Abstract
Invasive infection often begins with asymptomatic colonization of mucosal surfaces. A murine model of bacterial colonization with Streptococcus pneumoniae was used to study the mechanism for mucosal protection by immunoglobulin. In previously colonized immune mice, bacteria were rapidly sequestered within large aggregates in the nasal lumen. To further examine the role of bacterial agglutination in protection by specific antibodies, mice were passively immunized with IgG purified from anti-pneumococcal sera or pneumococcal type-specific monoclonal human IgA (hIgA1 or hIgA2). Systemically-delivered IgG accessed the mucosal surface and blocked acquisition of colonization and transmission between littermates. Optimal protection by IgG was independent of Fc fragment and complement and, therefore, did not involve an opsonophagocytic mechanism. Enzymatic digestion or reduction of IgG prior to administration showed that protection required divalent binding that maintained its agglutinating effect. Divalent hIgA1 is cleaved by the pneumococcal member of a family of bacterial proteases that generate monovalent Fabα fragments. Thus, passive immunization with hIgA1 blocked colonization by an IgA1-protease deficient mutant (agglutinated), but not the protease-producing wild-type parent (not agglutinated), whereas protease-resistant hIgA2 agglutinated and blocked colonization by both. Our findings highlight the importance of agglutinating antibodies in mucosal defense and reveal how successful pathogens evade this effect.
INTRODUCTION
Colonization of mucosal surfaces is often the first step in the pathogenesis of disease for many microbial infections. Immunoglobulin plays an important role in host defense at mucosal sites and is thought to act by preventing colonization of pathogens. Patients with hypogammaglobulinemia or agammaglobulinemia, for example, typically present in early childhood with recurrent respiratory tract infections, in particular with extracellular, encapsulated bacteria (1). Most mucosal antibodies that are actively transported into the lumen (IgA and IgM) are multivalent with 4 or 10 to 12 antigen-binding sites per molecule, respectively. IgG, with two binding sites per molecule, is not secreted by the same mechanism, but its extravasation from the abundant plasma pool results in effective levels on mucosal surfaces (2). Evidence for the importance of plasma antibody in protection against disease is demonstrated by the effectiveness of systemic immunization against several mucosal pathogens, which correlates with increased specific antibody titers. Polysaccharides that comprise the capsules of common respiratory pathogens (Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis) induce high levels of IgG when conjugated to an immunogenic protein carrier. This approach has been very successful for vaccines licensed to protect against invasive disease (3). Moreover, high plasma titers to these parenterally-administered conjugate vaccines correlate with decreased rates of colonization in immunized individuals – an effect attributed to reduced bacterial acquisition (4, 5). Lower rates of colonization in turn diminish the frequency of host-to-host transmission, amplifying the effectiveness of immunization by protecting the unvaccinated population (indirect protection or herd immunity). Extensive surveillance in the U.S. has revealed that much of the public health benefit of the pneumococcal conjugate vaccine is due to protection of unvaccinated adults and the decreased incidence of pneumonia in this population (6, 7).
Several distinct functions of immunoglobulin in supporting host defense against pathogens have been described (8). 1) Antibodies may act as opsonins, leading to enhanced phagocytic recognition or promoting the deposition of complement and subsequent lysis. 2) Antibodies may tag infected cells for destruction through antibody-dependent cell-mediated cytotoxicity (ADCC). 3) Antibodies may neutralize a pathogen by binding to its surface and inhibiting its growth. 4) Antibodies may coat a pathogen and prevent its adherence to host cells and tissues. 5) Antibodies, because of their di- or multi-valent binding properties, may agglutinate their target into larger clusters allowing for more effective recognition and mechanical clearance by the host. However, the specific mechanism(s) by which mucosal antibodies, including systemically-generated antibodies derived from plasma, ameliorate colonization by pathogens is poorly understood.
In this study, we used a murine model of S. pneumoniae colonization of the upper respiratory tract to characterize how immunoglobulin affects mucosal colonization. These experiments using passive immunization revealed that multivalency of antibodies was required to protect against colonization in vivo, an outcome that was dependent on their ability to agglutinate the infecting bacteria at the mucosal surface.
RESULTS
Serotype-specific IgG blocks the acquisition of bacterial colonization
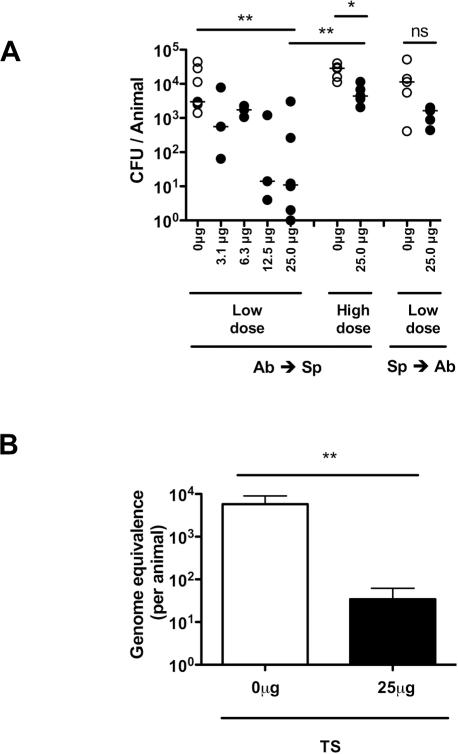
To examine the role of systemic antibody in mucosal protection, adult mice were passively immunized intraperitoneally (IP) with high-titer anti-pneumococcal rabbit sera and challenged intranasally (IN) with S. pneumoniae. Protection from the acquisition of colonization was determined by quantitative culture of upper respiratory tract lavages 20 hrs following bacterial challenge. An inoculum of 104 CFU was chosen because this dose was previously established as the 50% colonizing dose for this strain in experimental human carriage studies in healthy adults (9). Mice passively immunized 4 hrs prior to pneumococcal challenge with antisera raised to an isolate of the same capsular polysaccharide serotype as the challenge strain (TS, type-specific) were significantly protected from colonization (Fig. 1A). Protection against a low inoculum challenge was dose-dependent, requiring at least 25μg of specific antibody/animal for significant protection, an amount used in subsequent experiments (Fig. 1A). This mucosal protection detected using viable counts was confirmed using an independent non-culture based method - qPCR of nasal lavages amplifying pneumococcal gyrA DNA (Fig. 1B). The magnitude of protective effect of TS antiserum was diminished with a higher dose (106 CFU) bacterial challenge (Fig. 1A). The timing of antibody exposure was important as the protective effect of TS antiserum was no longer significant when mice were passively immunized 24 hrs after pneumococcal challenge, when stable colonization on the epithelial surface had already been established (10). These results demonstrate that specific antibody is most effective in blocking primary acquisition.
Figure 1. Protection against mucosal colonization by systemic antibody.
