Abstract
Background:
Maternal prenatal mental health has been shown to be associated with adverse consequences for the mother and the child. However, studies considering the effect of prenatal depressive symptoms are lacking. The aim of this study was to examine the influence of antenatal depressive symptoms on obstetric outcomes and to determine associations between antenatal and postpartum depressions.
Materials and Methods:
This was a prospective cohort study. The Edinburgh postnatal depression scale (EPDS) questionnaire was completed by pregnant women receiving obstetrical care at Seoul St. Mary's hospital in the third trimester of gestation. The electronic medical records were reviewed after delivery and perinatal outcomes were evaluated. The association between antenatal and postpartum depression was analyzed using the EPDS questionnaire, which was completed by the same women within 2 months of delivery.
Results:
Of the 467 participants, 26.34% (n = 123) had antenatal depressive symptoms, with EPDS scores of ≥10. There were no significant perinatal outcomes associated with antenatal depressive symptoms. During the postpartum period, 192 of the women in the initial study cohort were given the EPDS again as a follow-up. Of the 192 participants, 56 (29.17%) scored >10. Spearman correlation coefficient between the antenatal and postpartum EPDS scores was 0.604, which was statistically significant (P < 0.001).
Conclusion:
Antenatal depression does not lead to unfavorable perinatal outcomes. However, screening for antenatal depression may be helpful to identify women at risk of postpartum depression.
Keywords: Depression, postpartum, pregnancy, prenatal care, pregnancy outcome
INTRODUCTION
Young women are considered to be at high risk for depression.[1] The likelihood of developing depressive disorder is nearly double in women than in men, and the highest risk period for women occurs during the fertile years. Numerous epidemiological studies have shown that up to 10-15% of women of childbearing age show depressive symptoms.[2,3,4] In particular, depressive disorder occurs in about 10% of pregnant women.[2]
Despite the considerably high incidence of antenatal depression, little attention has been directed toward mood and anxiety symptoms during pregnancy. A plethora of studies have focused on the detection and treatment of postpartum mental health problems.[5,6] Recently, more attention has been paid to the connection between antenatal and postnatal depressive symptoms. Although one study found that the profiles of antenatal and postnatal depressive symptoms were different,[7] other studies have found that among women with postpartum depression, over 50% had depression identified either before or during pregnancy.[8]
The need to consider a perinatal spectrum of depressive symptoms is also underscored by the possibility that antenatal depression has been associated with obstetrical complications. Studies have repeatedly shown that mental health problems during pregnancy are associated with impaired obstetric and infant outcomes. Antenatal stress and depression, for example, have been linked to preterm birth, intrauterine growth restriction, and low-weight infants.[9,10,11] Studies indicate that expectant mothers with prenatal depression may be less likely to attend antenatal examinations, a situation that contributes to unfavorable pregnancy outcomes such as inadequate weight gain, preterm delivery, low birth weight, and poor neonatal adaptation.[12,13,14,15] The offspring of mothers with depressive symptoms may experience long-term sequelae such as impaired neurological, cognitive, emotional, and behavioral development.[2] Other studies, however, reportedly found no association between prenatal depression and obstetric outcome variables.[16,17,18]
Due to conflicting reports on the impact of antenatal depression and few studies for exploring the antenatal and postpartum depression in the Korean population, we evaluated a group of women during and after pregnancy to examine the influences of antenatal depressive symptoms on obstetric outcomes and the association between antenatal and postpartum depressions in Korean pregnant women.
MATERIALS AND METHODS
Participants and procedures
This was a prospective cohort study. All pregnant women in the third trimester of gestation who received antenatal care at Seoul St. Mary's Hospital in Seoul, Korea from July 2009 to June 2010 were asked to participate in this prospective study. The aims and procedures for the study were explained to the women when they visited the hospital for antenatal care. A total of 494 pregnant women agreed to join the study were initially enrolled, and written consent was obtained. Of the participants, 467 (94.5%) delivered their babies at participating hospital, and the electronic medical records of the participants and their newborns were obtained and reviewed after delivery. Women with missing information for the independent variables were excluded from the analysis.
The study was approved by the Ethics Committees at the Clinical Research Coordinating Center of the Catholic Medical Center during 2009 (KC09OIS01368).
Measures
Depressive symptoms were measured using the Edinburgh postnatal depression scale (EPDS), a 10-item self-reporting survey. This tool was designed by Cox et al. to screen for postpartum depression.[19] It is the most widely used screening questionnaire for postpartum depression[20] and is also used to screen for antenatal depressive symptoms.[21] Cutoff score of 9/10 was used in this study. A cutoff value of 10 in the second and third trimesters was validated as the best combination of sensitivity, specificity, and positive predictive value.[22] Cronbach's alpha coefficient of the EPDS in this study was 0.82, which suggests good internal consistency.
