-well plate), greatly increased the transfection efficiency while avoiding cytotoxicity. The optimal transfection efficiency with PEI resulted from the stepwise combining 1 × 106 ESCs resuspended in 300 µL Opti-MEM, followed by the addition of 2 µg pMaxGFP, then 100 µL PEI.
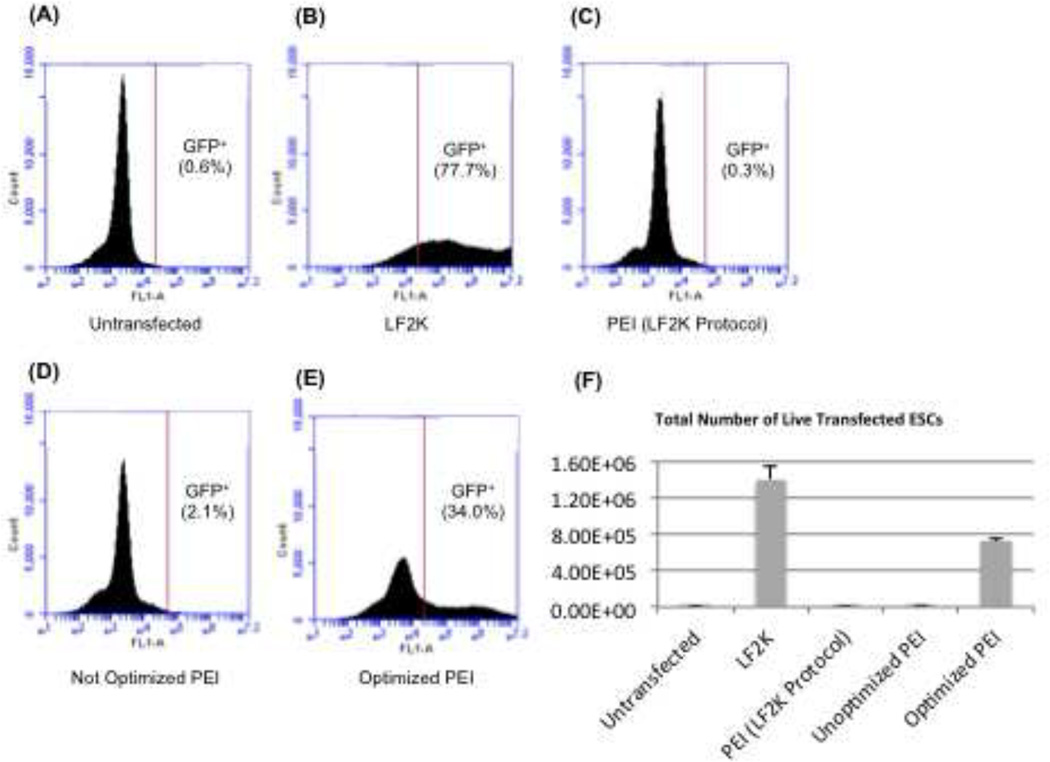
We performed flow cytometry to quantify the relative transfection efficiencies of PEI and LF2K using these optimized conditions. Using untransfected ESCs to gate for GFP-positive cells (Fig. 2A), we found that transfection with LF2K resulted in an average of 77.7% of GFP-positive ESCs (Fig. 2B). PEI-transfected cells, when substituted for LF2K in the same protocol, resulted in only 0.3% of GFP-positive ESCs (Fig. 2C), which was virtually the same percentage as untransfected ESCs (0.6%) (Fig. 2A). Increasing PEI 4-fold only increased the amount of GFP-positive ESCs to 2.1% (Fig. 2D). Once we completed our empirically determined optimization for PEI, the new protocol resulted in 34.0% of ESCs being GFP-positive (Fig. 2E), and this compared to a 77.7% transfection efficiency with LF2K (Fig. 2F).
Figure 2.