(A) Mice were passively immunized (IP) with rabbit antisera raised against S. pneumoniae either 4 hours before (Ab→Sp) or 24 hours after (Sp→Ab) pneumococcal challenge with a type 23F isolate. Increasing doses of serotype 23F capsular polysaccharide–specific antibody were tested for effects on colonization, which was determined by quantitative culture of upper respiratory tract lavages. Mice were challenged intranasally (IN) with either a low dose (104) or high dose (106) of S. pneumoniae. CFU/animal was determined 24 hours post immunization with type-specific antisera (closed symbols) or control (open symbols). n≥3 mice per group. ns, not significant. * P<0.05, ** P<0.01. (B) Bacterial load was confirmed in the nasal lavages of mice passively immunized 4 hours before challenge with low dose (104) S. pneumoniae by qPCR. Genomic equivalence per animal ± SEM was determined based on a standard curve of DNA of known bacterial quantity. n≥6 nasal lavage samples per group. ** P<0.01.
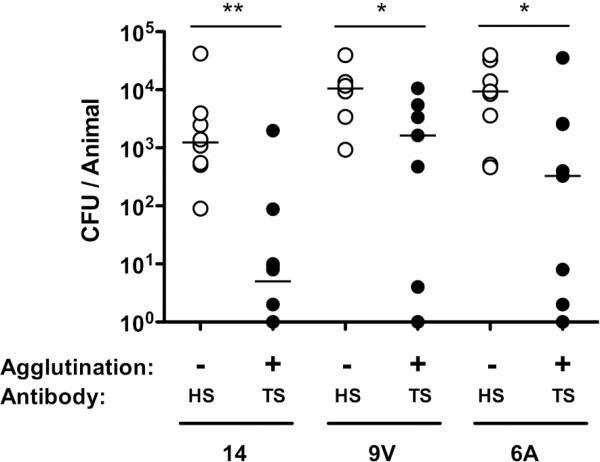
To confirm this model was broadly applicable, we tested protection by prior IP administration of antisera against low-dose challenge with isolates of three additional common pneumococcal serotypes (Fig. 2). In each case, protection was serotype-dependent, since protection was observed with TS antisera, but not antisera generated against an isolate of another capsular polysaccharide serotype (HS, heterologous-type).
Figure 2. Type-specific protection against multiple serotypes.
Mice were passively immunized with type-specific (TS) or an equivalent amount of antisera generated to a heterologous serotype (HS) and challenged with 104 pneumococci of the serotype indicated below 4 hours later. CFU/animal was determined 24 hours post immunization. n≥6 mice per group. * P<0.05, ** P<0.01. The agglutinating effect of TS and HS anti-pneumococcal rabbit antiserum was tested for each challenge strain.
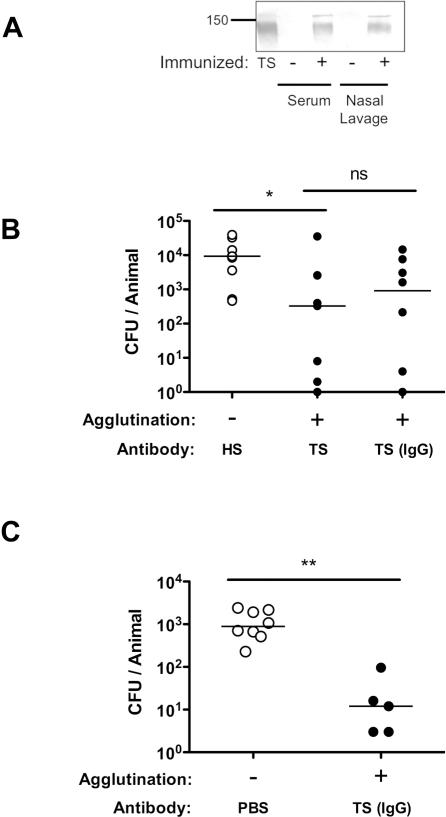
Following passive immunization and at the time of bacterial challenge, rabbit IgG was detected on the mucosal surface of the upper respiratory tract (Fig. 3A). Passively-administered purified serum TS IgG was sufficient for protection against mucosal colonization in adult mice (Fig. 3B). The effectiveness of TS IgG in blocking the acquisition of colonization was also demonstrated using infant mice (Fig. 3C).
Figure 3. IgG-mediated protection against colonization.
(A) Mice were passively immunized with anti-pneumococcal rabbit antisera (+) or PBS (-) and sacrificed 4 hours later to detect systemic (serum) and mucosal (nasal lavage) rabbit IgG. Rabbit IgG was detected by Western analysis with anti-rabbit IgG (whole molecule specific) using non-reducing conditions. An aliquot of antiserum was used as a positive control (TS). Size marker in kilodaltons. (B) Adult mice were passively immunized with equal amounts of HS or TS antisera or an equivalent amount of IgG purified from TS antisera and challenged with 104 pneumococci (type 6A) 4 hours later. CFU/animal was determined 24 hours post immunization. n≥ 8 mice per group. (C) 2 week old infant mice were passively immunized with IgG purified from TS or PBS control and 4 hours later challenged IN with 103 pneumococci (type 2). CFU/animal was determined 24 hours post immunization. The agglutinating effect of each serum tested was compared. n≥ 5 mice per group. * P<0.05, ** P<0.01.
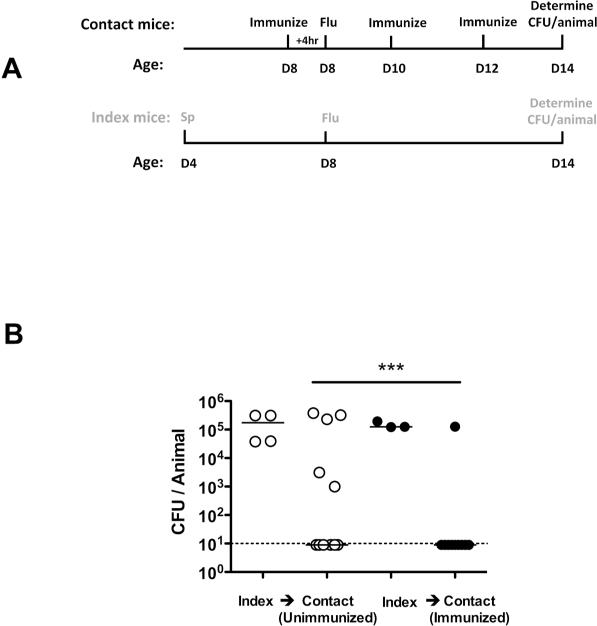
In addition to its role in preventing primary colonization, we investigated the role of antibody in blocking pneumococcal transmission from an infected host. We used a previously described infant mouse model of colonization in the setting of influenza A co-infection (11). At age 4 days, one in four mice within a litter (index mice) were colonized with S. pneumoniae, followed by influenza A infection of all mice in the litter at age 8 days (Fig. 4). The acquisition of pneumococci by day 14 in uncolonized littermates (contact mice) though intra-litter transmission was reduced from 5/11 (45%) in unimmunized contact pups to 1/10 (10%) in TS antisera immunized contact pups.
Figure 4. Protection against bacterial transmission.
(A) Schematic of inoculation and immunization schedule. S. pneumoniae (Sp) (type 23F) (index mice only) and influenza virus (Flu) (index and contact mice) were administered intranasally to infant mice. In the ‘immunized’ group the contact mice were passively immunized IP with TS antisera. Black text refers to contact mice and gray text refers to index mice. (B) Infant mice were infected and sacrificed as described above. CFU/animal in nasal lavage fluid are shown. Open circles designate unimmunized group, and filled represent TS immunized group. n≥ 10 mice per group. *** P<0.001. Dotted line indicates limit of detection (10 CFU).