The Korean version of the EPDS was filled out by the participants. They also completed a questionnaire that recorded sociodemographic data and obstetrical characteristics. The EDPS questionnaire was administered once more after the women visited the hospital for postpartum care within 2 months of delivery.
The sociodemographic and obstetrical risk factors questionnaire included questions on age, pregestational body weight (underweight, BMI [body mass index] <18.5; normal, 18.5 ≤ BMI < 23; overweight, 23 ≤ BMI < 25; and obese, 25 ≤BMI, according to WHO definitions for Asian populations[23]) parity, gestational age, the highest level of education completed, occupation, alcohol habits, past and/or current health problems, history of adverse obstetrical outcomes such as abortion or preterm delivery, marital status, monthly income ($2000-5000) was the range considered to be an average monthly income, based on the median monthly domestic household income in Korea; Statistics Korea 2007), family composit ion (extended family or nuclear family), and if the pregnancy was a planned one.
We analyzed the association between antenatal depression and perinatal outcomes on the basis of maternal and neonatal factors, respectively. The maternal factors included were gestational age at delivery, maternal gestational weight gain, cesarean section rate, preterm delivery, and oligohydramnios, and the neonatal factors included were neonatal birth weight, Apgar score. The criterion for birth weight was based on the gestational age at delivery, where cases with weights below the 10th percentile or above the 90th percentile were classified as small for gestational age (SGA) and large for gestational age, respectively; this criterion was derived from a worldwide study conducted by Alexander et al.[24] All of the data about perinatal outcomes were obtained from medical records.
Sample size determination and statistical analysis
The required sample size was determined based on the correlation between the antenatal and postpartum EPDS scores as follows. First, we decided on a correlation coefficient of P = 0.3, which was suggested by Cohen as a medium effect, along with a two-sided significance level of 0.05 and 95% power.[25] Based on these values, we found that at least 138 patients were needed to obtain statistically significant results; however, we also suspected that there would be a high-drop-out rate (>70%), and therefore, we enrolled more than 460 patients in the present study.
The statistical software package SPSS 13.0 was used for analysis. The continuous variables were presented as mean ± standard deviation and analyzed using an independent t-test. In addition, the clinical characteristics and obstetric categorical outcomes were presented as frequency (%) and analyzed using Pearson chi-square or Fisher exact test, as appropriate. Gamma and Spearman correlation coefficient were used to analyze the relationship between the antenatal and postpartum EPDS scores. We considered two-sided P < 0.05 as statistically significant.
RESULTS
Characteristics of study population
A total of 1052 pregnant women visited our hospital during their third trimester of gestation. Among them, 467 pregnant women (44.4%) were included in the study and underwent the delivery at the participating hospital. Participants were divided into two groups on the basis of EPDS scores: A nondepressed group (n = 344, EPDS score ≤9) and depressed group (n = 123, EPDS score ≥10).
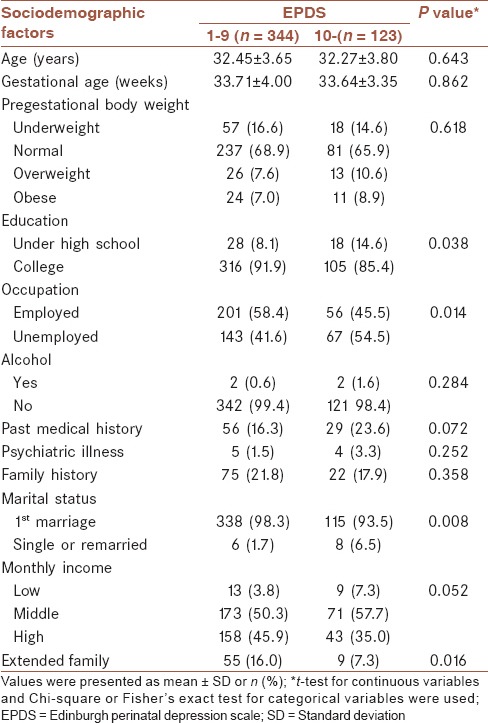
Table 1 presents the sociodemographic characteristics of the participants. More than half of the participants were between the ages of 30 and 35 years. There were significant differences regarding the women's educational and occupational status. There were more pregnant women with low levels of education in the depressed group than in the nondepressed group (14.6% vs. 8.1%, respectively, P = 0.038). There were also more unemployed women in the depressed group than in nondepressed groups (54.5% vs. 41.6%, respectively, P = 0.014). A significant difference was noted in the marital status between the two groups; that is, the depressed group included more women who were unmarried or had remarried than did the nondepressed group (6.5% vs. 1.7%, respectively, P = 0.08). The nondepressed group had more women who were in extended families than those in the depressed groups (16.0% vs. 7.3%, respectively, P = 0.016).