Quantification of transfected cells. This data represents flow cytometry analysis of WT mouse ESCs transfected with 2 µg pMaxGFP. All analyses were performed on 3 independent ESC transfections. For images A – E, the x-axis represents fluorescence and the y-axis represents the number of cells detected at a certain fluorescence by the flow cytometer. (A) Untransfected mouse ESCs were used as a reference and the gating was set such that 0.6% of cells were untransfected. (B) LF2K transfection efficiency averaged 77.7% (n=3). (C) PEI substituted in the LF2K protocol resulted in 0.3% transfected cells (n=3). (D) Increasing the PEI concentration achieved an average of 2.1% transfected ESCs (n=3). (E) The optimized PEI protocol achieved 34% transfected ESCs (n=3). (F) This graph shows the percentage of GFP+ cells determined in (A-E). The x-axis represents the conditions compared in this manuscript, and the y-axis represents percent transfection as determined by flow cytometry. Error bars represent the standard error of the mean (SEM).
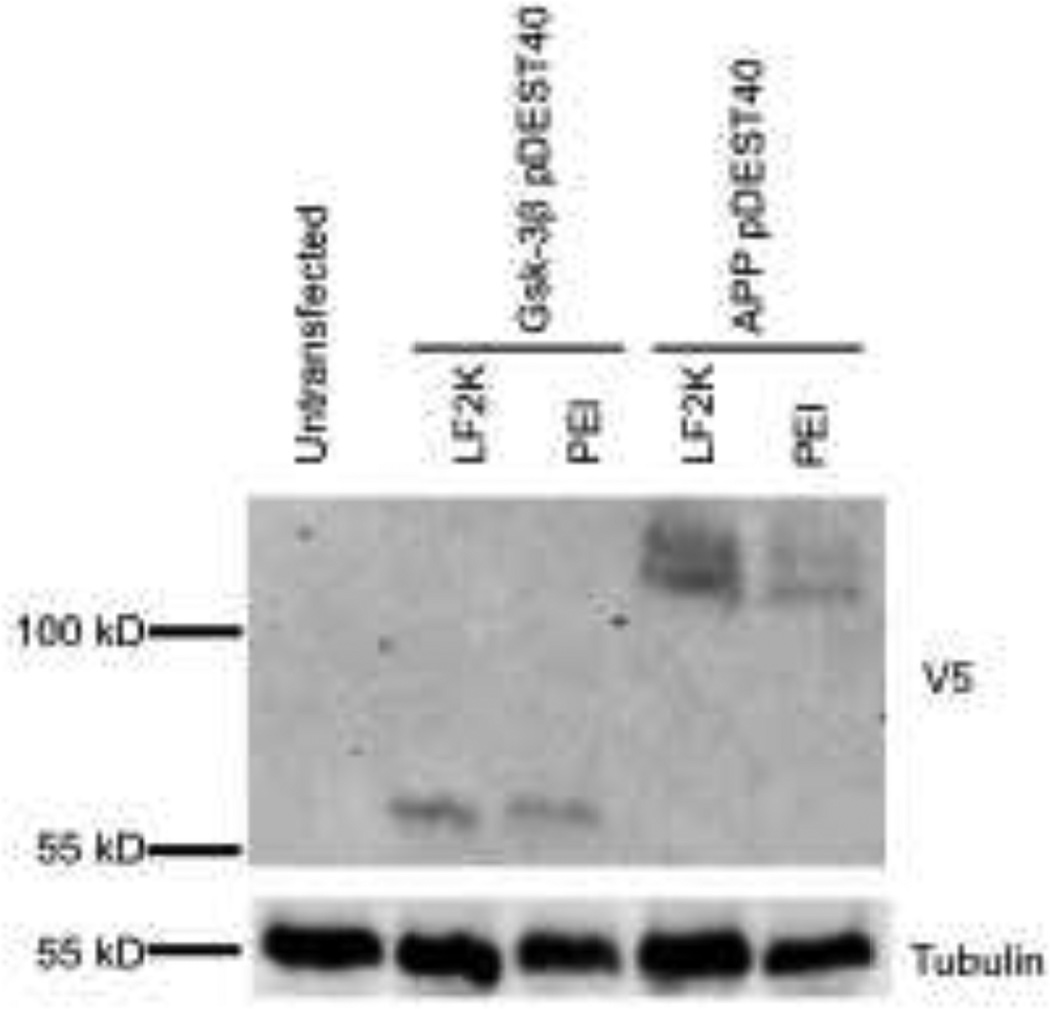
We next wanted to demonstrate that PEI transfections of mouse ESCs were not limited to pMaxGFP. Therefore, mouse ESCs were transfected with mammalian expression constructs encoding V5-tagged glycogen synthase kinase-3β (Gsk-3β) [13] and amyloid precursor protein (APP). Western blotting using a V5 antibody shows that the expression of Gsk-3β is comparable between PEI and LF2K, while APP was expressed at a lower level with PEI compared to LF2K (Fig. 3).