Thus, our model using passive immunization recapitulated many of the clinical observations that suggest that systemic antibody protects against mucosal pneumococcal infection of adults and infants. These included the requirement for TS antibody and the importance of diffusion of IgG from the plasma pool to the mucosal surface in blocking the acquisition of carriage and transmission.
The agglutinating effect of antibody enhances mucosal protection
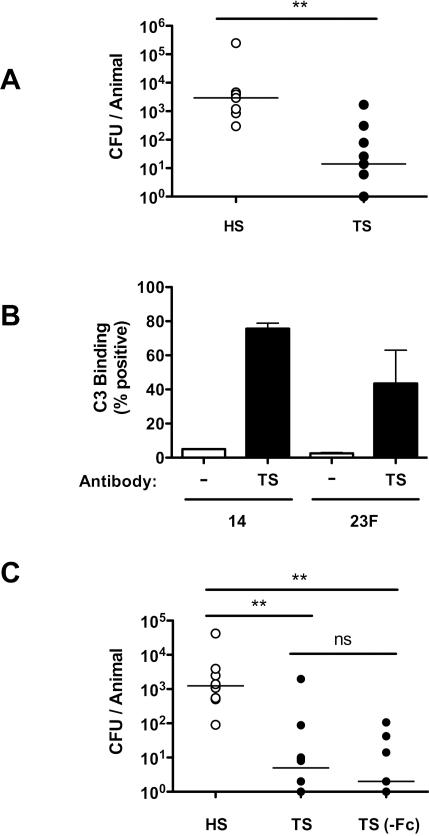
To determine the mechanism of systemic IgG-mediated mucosal protection, we considered factors required for opsonophagocytosis of pneumococci, since this is the primary mechanism of its clearance from the bloodstream (12). Phagocytes recognize the Fc portion of bound antibody and their recognition is enhanced by the deposition and activation of complement through binding of products derived from complement component 3 (C3). However, TS antiserum was still protective against colonization in complement-deficient (C3−/−) mice (Fig. 5A). Additionally, we confirmed that TS rabbit antisera promotes the deposition of mouse C3 on S. pneumoniae (Fig. 5B), although this process is not required for antibody-dependent protection against colonization. Furthermore, mice passively immunized with TS IgG that had been cleaved to completely remove its Fc-domain were also protected, excluding a role for direct Fc-binding in the mechanism of antibody-mediated protection (Fig. 5C). Together these findings suggested that the protective effect of mucosal IgG does not require opsonization. The Fc-independence of protection also suggested that antibody-dependent cell-mediated cytotoxity did not contribute to protection in this model.
Figure 5. Complement, and Fc-fragment, -independent mucosal protection.
(A) C3−/− mice were immunized with HS or TS anti-pneumococcal rabbit antisera and challenged with 104 pneumococci (type 23F) 4 hours later. CFU/animal was determined 20 hours post challenge. n≥ 7 mice per group. (B) Serotype 14 or 23F pneumococci were incubated with fresh mouse sera as a complement source and TS anti-pneumococcal rabbit antisera (black bars) or buffer control (white bars). Deposition of murine C3 was detected using FITC-conjugated anti-mouse C3 by flow cytometry to quantify the percent of events above background ± SEM. (C) Mice were immunized with HS, TS, or an equivalent amount of TS anti-pneumococcal rabbit antisera treated with pepsin under conditions that completely digested the Fc-fragment and challenged with 104 pneumococci (type 14) 4 hours later. CFU/animal was determined 24 hours post immunization. n≥ 7 mice per group. ** P<0.01.
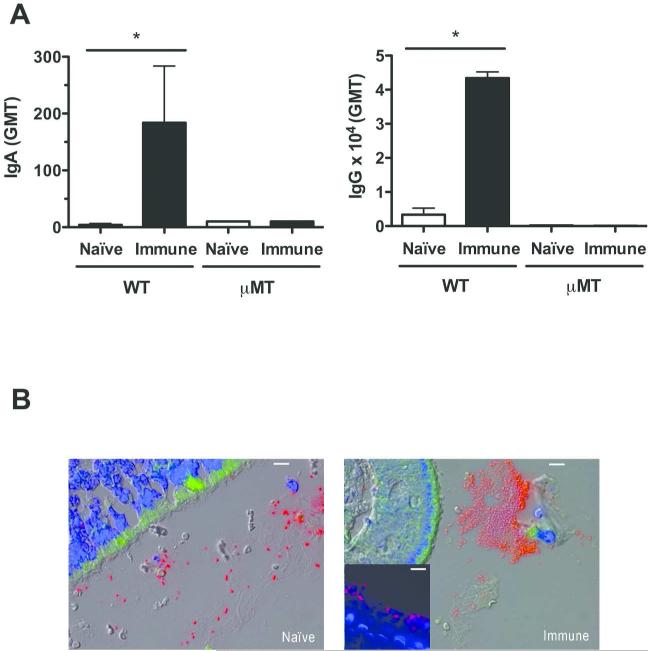
Because of the low inoculum required for protection from passively-administered antibody, we were unable to detect bacteria in the upper respiratory tract in tissue sections using this model. However, we visualized the effect of antibody during the early events in colonization following a larger inoculum (107 CFU) by comparing previously colonized (immune) and sham-colonized (naïve) mice using immunofluorescence on nasal tissue sections obtained during the initial 24 hrs post-challenge. Immune mice expressed higher antibody levels at the nasal mucosa (IgA) and in serum (IgG) (Fig. 6A) and have previously been shown to be less susceptible to secondary high-dose IN challenge in an antibody-dependent manner (13). As early as 30 mins post challenge, S. pneumoniae was found in the lumen predominantly in large aggregates in immune mice, whereas in naïve mice the bacteria in these spaces were mostly single or in pairs (Fig. 6B). Consistent with results in naïve immunocompetent animals, large aggregates of pneumococci were not seen in previously colonized μMT mice (Fig. 6B Insert), which do not generate specific antibody and remain susceptible to secondary challenge. The observation that the presence of local and systemic antibody was associated with agglutination in vivo led us to investigate whether the agglutinating effect of antibody was responsible for mucosal protection.
Figure 6. Agglutination of S. pneumoniae in mice with mucosal immunity.
Wild-type (WT) C57Bl6/J or μMT mice were serially immunized with 3 doses of colonizing pneumococci (immune) or PBS (naïve) and rechallenged IN with 107 CFU of the same isolate (type 4) 2 weeks after final immunization. Mice were sacrificed at 30 mins post-challenge to visualize early events during colonization. (A) Pneumococcal-specific IgA in lavage fluid and IgG in serum from naïve (white bars) and immune (black bars) mice was quantified by ELISA and expressed as geometric mean titer (GMT) ± SEM. n=4 for WT mice, and a representative sample for μMT mice. * P<0.05. (B) Immunofluorescent images of nasal tissue sections from naïve and immune WT mice 30 min post-challenge stained with anti-capsular antibodies for pneumococci (red) and host cells with DAPI (blue). Tissue autofluorescence was also detected (green). Representative images from mice in triplicate. Images taken at 400× magnification, with 10micron scale bars. Insert: Immunized μMT mice at 24 hours post pneumococcal challenge at 400x magnification, with 10 micron scale bar.