Table 1.
Sociodemographic factors according to antenatal depressive symptoms
Obstetrical characteristics in the two groups of women were also compared [Table 2]. The depressed group had more multiparous pregnant women more than the nondepressed groups (49.6% vs. 39.0%, respectively, P = 0.040). In the depressed groups, there were more women who had adverse obstetrical histories than in the nondepressed group; these included abortion (39.0% vs. 14.0%, respectively, P < 0.001) and fetal abnormal findings (10.6% vs. 4.9%, respectively, P = 0.029).
Table 2.
Obstetrical characteristics according to antenatal depressive symptoms

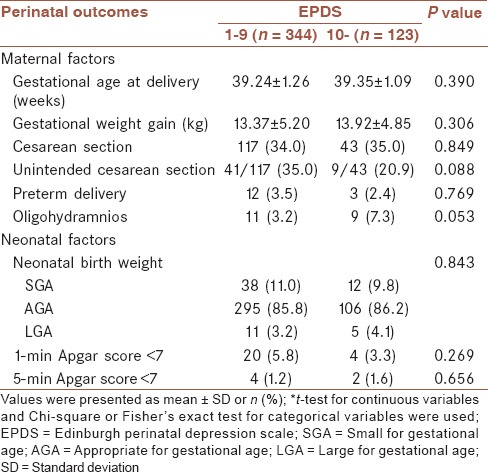
Perinatal outcomes
We reviewed the delivery data pertaining to the general health of the mother-newborn pairs in the immediate postpartum period. As shown in Table 3, no significant differences were found for gestational age at delivery (P = 0.390), gestational weight gain (P = 0.306), cesarean section rate (P = 0.849), unintended cesarean section rate (P = 0.088), preterm delivery (P = 0.769). Oligohydramnios occurred more frequently in the depressed group than in the nondepressed group (7.3% vs. 3.2%, respectively, P = 0.053). However, it was not statistically significant. We also found that antenatal depression was not significantly associated with adversely neonatal outcomes such as neonatal birth weight (P = 0.843), low 1- and 5-min Apgar score (P = 0.269 and 0.656).
Table 3.
Perinatal outcomes according to antenatal depressive symptoms
Antenatal and postnatal Edinburgh postnatal depression scale
The scores of 123 out of 467 women (26.3%) were ≥10 points during their third trimester. Of the 467 pregnant women, 192 were followed-up during the postpartum period. Out of 192 women, 56 (29.2%) scored >10 points. The prevalences of antenatal and postnatal depression were similar as assessed by self-reported survey. Women in the antenatal depressed groups developed postpartum depressive symptoms more frequently than those in the antenatal nondepressed group (59.6% vs. 17.9%, respectively, P < 0.001). A highly significant association was noted between the antenatal and postnatal EPDS scores, with a Spearman correlation coefficient of 0.604 and gamma value of 0.743 [Table 4].
Table 4.
Association between antenatal and postpartum depressive symptoms (n = 192)

Drop-out analysis
Of the 467 pregnant women who underwent the delivery at the participating hospital, 192 (41.1%) were administered the EPDS once more during the postpartum period. A busy clinical setting may have contributed to the high-drop-out rate. We compared the basic characteristics between “postpartum followed-up participants” and “drop-out participants” to rule out any selection bias. Because no significant differences were observed in the sociodemographic and obstetric data including the prevalence of psychiatric illness between the two groups, we concluded that “postpartum followed-up participants” could be used as a representative sample of participants in the third trimester.
DISCUSSION
This study clearly demonstrates a strong relationship between perinatal depressive symptoms in the third trimester and postpartum although antenatal depression does not lead to unfavorable perinatal outcomes. These findings support the existence of a continuum in prenatal depressive symptoms from the third trimester to the postpartum period and indicate that the EPDS has good predictive power to identify women in the third trimester of pregnancy who are at high risk for elevated postpartum EPDS scores.
Senturk et al. analyzed the relationship between social support and antenatal depression in 772 women in their third trimester of pregnancy. They reported that predisposing factors for antenatal depression included a previous history of depression or mental disorder; moreover, lack of social support was strongly implicated in the etiology of perinatal depression. Particularly, lower quality relationships between key family members were strongly associated with third-trimester depression.[26] Our findings not only support this result but also implicate further risk factors associated with antenatal depression. Our data show that socioeconomic status and the burden of childcare were linked to depressive symptoms. The quality of marital life and familial support could be considered important correlates.