Figure 3.
Confirmed transfection of additional mammalian expression constructs. Western blot analysis confirmed PEI transfection of mouse ESCs transfected with V5-tagged (Gsk-3β pDEST40) and V5-tagged APP (APP pDEST40) using either LF2K or the optimized PEI protocol. Tubulin expression served as a loading control.
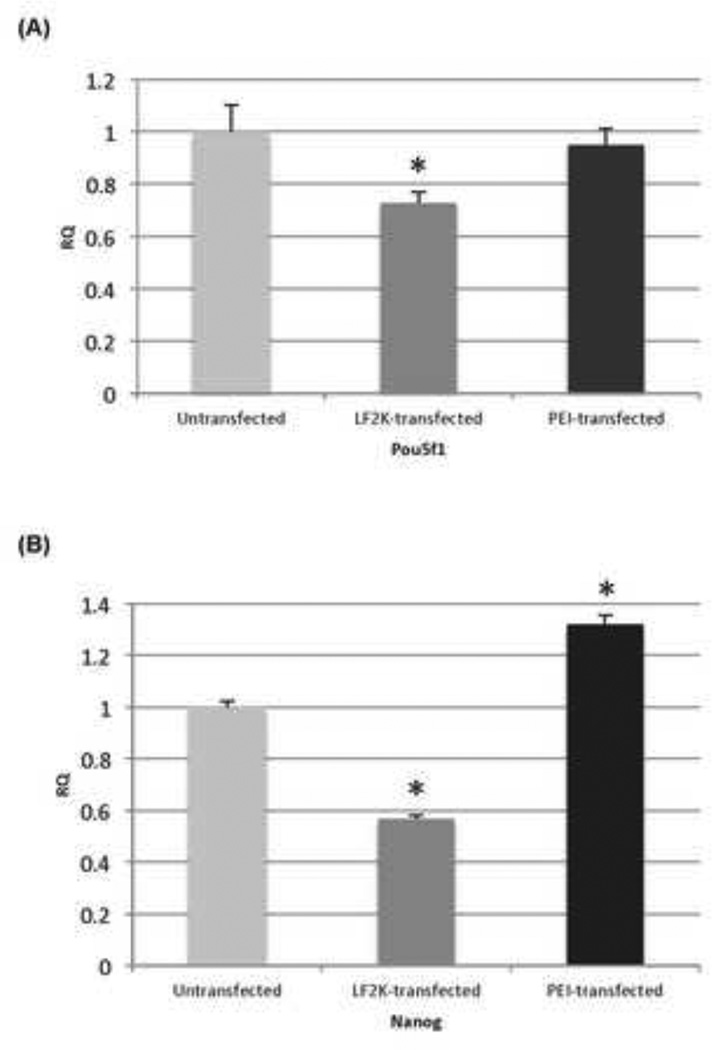
Finally, we wished to verify that transfection of mouse ESCs with PEI did not affect the pluripotency of the stem cells. Therefore, we used quantitative RT-PCR to detect the expression of endogenous Oct4 (Pou5f1) and Nanog, markers of ESC pluripotency [14]. We found that Oct4 expression 48-hours post PEI transfection is very similar to untransfected WT ESCs (less than 5% difference), while Nanog expression is actually higher than untransfected ESCs. Surprisingly, we found that Oct4 and Nanog expression were reduced by approximately 30% and 45%, respectively, in ESCs transfected with LF2K (Fig. 4). In summary, PEI does not appear to affect the pluripotent characteristic of ESCs, and we conclude that the ESCs remain undifferentiated post transfection.
Figure 4.
Effect of transfection on ESC pluripotency. Quantitative RT-PCR of (A) Oct4 (Pou5f1) and Nanog(B), comparing untransfected ESCs with ESCs transfected with pMaxGFP using either PEI or LF2K. Gapdh served as the internal control for quantification. Asterisks represent p<0.05; Student’s t-test.