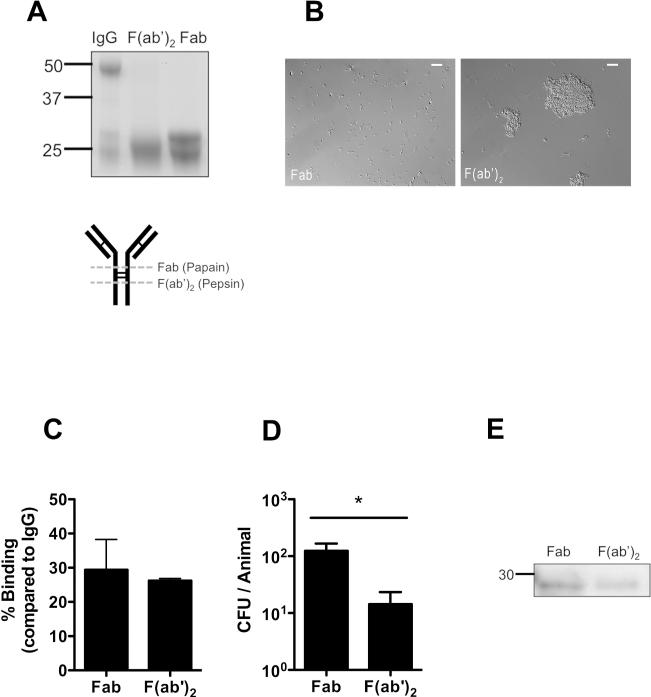
Each of the TS antiserum tested agglutinated the corresponding protected challenge strain, whereas the HS antiserum did not protect or agglutinate this strain (Fig.2 and 3B,C). Importantly, agglutination did not affect our ability to accurately assess colonization density on the mucosal surface as qPCR data correlated with CFU counts (Fig. 1B). To further examine the contribution of agglutination, TS IgG was cleaved into Fab (monovalent) and F(ab’)2 (divalent) fragments with papain or pepsin, respectively (Fig. 7A). Pneumococci incubated with divalent F(ab’)2, but not monovalent Fab, were heavily agglutinated in vitro (Fig. 7B), although both bound to homologous capsular polysaccharide with similar titers using an ELISA detecting rabbit light chains (Fig. 7C). Mice passively immunized with these fragments showed a difference in protection, with the agglutinating F(ab’)2 significantly more effective than equivalent amounts of non-agglutinating Fab (Fig. 7D). Only two groups, Fab versus F(ab’)2, were compared in this experiment as whole IgG bound ~3-fold more efficiently to bacteria than fragments (Fig. 7C). This difference in protection was not due to any differences in the ability of fragments to access the nasal mucosa as both were detected in nasal lavages (Fig. 7E).
Figure 7. Agglutination enhances protection by antibody.
TS IgG was cleaved into either F(ab’)2 or Fab fragments by pepsin or papain, respectively. (A) IgG, Fab and F(ab’)2 fragments were separated on a reducing SDS-PAGE gel and detected by coomassie to determine the completeness of digestion. Size markers in kilodaltons. Schematic of IgG digestion is shown, with dashed line indicating enzymatic cleavage sites relative to disulphide bonds (thin lines). (B) S. pneumoniae was incubated with Fab or F(ab’)2 fragments and agglutination was visualized by Nomarski microscopy with 200x magnification, with 20micron scale bars. (C) Binding of Fab and F(ab’)2 fragments to immobilized capsular polysaccharide was quantified by ELISA, and shown as the % binding compared to whole IgG ± SEM. (D) Mice were passively immunized with equivalent concentrations of IgG fragments and challenged with 104 pneumococci (type 14) 4 hours later. CFU/animal was determined 24 hours post immunization ± SEM. n≥ 12 mice per group. * P<0.05. (E) Western blot analysis of nasal lavages from mice immunized 4 hrs prior with either Fab or F(ab’)2. Antibody fragments were detected with anti-rabbit IgG (F(ab’)2 specific) using horseradish peroxidase under reducing conditions. Size marker in kilodaltons.
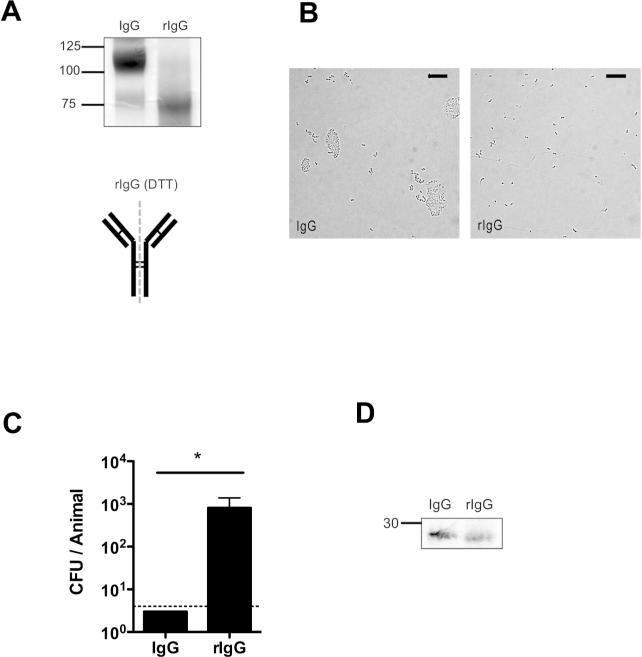
To further examine the role of agglutination in protection, we took advantage of the susceptibility of IgG to partial reduction. Treatment with a mild reducing agent, such as DTT, preferentially reduces the hinge region disulphide bonds to generate rIgG (or half IgG), which is monovalent and no longer agglutinates its target, but does not affect antigen binding (14) (Fig. 8A and B). In contrast to results with the whole IgG molecule, rIgG was not protective (Fig 8C), although both accessed the mucosal surface in similar quantities (Fig. 8D). These data demonstrate that agglutination resulting from the multivalent character of immunoglobulin promotes mucosal protection.
Figure 8. Protection by reduced IgG antibody.
IgG was partially reduced to rIgG by incubation with DTT. (A) Whole IgG and rIgG fragments were separated by SDS-PAGE gel and detected by coomassie staining to determine the purity of rIgG under non-reducing conditions. Size markers in kilodaltons. Schematic of IgG reduction is shown, with dashed line indicating separation site and disulphide bonds represented by thin lines. (B) S. pneumoniae was incubated with whole IgG or rIgG and agglutination was visualized by Nomarski microscopy with 200× magnification, with 20micron scale bars. (C) Mice were immunized with equal amounts of whole IgG or rIgG, and challenged with 104 pneumococci (type 6A) 4 hours later. CFU/animal was determined 24 hours post immunization + SEM. n≥ 4 mice per group. * P<0.05. Dotted line indicates limit of detection (4 CFU). (D) Western blot analysis of nasal lavages from mice immunized 4 hrs prior detecting rabbit antibody with anti-rabbit IgG (F(ab’)2 specific) using alkaline phosphatase. Size marker in kilodaltons.
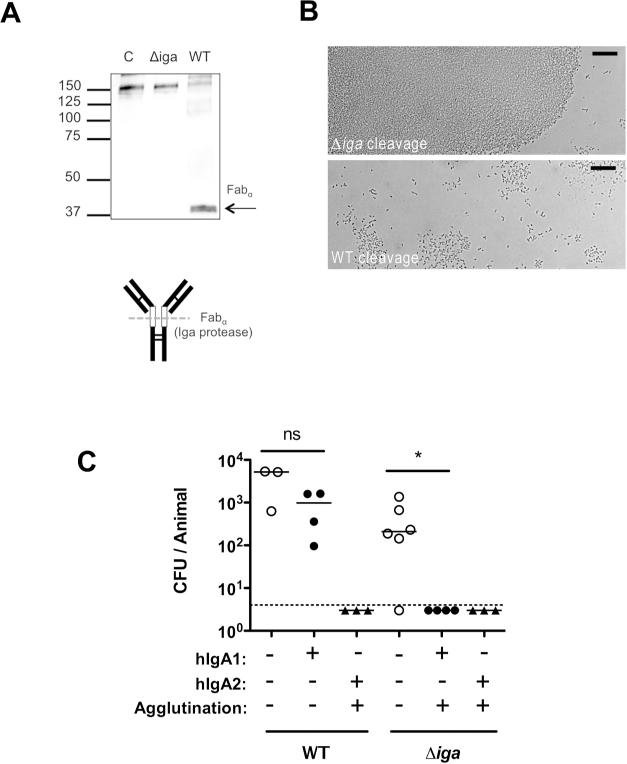
Many successful respiratory pathogens express a protease with specificity for bonds within the hinge region of human IgA1, the most abundant immunoglobulin subclass on airway mucosal surfaces (15, 16). Cleavage of di- or polyvalent IgA1 generates monovalent Fabα fragments. In the case of the pneumococcus, its IgA1-protease (Iga) is cell-surface attached and preferentially cleaves bound antibody (17). To determine whether Iga functions by abrogating the agglutinating effect of human IgA1, previously described human IgA (both IgA1 and IgA2) mAbs generated to pneumococcal capsular polysaccharide (18) were tested for protection against a protease-expressing wild-type strain (WT) and an isogenic mutant lacking the protease (Δiga). Cleavage of the hIgA1 mAb correlated with a loss of its ability to agglutinate pneumococci of the serotype recognized by the antibody (Fig. 9A and B). Mice were then passively immunized with the TS hIgA1 mAb or an equivalent concentration of an agglutinating TS hIgA2 mAb, which is not cleaved by the protease and served as a control (Fig 9C). Expression of the protease eliminated the protective effect of the IgA1 on the WT strain – an effect that correlated with the loss of agglutination after exposure to the protease. In contrast, protection was observed against the Δiga strain immunized with the TS hIgA1 mAb, and the protease had no effect on protection by the TS hIgA2 mAb. Since the protease preferentially cleaves antibody bound to the bacterial surface, its ability to mediate evasion of protective protease-sensitive antibody was unlikely to be caused by differences in the binding of intact versus digested antibody or their ability to access the mucosal surface. This data provided further evidence for the role of agglutination in antibody-mediated protection against colonization and also highlighted a common bacterial mechanism for overcoming this host defense.
Figure 9. Bacterial IgA1 protease eliminates mucosal protection by human IgA1.
(A) Human monoclonal IgA1 was incubated overnight with wild-type (WT) or IgA1 protease deficient (Δiga) pneumococci (type 2). The supernatant was analyzed by Western blot detecting hIgA1 by anti-human IgA antibody (α-chain specific) using alkaline phosphatase under non-reducing conditions. Size markers in kilodaltons. Schematic of IgA1 digestion to generate fabα is shown, with open boxes indicating the hinge region, and dashed line indicating protease cleavage site relative to disulphide bonds (thin lines). (B) S. pneumoniae was incubated with TS hIgA1 mAb following cleavage from WT or Δiga pneumococci, and agglutination was then visualized using Nomarski microscopy with 200× magnification, with 20micron scale bars. (C) Mice were passively immunized with 25μg/animal anti-type 2 hIgA1 or hIgA2 mAbs (or PBS vehicle control) and challenged 4 hours later with 103 WT or Δiga pneumocci (type 2). CFU/animal was determined 20 hours post challenge. The dashed line represents the limit of detection (4 CFU). n≥ 3 mice per group. ns, not significant. * P<0.05.
Discussion
The agglutinating effect of immune serum was first cited in 1891 by Élie Metchnikoff, who described large ‘packets’ of streptococci in the blood of vaccinated rabbits (19). These observations on ‘clumping’ as a marker of immunity would soon lead Neufeld to use it to develop the Quelling reaction, an assay that was the basis for the selection of therapeutic sera in the pre-antibiotic era and is still used today for typing pneumococci (20). Findings in our report suggest that the agglutinating effect of antibody, in fact, plays a major role in host defense during the initial phase of infection, colonization of mucosal sites. Many studies have correlated agglutination and protection, but the contribution of the agglutinating effect of antibody has largely been assumed, and to our knowledge never directly demonstrated (21-23). In the case of H. influenzae, for instance, protection against colonization by antibody was also shown to be Fc-independent, although the mechanism of this effect with F(ab’)2 was not analyzed (24).
Several independent lines of experimental evidence contributed to our conclusions. First, only specific antibody that agglutinated the challenge strain was protective against that strain. Second, protection of previously colonized immune animals correlated with the rapid agglutination of bacteria in vivo during rechallenge. Third, prior treatment of IgG to remove its divalent binding, either by generating Fab or rIgG fragments, prevented both agglutination and protection but still allowed binding to the organism. Fourth, the bacterial protease that cleaves human IgA1 to yield monovalent Fabα fragments also eliminated both agglutination and protection.
Our findings demonstrate that agglutinating antibody contributes significantly to mucosal defense by blocking the acquisition of colonization, including through host-to-host transmission. We cannot rule out a lesser role of Fab fragments in mucosal protection, it is, however, difficult to assess any potential effects on bacterial adherence to the epithelium or neutralization with in vivo studies. Passive immunization with agglutinating antibody that protected against primary colonization had little impact once colonization was established. This temporal difference is distinct from the requirement for Th17-dependent cell-mediated immune clearance of established colonization (25, 26). Our observations suggest that agglutination may prevent bacteria from accessing the epithelial surfaces where stable colonization occurs. Large aggregates of bacteria could be more easily swept away by mucociliary flow or other mechanical factors. Bacterial characteristics that promote association with mucus have previously been shown to enhance their removal (27). We cannot exclude the possibility that bacteria are also neutralized more effectively within aggregates, since it is difficult to compare growth characteristics under agglutinating conditions. Although we have previously reported that pneumococci that grow as chains of increased length, with more surface area/particle, adhere better to epithelial cells in vitro and colonize more efficiently, these forms are far smaller than the large aggregates described in the current study (28). We have also reported that aggregation of bacteria promotes complement activation and complement-dependent killing and that this effect is enhanced by agglutinating antibody (29). However, in the current study mucosal protection by passively administered antibodies did not require complement or Fc components required for phagocytic killing.