Discussion
The use of mouse ESCs as an in vitro model is becoming more commonplace as a priority has been placed on understanding the fundamental aspects regulating the maintenance of pluripotency due to the implications for the future of regenerative medicine [15]. Also driving the increased use of mouse ESCs is the growing number of cell lines containing genetic deletions [16]. While reagents capable of efficiently transfecting mouse ESCs are commercially available, one drawback for labs performing large-scale screens, or for labs with limited funds, is the cost of these reagents. It is in this context that we present our optimized protocol for transfecting mouse ESCs efficiently and inexpensively.
PEI alone has been shown to transfect other types of murine stem cells, including mesenchymal stem cells [17–32] and neural stem cells [26, 33], but we are unaware of any reports demonstrating the successful use of PEI alone in mouse ESCs. One group set out to compare PEI chemical transfection efficiencies to nucelofection in mammalian cell lineages. While they found that mouse ESCs could be transfected with 62% efficiency and 66% viability by nucelofection of 2 µg pMaxGFP, they reported an inability to transfect ESCs using PEI due to high cytotoxicity and/or senescence post transfection [3]. We did not encounter any issues with either cytotoxicity or senescence. In fact, using the conditions presented here, PEI can transfect mouse ESCs with minimal cell death after reagent exposure. In addition, we demonstrated that successful transfection of mouse ESCs does not affect expression of Oct4 (Pou5f1) nor Nanog, an important markers for pluripotency of ESCs [14]. Another group further demonstrated that nucleofection transfected mouse ESCs with higher efficiency than electroporation. Nucleofection of mESCs with eGFP resulted in 63.66% transfection efficiency, compared with only 6.41% transfected cells with electroporation. Nucleofection also proved more efficient than electroporation in producing stable cell lines [34]. An important technical note is that, similar to a previous report optimizing PEI for the transfection of HeLa cells [38], reconstituted PEI should be kept frozen until ready for usage, as the efficiency of transfection dissipates over time if refrigerated. Frozen stocks of PEI do not show any reduction in transfection efficiency after prolonged storage (data not shown).
Our protocol is not the first to show that PEI can successfully transfect mouse ESCs. One group has devised a method to transfect mouse ESCs using magnetofection [8], which combines PEI with superparamagnetic iron oxide to deliver plasmid DNA into ESCs. The vector/iron oxide complex is coated with PEI, and a magnetic force is applied to cells, facilitating transfection to occur [35–37]. Magnetofection was compared to a liposomal-based transfection reagent, FuGENE 6, and it was shown that FuGENE 6 transfects an average of 15% ESCs, while magnetofection transfected ESCs with an average of 45% efficiency [8], which is similar to our protocol using PEI alone. In addition, PEI has been shown to transfect mouse ESCs using a microfluidic hydrodynamic focusing device to prepare PEI/plasmid DNA complexes [9]. While the use of PEI for transfecting mouse ESCs is not entirely novel, the protocol we have devised has the added advantages of being it is less time-consuming, less costly, and overall less complicated than using magnetofection and microfluidic hydrodynamic focusing in combination with PEI.
PEI transfection of mouse ESCs has several practical advantages over commercially available lipid-based transfection reagents. Two grams of PEI (~$100) supplies enough reagent for 2 liters of solution, or 20,000 reactions (at 100 µL per 1 reaction of a 6-well plate). For the same price as a 1 mL vial of LF2K (250 reactions), we are able to perform approximately 82,000 PEI reactions. Not only is PEI a cost-efficient alternative, the protocol that we have devised is simple and produces results faster than the recommended protocol for LF2K. Previous ESC protocols required plating 24-hours before transfection, followed by transfection the following day, then waiting 24 – 48 hours before visualization and analysis. This resulted in a 3- to 4-day long transfection procedure. In our protocol, we transfect and plate the cells in the same day, visualizing and analyzing 24 – 48 hours later. We used a 24-hour time frame in our experiments, showing it is sufficient for the detection of transfected plasmids in ESCs. In addition, both the manufacturer’s protocol for LF2K and the modified Bugeon et. al protocol requires preparing DNA and LF2K complexes prior to the resuspension of cells. Our protocol simplifies this step, requiring only the direct addition of DNA and PEI to cells in suspension, limiting the need for any excess materials or instruments, and making the process simpler for large-scale reactions. Lastly, using PEI does not appear to reduce the expression of pluripotency-related genes in ESCs [14]. The maintenance of undifferentiated ESCs post-transfection is critically important for most downstream analyses, as well as for the creation of stable cell lines, making PEI a particularly suitable transfection reagent for mouse ESCs. It is our hope that this protocol for mouse ESC transfection by PEI can be applicable to many labs as a cost-efficient transfection alternative to more expensive commercial reagents.