Our observations were based largely on the use of passive immunization with IgG, which can bridge its targets due to its divalent binding characteristic. Most mucosal antibody, however, is secretory polymeric IgA (tetravalent) or IgM (decaor dodecavalent), which should agglutinate bacteria even more efficiently. Polymeric anti-capsular IgA has been shown to mediate agglutination of pneumococci more effectively than its monomeric form (30). Our data confirm that bacterial IgA1 protease hinders the agglutinating and protective effect of protease-sensitive human IgA1 and extend these results by confirming this effect in vivo on mucosal surfaces of the upper respiratory tract. The importance of circumventing IgA1, which accounts for ~90% of airway immunoglobulins in humans, may contribute to the successful colonization by members of the genera Streptococcus, Haemophilus and Neisseriae, each of which express an IgA1 protease (15, 16). These proteases cleave only within the hinge region to generate non-agglutinating Fabα. Despite their shared function, these proteins encompass different enzymatic classes and cleave different peptide bonds, suggesting that the bacterial proteases that relieve the agglutinating effect of human IgA1 are an example of convergent evolution. Because protease-expressing organisms are able to evade the protective effect of IgA1, other antibodies on mucosal surfaces, such as IgG derived from the plasma pool, are critical for mucosal defense. Note that our experiments using immune mice, which generated anti-pneumococcal IgA, would not have allowed for evasion of protection by the protease since mouse IgA lacks the enzymatic target.
Encapsulation by a thick layer of polysaccharide protects organisms from recognition of its underlying structures by antibody. However, the capsule is also the Achilles heel of encapsulated bacteria, since it is an abundant antigen that induces agglutinating antibody. When given systemically, sufficient amounts of protease-resistant immunoglobulin are generated to access and protect mucosal surfaces either by passive transudation or possibly via active transport of IgG by FcRn. In response to the selective pressure of immunoglobulin, encapsulated bacteria vary the composition of their surface polysaccharide (antigenic variation). Our results suggest that alternative vaccine strategies to target more conserved bacterial antigens would similarly need to generate agglutinating antibody in order to provide comparable levels of mucosal protection. Moreover, protective immune responses to other pathogens might also benefit from strategies that induce agglutinating antibodies.
METHODS
Bacterial strains and culture conditions
S. pneumoniae strains were grown in tryptic soy broth (BD, Franklin Lakes, NJ) at 37°C in a non-shaking water bath to an O.D.620=0.5. Strains used were clinical isolates selected for their ability to colonize the murine nasopharynx and included pneumococcal serotypes 2 (D39) (31), 4 (TIGR4) (32), 6A (13), 9V (Collection of R. Austrian), 14 (Collection of E. Janoff) and 23F (9). D39Δiga, an insertion-deletion mutant lacking the pneumococcal IgA1-protease, was previously described (33). All strains were passaged intranasally (IN) in mice prior to preparation of frozen stocks.
Immunization reagents
Hyperimmune rabbit anti-pneumococcal sera were obtained from the collection of the late Dr. Robert Austrian (University of Pennsylvania). Anti-sera were generated by serial immunization of rabbits with formalin-treated heat-killed whole bacteria of the specified type as previously described (34). Each strain used for generating anti-pneumococcal serum was distinct from the type-matched challenge isolates described above, and would be recognized by anti-capsular antibodies but not necessarily antibodies against other underlying antigens.
IgG was purified from rabbit anti-pneumococcal sera using a HiTrap Protein G column and AKTA FPLC (GE Healthcare Life Sciences, Pittsburgh, PA). IgG was buffer exchanged into PBS using Amicon 10k MWCO centrifugal filters (Millipore, Billerica, MA).
F(ab’)2 fragments were prepared from IgG purified from rabbit anti-pneumococcal serum using immobilized pepsin (Thermo Fisher Scientific, Waltham, MA) which digests the Fc fragment into small peptides. Briefly, IgG was incubated with immobilized pepsin at 37 °C for 5.5 hrs with continuous end-over-end rotation. Beads were removed by centrifugation and undigested IgG was removed by Protein A Nab column (Thermo Fisher Scientific). F(ab’)2 fragments were buffer exchanged into PBS and concentrated using Amicon 10k MWCO spin filters.
Fab fragments were prepared from IgG purified from rabbit anti-pneumococcal serum using immobilized papain (Thermo Fisher Scientific). Briefly, IgG was incubated with immobilized papain at 37 °C for 17 hrs with continuous end-over-end rotation. Beads were removed by centrifugation and undigested IgG was removed by size exclusion chromatography using a HiPrep 26/60 Sephacryl S200 HR column (GE Healthcare Life Sciences) and an AKTA FPLC. Due to the similar size of cleaved Fc and Fab, these fragments co-eluted. In pilot experiments, in which the papain-digested IgG was serially passed through a Protein A column until all Fc fragments were removed, there was no difference in protection in the presence or absence of this fragment.
Monovalent, reduced IgG (rIgG) was generated from rabbit anti-pneumococcal serum by partial reduction under mild reducing conditions that preferentially reduces the hinge region disulphide bonds (14, 35). Briefly, whole IgG was incubated in 25mM DTT for 1.5 hr at 37 °C. The rIgG was then alkylated with 25mM iodoacetamide for 30min at 37°C to prevent re-oxidation to whole IgG. Undigested IgG and overly reduced IgG species were then removed using size exclusion chromatography as described above. The ~75kD rIgG fraction consisting of one heavy and one light chain was buffer exchanged into PBS.
Human mAbs specific for S. pneumoniae serotypes 2 (IgA1 and IgA2), and serotype 8 (IgA1) were purified to >98.5% purity from B cells of immunized healthy adults that had been fused with the K6H6/B5 mouse–human heteromyeloma, as previously described (30, 33).
Passive immunization experiments
C57Bl/6J (Jackson Laboratories, Bar Harbor, ME) and B6;129S4-C3tm1Crr/J (C3-/-, kind gift of Elizabeth Grice, University of Pennsylvania) mice were housed in accordance with Institutional Animal Care and Use Committee protocols. 5-6 weeks old adult mice were immunized intraperitoneally (IP) with rabbit anti-pneumococcal sera, IgG, IgG fragments, mAbs, or vehicle controls. Unless specified otherwise, 4 hrs post-immunization mice were inoculated intranasally (IN), with 10μl containing approximately 2 × 104 CFU of the pneumococcal strain indicated in PBS. At 20 hours post pneumococcal challenge, mice were euthanized, the trachea cannulated, and 200μl of PBS instilled and lavage fluid was collected from the nares for quantitative culture. Lavage fluid was vortexed vigorously prior to plating to ensure any bacterial aggregates were dissociated. In pilot experiments we confirmed that agglutination by TS antiserum did not reduce colony counts using this plating procedure. Selective media was used to inhibit the growth of contaminants; neomycin (5μg/ml) for wild-type strains or spectinomycin (200μg/ml) for the Δiga mutant. The limit of detection was 2 CFU/animal, unless otherwise indicated. The success of passive immunization was confirmed by Western analysis of serum obtained at the time of sacrifice for each animal tested. The infant mouse protection experiment was performed on 14-day-old mice passively immunized IP with a volume of 25μl or less and challenged IN with 3μl containing 1-2 × 103 CFU. Protection was assessed as described above for adult mice.