Figure 5.
Diagram of transfection scheme. This transfection schematic illustrates the ease of transfecting mouse ESCs with PEI.
Highlights.
Polyethylenimine efficiently transfects mouse embryonic stem cells.
Exposure of cells to polyethylenimine does not reduce pluripotency.
Polyethylenimine is an economical alternative to other lipid-based
Acknowledgments
We would like to thank Dr. Brad Doble (McMaster University) for providing us with modified Bugeon et. al protocol for transfecting mouse ESCs. This work was supported by NIH R01AG031883 and funds from the University of Colorado Denver.
Abbreviations
- ESCs
Embryonic stem cells
- PEI
Polyethylenimine
- LF2K
Lipofectamine 2000™
- GFP
green fluorescent protein
- HEK-293T
human embryonic kidney 293T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future. Analytical and Bioanalytical Chemistry. 2010;397:3173–3178. doi: 10.1007/s00216-010-3821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huh SH, Do HJ, Lim HY, Kim DK, Choi SJ, Song H, Kim NH, Park JK, Chang WK, Chung HM, Kim JH. Optimization of 25 kDa linear polyethylenimine for efficient gene delivery. Biologicals : journal of the International Association of Biological Standardization. 2007;35:165–171. doi: 10.1016/j.biologicals.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Maurisse R, De Semir D, Emamekhoo H, Bedayat B, Abdolmohammadi A, Parsi H, Gruenert DC. Comparative transfection of DNA into primary and transformed mammalian cells from different lineages. BMC Biotechnology. 2010;10:9. doi: 10.1186/1472-6750-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspensiongrowing human 293-EBNA1 cells. Nucleic Acids Research. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backliwal G, Hildinger M, Hasija V, Wurm FM. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnology and Bioengineering. 2008;99:721–727. doi: 10.1002/bit.21596. [DOI] [PubMed] [Google Scholar]
- 6.Baldi L, Muller N, Picasso S, Jacquet R, Girard P, Thanh HP, Derow E, Wurm FM. Transient gene expression in suspension HEK-293 cells: application to large-scale protein production. Biotechnology Progress. 2005;21:148–153. doi: 10.1021/bp049830x. [DOI] [PubMed] [Google Scholar]
- 7.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CH, Kim EY, Jeon K, Tae JC, Lee KS, Kim YO, Jeong MY, Yun CW, Jeong DK, Cho SK, Kim JH, Lee HY, Riu KZ, Cho SG, Park SP. Simple, efficient, and reproducible gene transfection of mouse embryonic stem cells by magnetofection. Stem Cells and Development. 2008;17:133–141. doi: 10.1089/scd.2007.0064. [DOI] [PubMed] [Google Scholar]
- 9.Koh CG, Kang X, Xie Y, Fei Z, Guan J, Yu B, Zhang X, Lee LJ. Delivery of polyethylenimine/DNA complexes assembled in a microfluidics device. Molecular Pharmaceutics. 2009;6:1333–1342. doi: 10.1021/mp900016q. [DOI] [PubMed] [Google Scholar]
- 10.DPN Gearing NA, Metcalf D, Foote S, Wilson TA, Gough NM, Williams RL. Production of leukemia inhibitory factor in Escherichia coli by a novel procedure and its use in maintaining embryonic stem cells in culture. Bio/Technology. 1989;7:1157–1161. [Google Scholar]
- 11.Bugeon L, Syed N, Dallman MJ. A fast and efficient method for transiently transfecting ES cells: application to the development of systems for conditional gene expression. Transgenic Research. 2000;9:229–232. doi: 10.1023/a:1008990510849. [DOI] [PubMed] [Google Scholar]