Quantitative real-time PCR (qPCR)
In order to confirm bacterial load in nasal lavages pneumococcal gyrA was amplified by qPCR using the following primers: forward, CCCTTTGGCAGTCCGACCA; reverse, ACGTGGGGGTCGTGGTGTCC. 2μl of nasal lavages from passive immunization experiments were added directly to a qPCR reaction. The qPCR reaction was performed in a total volume of 20 μl using the Power SYBR Green PCR Master Mix (Life Technologies, Grand Island, NY), containing 60 nM of each primer. The reaction conditions for amplification of DNA were 95°C for 10 min, and 40 cycles of 95°C for 15 sec and 60°C for 1 min, run on a StepOne Plus Real Time PCR System (Life Technologies). All reactions were performed in duplicate and mean values were calculated based on a standard curve of DNA from known bacterial quantities. Data were analyzed using StepOne Software v2.0 (Life Technologies).
Pneumococcal transmission
1 in 4 mice per litter of 4-day-old pups were inoculated IN with 1-2 × 103 CFU of the serotype 23F isolate. These mice were considered ‘index’ mice, and the remaining un-inoculated mice in the litter ‘contact’ mice. In the immune group, on day 8, 10 and 12 (age), contact mice were passively immunized IP with rabbit anti-pneumococcal antisera (25μl). Both index and contact mice received 2×104 TCID50 of influenza A strain X31, a H3N2 recombinant of mouse adapted PR8 in 3μl IN on day 8 (age) to promote nasal shedding. Influenza A is required for pneumococcal transmission between littermates (11) and was only used in transmission experiments. All mice were sacrificed on day 14 (age) and lavages were plated to determine pneumococcal transmission from index to contacts.
Western blot analysis
Mouse samples (10μl serum diluted 1:1000, or 20μl undiluted nasal lavage) were resolved on a 10% Mini-PROTEAN®TGX™ gel using a Tetra cell (Bio-Rad, Hercules, CA). Reduced samples were prepared with Laemmli sample buffer containing β-mercaptoethanol (βME) and boiled for 5 mins. Non-reduced samples were prepared without βME and boiling. Gels were transferred onto PVDF membranes by semi-dry transfer apparatus (Bio-Rad). Membranes were blocked in Pierce Protein-Free (TBS) Blocking Buffer (Thermo Fisher Scientific). Whole rabbit IgG was detected in lavages using anti-rabbit IgG (whole molecule) (Sigma, St. Louis, MO). Rabbit IgG fragments were detected using anti-rabbit IgG (F(ab’)2 specific) (Jackson ImmunoResearch Laboratories, West Grove, PA). hmAbs were detected using anti-human IgA (α-chain specific) (Sigma).
Enzyme-linked immunosorbent assays
To determine target-specific (anti-capsular) antibody binding, capsular polysaccharide of the type specified (ATCC) was coated (2.5μg/ml) onto Immulon 1B 96 well plates (Thermo Fisher Scientific) for 5 hours at 37°C. TS antisera, intact IgG or IgG fragments were added in doubling dilutions and incubated at room temperature overnight. Binding of antibody or fragments was detected with biotinconjugated anti-rabbit IgG (light chain specific) (Cell Signaling Technology, Danvers, MA) followed by streptavidin conjugated to alkaline phosphatase. Signal was developed with p-nitrophenyl phosphate (Sigma), and the absorbance at 415 nm was recorded after a standardized period of 30min. A capture ELISA was used to calculate the concentration of total rabbit IgG in TS antisera. Briefly, Immulon 2HB plates (Thermo Fisher Scientific) were coated with anti-rabbit IgG (light chain specific) (2.5μg/ml) overnight at 4°C. TS rabbit antisera were added in doubling dilutions overnight at room temperature. Bound total rabbit IgG was detected by anti-rabbit IgG (whole molecule specific) conjugated to alkaline phosphatase (Sigma). Polysaccharide capsule-specific antibody titer was then calculated by comparing the titers of total rabbit IgG to target-specific IgG. Murine titers of IgA in lavage fluid and IgG in serum were determined as previously described (13). Human type 2 capsule-specific IgA was measured as described (30).
Agglutination assays
To compare Fab and F(ab’)2, the serotype 14 isolate was diluted in tryptic soy broth to O.D.620=0.1 and incubated with 100μg/ml of fragments for 1 hour at 37°C. Samples were resuspended and placed onto glass slide to visualize agglutination. For rIgG the serotype 6A isolate was grown to mid-log phase and mixed with antibody (at 3.5mg/ml) on glass slide at 3:1 (v:v) ratio. Agglutination was visualized at 200× magnification on a Nikon E600 Eclipse microscope equipped with a liquid crystal (Micro-color RGB-MS-C, CRI Inc., Boston, MA) and a high-resolution charge-coupled device (CCD) digital camera (CoolSnap CF, Roper Scientific, Tucson, AZ) with Nomarski optics. All image analysis was performed with iVision-Mac™ (BioVision Technologies, Exton, PA).
Cleavage of human IgA in vitro
hMAb (IgA1 to serotype 8, 960μg/ml) was incubated with mid-log phase D39 or D39Δiga at a ratio of 1:30 (v:v), and incubated at 37°C overnight. Cultures were centrifuged to pellet bacteria and supernatant was collected and separated on non-reducing gel to identify pneumococcal IgA1-protease dependent cleavage of unbound IgA1 (see Western protocol) (17). Supernatant was also used for agglutination assay as described above for rIgG samples.
Immunofluorescence staining of mouse nasal tissue
C57Bl/6J and B6.129S2-Ighmtm1Cgn/J (μMT) mice (Jackson Laboratories) were immunized IN with 3 doses of strain TIGR4 (106-107 CFU/dose) (immune) or PBS (naïve) on day 0, 7 and 14. Two weeks after the final dose, when initial colonizing strain has been cleared, mice were challenged IN with 107 CFU strain TIGR4. At 30 mins post-challenge, the mice were sacrificed, heads collected, fixed, decalcified, frozen embedded and 5μm nasal tissue sections were cut. Frozen tissue sections were stained as previously described (27). Briefly, sections were fixed in 1:1 acetone:methanol and blocked with StartingBlock protein blocker (Thermo Fisher Scientific). Bacteria were stained with typing sera (Staten Serum Institut, Copenhagan, Denmark) and detected with donkey anti-rabbit cy3. Host cells were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (Life Technologies). Fluorescent image acquisition was performed as described above for agglutination assays.
Complement fixation assay
Serotype 14 and 23F isolates were incubated with 2% fresh mouse serum as a source of complement, with or without TS antisera (1:1000 dilution) and incubated at 37°C for 30mins. Bacteria were washed and C3 deposited on the bacteria was detected by FITC-conjugated anti-mouse C3 (Cedarlane, Burlington, Canada) using a FacsCalibur (Becton Dickinson, Franklin Lakes, NJ). Inactivated complement, heated to 56°C for 30mins, and serum from C3−/− mice were used as negative controls. Flow cytometry data was analyzed using FlowJo 10 (Tree Star, Ashland, OR).
Animal study approval
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Statistical Analysis
Colonization density was expressed as the log CFU/animal for calculation of medians. Mann-Whitney test was performed when two groups were compared; Kruskal-Wallis test with Dunn's post-test was performed when groups of three or more were compared (GraphPad Prism 5, GraphPad Software, Inc, La Jolla, CA).