- 12.Buescher JL, Phiel CJ. A noncatalytic domain of glycogen synthase kinase-3 (GSK-3) is essential for activity. The Journal of Biological Chemistry. 2010;285:7957–7963. doi: 10.1074/jbc.M109.091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeidner LC, Buescher JL, Phiel CJ. A novel interaction between Glycogen Synthase Kinase-3alpha (GSK-3alpha) and the scaffold protein Receptor for Activated C-Kinase 1 (RACK1) regulates the circadian clock. International Journal of Biochemistry and Molecular Biology. 2011;2:318–327. [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136:2311–2322. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum HE. Advances in individualized and regenerative medicine. Advances in Medical Sciences. 2014;59:7–12. doi: 10.1016/j.advms.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Ayadi A, Birling MC, Bottomley J, Bussell J, Fuchs H, Fray M, Gailus-Durner V, Greenaway S, Houghton R, Karp N, Leblanc S, Lengger C, Maier H, Mallon AM, Marschall S, Melvin D, Morgan H, Pavlovic G, Ryder E, Skarnes WC, Selloum M, Ramirez-Solis R, Sorg T, Teboul L, Vasseur L, Walling A, Weaver T, Wells S, White JK, Bradley A, Adams DJ, Steel KP, Hrabe de Angelis M, Brown SD, Herault Y. Mouse large-scale phenotyping initiatives: overview of the European Mouse Disease Clinic (EUMODIC) and of the Wellcome Trust Sanger Institute Mouse Genetics Project. Mammalian Genome : Official Journal of the International Mammalian Genome Society. 2012;23:600–610. doi: 10.1007/s00335-012-9418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tong H, Wang C, Huang Y, Shi Q, Fernandes JC, Dai K, Tang G, Zhang X. Polyethylenimine600-beta-cyclodextrin: a promising nanopolymer for nonviral gene delivery of primary mesenchymal stem cells. International Journal of Nanomedicine. 2013;8:1935–1946. doi: 10.2147/IJN.S43074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tierney EG, Duffy GP, Hibbitts AJ, Cryan SA, O'Brien FJ. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. Journal of Controlled Release: Official Journal of the Controlled Release Society. 2012;158:304–311. doi: 10.1016/j.jconrel.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Deng W, Fu M, Cao Y, Cao X, Wang M, Yang Y, Qu R, Li J, Xu X, Yu J. Angelica sinensis polysaccharide nanoparticles as novel non-viral carriers for gene delivery to mesenchymal stem cells. Nanomedicine : Nanotechnology, Biology, and Medicine. 2013;9:1181–1191. doi: 10.1016/j.nano.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Tokatlian T, Cam C, Siegman SN, Lei Y, Segura T. Design and characterization of microporous hyaluronic acid hydrogels for in vitro gene transfer to mMSCs. Acta Biomaterialia. 2012;8:3921–3931. doi: 10.1016/j.actbio.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SJ, Na K. The transfection efficiency of photosensitizer-induced gene delivery to human MSCs and internalization rates of EGFP and Runx2 genes. Biomaterials. 2012;33:6485–6494. doi: 10.1016/j.biomaterials.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 22.Park JS, Na K, Woo DG, Yang HN, Kim JM, Kim JH, Chung HM, Park KH. Non-viral gene delivery of DNA polyplexed with nanoparticles transfected into human mesenchymal stem cells. Biomaterials. 2010;31:124–132. doi: 10.1016/j.biomaterials.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Needham CJ, Williams AK, Chew SA, Kasper FK, Mikos AG. Engineering a polymeric gene delivery vector based on poly(ethylenimine) and hyaluronic acid. Biomacromolecules. 2012;13:1429–1437. doi: 10.1021/bm300145q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu YL, Huang B, Zhang TY, Miao PH, Tang GP, Tabata Y, Gao JQ. Mesenchymal stem cells as a novel carrier for targeted delivery of gene in cancer therapy based on nonviral transfection. Molecular Pharmaceutics. 2012;9:2698–2709. doi: 10.1021/mp300254s. [DOI] [PubMed] [Google Scholar]
- 25.Delyagina E, Schade A, Scharfenberg D, Skorska A, Lux C, Li W, Steinhoff G. Improved transfection in human mesenchymal stem cells: effective intracellular release of pDNA by magnetic polyplexes. Nanomedicine. 2014;9:999–1017. doi: 10.2217/nnm.13.71. [DOI] [PubMed] [Google Scholar]
- 26.Shakhbazau AV, Shcharbin DG, Goncharova NV, Seviaryn IN, Kosmacheva SM, Kartel NA, Bryszewska M, Majoral JP, Potapnev MP. Neurons and stromal stem cells as targets for polycation-mediated transfection. Bulletin of Experimental Biology and Medicine. 2011;151:126–129. doi: 10.1007/s10517-011-1273-4. [DOI] [PubMed] [Google Scholar]
- 27.King WJ, Kouris NA, Choi S, Ogle BM, Murphy WL. Environmental parameters influence non-viral transfection of human mesenchymal stem cells for tissue engineering applications. Cell and Tissue Research. 2012;347:689–699. doi: 10.1007/s00441-011-1297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Lee ES, Noh MY, Koh SH, Lim EK, Yoo AR, Lee K, Suh JS, Kim SH, Haam S, Huh YM. Ambidextrous magnetic nanovectors for synchronous gene transfection and labeling of human MSCs. Biomaterials. 2011;32:6174–6182. doi: 10.1016/j.biomaterials.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Yang HN, Park JS, Woo DG, Jeon SY, Do HJ, Lim HY, Kim JH, Park KH. C/EBP-alpha and C/EBP-beta-mediated adipogenesis of human mesenchymal stem cells (hMSCs) using PLGA nanoparticles complexed with poly(ethyleneimmine) Biomaterials. 2011;32:5924–5933. doi: 10.1016/j.biomaterials.2011.04.072. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Li W, Ou L, Flick E, Mark P, Nesselmann C, Lux CA, Gatzen HH, Kaminski A, Liebold A, Lutzow K, Lendlein A, Li RK, Steinhoff G, Ma N. Polyethylenimine-mediated gene delivery into human bone marrow mesenchymal stem cells from patients. Journal of Cellular and Molecular Medicine. 2011;15:1989–1998. doi: 10.1111/j.1582-4934.2010.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saraf A, Hacker MC, Sitharaman B, Grande-Allen KJ, Barry MA, Mikos AG. Synthesis and conformational evaluation of a novel gene delivery vector for human mesenchymal stem cells. Biomacromolecules. 2008;9:818–827. doi: 10.1021/bm701146f. [DOI] [PubMed] [Google Scholar]
- 32.Song H, Wang G, He B, Li L, Li C, Lai Y, Xu X, Gu Z. Cationic lipidcoated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. International Journal of Nanomedicine. 2012;7:4637–4648. doi: 10.2147/IJN.S33923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falk A, Holmstrom N, Carlen M, Cassidy R, Lundberg C, Frisen J. Gene delivery to adult neural stem cells. Experimental Cell Research. 2002;279:34–39. doi: 10.1006/excr.2002.5569. [DOI] [PubMed] [Google Scholar]
- 34.Lakshmipathy U, Pelacho B, Sudo K, Linehan JL, Coucouvanis E, Kaufman DS, Verfaillie CM. Efficient transfection of embryonic and adult stem cells. Stem Cells. 2004;22:531–543. doi: 10.1634/stemcells.22-4-531. [DOI] [PubMed] [Google Scholar]
- 35.Plank C, Schillinger U, Scherer F, Bergemann C, Remy JS, Krotz F, Anton M, Lausier J, Rosenecker J. The magnetofection method: using magnetic force to enhance gene delivery. Biological Chemistry. 2003;384:737–747. doi: 10.1515/BC.2003.082. [DOI] [PubMed] [Google Scholar]
- 36.Plank C, Scherer F, Schillinger U, Bergemann C, Anton M. Magnetofection: enhancing and targeting gene delivery with superparamagnetic nanoparticles and magnetic fields. Journal of Liposome Research. 2003;13:29–32. doi: 10.1081/lpr-120017486. [DOI] [PubMed] [Google Scholar]
- 37.Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, Gansbacher B, Plank C. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Therapy. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 38.Fukumoto Y, Obaya Y, Ishibashi K, Tamura N, Kikuchi I, Aoyama K, Hattori Y, Tsuda K, Nakayama Y, Yamaguchi N. Cost-effective gene transfection by DNA compaction at pH 4.0 using acidified, long shelf-life polyethylenimine. Cytotechnology. 2010;62:73–82. doi: 10.1007/s10616-010-9259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]