ACKNOWLEDGEMENTS
We thank Jan Erikson from The Wistar Institute for providing X31 Influenza virus. This work was supported by the US Public Health Service, grant number AI38446 and AI05168 (J.N.W.), AI092468 and AI108479 (E.N.J) and the Robert Austrian Research Award in Pneumococcal Vaccinology (A.M.R). Additional support was provided by the NIH/NIDDK Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306) and its Molecular Pathology and Imaging core facilities, and the Mucosal and Vaccine Research Program Colorado (MAVRC).
Footnotes
The authors have no conflict of interest to declare.
REFERENCES
- 1.Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat. Rev. Immunol. 2013;13(7):519–33. doi: 10.1038/nri3466. [DOI] [PubMed] [Google Scholar]
- 2.Kauppi M, Eskola J, Kayhty H. Anti-capsular polysaccharide antibody concentrations in saliva after immunization with Haemophilus influenzae type b conjugate vaccines. Pediatr Infect Dis J. 1995;14(4):286–94. doi: 10.1097/00006454-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Whitney C, Farley M, Hadler J, Harrison L, Bennett N, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 4.Dagan R, Givon-Lavi N, Fraser D, Lipsitch M, Siber GR, Kohberger R. Serum serotype-specific pneumococcal anticapsular immunoglobulin G concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J. Infect. Dis. 2005;192(3):367–76. doi: 10.1086/431679. [DOI] [PubMed] [Google Scholar]
- 5.Trotter CL, McVernon J, Ramsay ME, Whitney CG, Mulholland EK, Goldblatt D, et al. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine. 2008;26(35):4434–45. doi: 10.1016/j.vaccine.2008.05.073. [DOI] [PubMed] [Google Scholar]
- 6.Lexau C, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley M, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 7.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N. Engl. J. Med. 2013;369(2):155–63. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiser JN, Nahm MN. Immunity to Extracellular Bacteria. In: Paul WE, editor. Fundamental Immunology. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 1182–203. [Google Scholar]
- 9.McCool T, Cate T, Moy G, Weiser J. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 2002;195:359–65. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2008;6(4):288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 11.Diavatopoulos DA, Short KR, Price JT, Wilksch JJ, Brown LE, Briles DE, et al. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J. 2010;24(6):1789–98. doi: 10.1096/fj.09-146779. [DOI] [PubMed] [Google Scholar]
- 12.Johnson S, Rubin L, Romero-Steiner S, Dykes J, Pais L, Rizvi A, et al. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J. Infect. Dis. 1999;180:133–40. doi: 10.1086/314845. [DOI] [PubMed] [Google Scholar]
- 13.Roche AM, King SJ, Weiser JN. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect. Immun. 2007;75(5):2469–75. doi: 10.1128/IAI.01972-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber JR, Barrus VA, Siber GR. Decreased protective efficacy of reduced and alkylated human immune serum globulin in experimental infection with Haemophilus influenzae type b. Infect. Immun. 1985;47(1):142–8. [Google Scholar]
- 15.Kilian M, Reinholdt J. Immunoglobulin A1 proteases of pathogenic and commensal bacteria of the respiratory tract. In: Nataro JP, Cohen PS, Mobley HLT, Weiser JN, editors. Colonization of Mucosal Surfaces. ASM Press; Washington, DC: 2005. pp. 119–30. [Google Scholar]
- 16.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal immunol. 2011;4(6):590–7. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 17.Bender MH, Weiser JN. The atypical amino-terminal LPNTG-containing domain of the pneumococcal human IgA1-specific protease is required for proper enzyme localization and function. Mol. Microbiol. 2006;61(2):526–43. doi: 10.1111/j.1365-2958.2006.05256.x. [DOI] [PubMed] [Google Scholar]
- 18.Janoff EN, Rubins JB, Fasching C, Charboneau D, Rahkola JT, Plaut AG, et al. Pneumococcal IgA1 protease subverts specific protection by human IgA1. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.41. doi: 10.1038/mi.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metchnikoff E. Etudes sur l'immunité. IV. L'immunité des cobayes vaccinées contre le Vibrio Metchnikowii. Ann. Inst. Pasteur (Paris) 1891;5:465–78. [Google Scholar]
- 20.Neufeld F. Verber die Agglutination der Pneumokokken und uber die Theorie der Agglutination. Z. Hyg. Infektionskr. 1902;40:54. [Google Scholar]
- 21.Sawada S, Suzuki M, Kawamura T, Fujinaga S, Masuho Y, Tomibe K. Protection against infection with Pseudomonas aeruginosa by passive transfer of monoclonal antibodies to lipopolysaccharides and outer membrane proteins. J. Infect. Dis. 1984;150(4):570–6. doi: 10.1093/infdis/150.4.570. [DOI] [PubMed] [Google Scholar]
- 22.Cherry JD, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. 1998;16(20):1901–6. doi: 10.1016/s0264-410x(98)00226-6. [DOI] [PubMed] [Google Scholar]
- 23.Mathias A, Longet S, Corthesy B. Agglutinating secretory IgA preserves intestinal epithelial cell integrity during apical infection by Shigella flexneri. Infect. Immun. 2013;81(8):3027–34. doi: 10.1128/IAI.00303-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kauppi M, Saarinen L, Kayhty H. Anti-capsular polysaccharide antibodies reduce nasopharyngeal colonization by Haemophilus influenzae type b in infant rats. J. Infect. Dis. 1993;167(2):365–71. doi: 10.1093/infdis/167.2.365. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 2009;119(7):1899–909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malley R, Trzcinski K, Srivastava A, Thompson C, Anderson P, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA. 2005;102:4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 2007;75(1):83–90. doi: 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez JL, Dalia AB, Weiser JN. Increased chain length promotes pneumococcal adherence and colonization. Infect. Immun. 2012;80(10):3454–9. doi: 10.1128/IAI.00587-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalia AB, Weiser JN. Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell host & microbe. 2011;10(5):486–96. doi: 10.1016/j.chom.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fasching CE, Grossman T, Corthesy B, Plaut AG, Weiser JN, Janoff EN. Impact of the molecular form of immunoglobulin A on functional activity in defense against Streptococcus pneumoniae. Infect. Immun. 2007;75(4):1801–10. doi: 10.1128/IAI.01758-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avery OT, MacLeod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med. 1944;79:137–57. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293(5529):498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 33.Weiser J, Bae D, Fasching C, Scamurra R, Ratner A, Janoff E. Antibody-enhanced pneumococcal adherence requires IgA1 protease. Proc. Natl. Acad. Sci. USA. 2003;100:415–20. doi: 10.1073/pnas.0637469100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiffman G, Bornstein DL, Austrian R. Capsulation of Pneumococcus with soluble cell wall-like polysaccharide. II. Nonidentity of cell wall and soluble cell wall-like polysaccharides derived from the same and different pneumococcal strains. J. Exp. Med. 1971;134(3):600–17. doi: 10.1084/jem.134.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machino Y, Ohta H, Suzuki E, Higurashi S, Tezuka T, Nagashima H, et al. Effect of immunoglobulin G (IgG) interchain disulfide bond cleavage on efficacy of intravenous immunoglobulin for immune thrombocytopenic purpura (ITP). Clin. Exp. Immunol. 2010;162(3):415–24. doi: 10.1111/j.1365-2249.2010.04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]