In our study, antenatal depression had no immediate impact on perinatal outcomes. Chung et al. reported that women who showed a significant degree of depression in the third trimester of pregnancy had an increased risk of requiring epidural anesthesia, cesarean section, or instrument-assisted vaginal delivery during childbirth compared with less depressed mothers. The infants born to these depressed mothers were more likely to be admitted to a neonatal intensive care unit than were neonates born to less depressed mothers.[27] However, Andersson et al. reported in a population-based study that depressive or anxiety disorders were not associated with preterm delivery or SGA birth in Sweden.[16] Perkin et al. did not find maternal depression to be associated with such obstetric complications as preterm delivery, labor induction, labor acceleration, analgesia use, and nonspontaneous delivery.[17] Suri et al. also found no significant impact of prenatal depression on infant gestational age, birth weight, Apgar score, or admission to the neonatal intensive care unit.[18] These findings support the results of our study.
There are some limitations of our study. First, the EPDS has well-known limitations, as it cannot diagnose depression. However, although this self-reported questionnaire is not diagnostic, it is sensitive to depressive symptoms. Second, it is possible that some participants may have under-reported their depressive symptoms on the screening questionnaire. Furthermore, more pregnant women who have depressive symptoms may be dropped from the study because only those who agreed to join the study filled out a questionnaire. A third limitation is that not all participants were actively followed up during the postpartum period. However, the similarity of basic characteristics between the “postpartum followed-up” and “drop-out” groups would indicate that “postpartum followed-up participants” could be used as a representative sample.
Despite these limitations, our study's strength is that it provides a comprehensive understanding of antenatal depression. This study was designed to explore the influences of sociodemographic factors as potential confounders of antenatal depression. Moreover, other important dimensions such as obstetrical factors have been considered.
The findings of our study contribute to an understanding of antenatal depression and the importance of being adequately attentive to maternal mental health and well-being during pregnancy. The direction of research and clinical efforts needs to move towards understanding and managing antenatal depression. It has to be recognized that identifying antenatal depression and referring high-risk women to appropriate care are the duties of the obstetricians as primary healthcare providers for pregnant women. Identification of depression during pregnancy enables healthcare providers to follow up high-risk women and implement preventive interventions or clinical interviews to establish the diagnosis of antenatal depression.
AUTHORS’ CONTRIBUTION
SKCh contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work JCSh contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work YGP contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. IYP and HYK contributed in the conception of the work, and agreed for all aspects of the work.
ACKNOWLEDGMENT
We are grateful to all of the participants of this study. We also thank the staff at the Department of Biostatistics, College of Medicine, Catholic University of Korea.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lewinsohn PM, Rohde P, Seeley JR, Fischer SA. Age-cohort changes in the lifetime occurrence of depression and other mental disorders. J Abnorm Psychol. 1993;102:110–20. doi: 10.1037//0021-843x.102.1.110. [DOI] [PubMed] [Google Scholar]
- 2.Haas DM, Weida J, Smith R, Abernathy MP. A comparison of depression symptoms and histories in pregnant women. J Reprod Med. 2011;56:39–43. [PubMed] [Google Scholar]
- 3.Vollebergh WA, Iedema J, Bijl RV, de Graaf R, Smit F, Ormel J. The structure and stability of common mental disorders: The NEMESIS study. Arch Gen Psychiatry. 2001;58:597–603. doi: 10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- 4.Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: Cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 5.Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323:257–60. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: A systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 7.Kammerer M, Marks MN, Pinard C, Taylor A, von Castelberg B, Künzli H, et al. Symptoms associated with the DSM IV diagnosis of depression in pregnancy and post partum. Arch Womens Ment Health. 2009;12:135–41. doi: 10.1007/s00737-009-0062-9. [DOI] [PubMed] [Google Scholar]
- 8.Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164:1515–20. doi: 10.1176/appi.ajp.2007.06111893. [DOI] [PubMed] [Google Scholar]
- 9.Dayan J, Creveuil C, Marks MN, Conroy S, Herlicoviez M, Dreyfus M, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: A prospective cohort study among women with early and regular care. Psychosom Med. 2006;68:938–46. doi: 10.1097/01.psy.0000244025.20549.bd. [DOI] [PubMed] [Google Scholar]
- 10.Lau Y, Wong DF, Chan KS. The utility of screening for perinatal depression in the second trimester among Chinese: A three-wave prospective longitudinal study. Arch Womens Ment Health. 2010;13:153–64. doi: 10.1007/s00737-009-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–8. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 12.Da Costa D, Larouche J, Dritsa M, Brender W. Psychosocial correlates of prepartum and postpartum depressed mood. J Affect Disord. 2000;59:31–40. doi: 10.1016/s0165-0327(99)00128-7. [DOI] [PubMed] [Google Scholar]
- 13.Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal depression restricts fetal growth. Early Hum Dev. 2009;85:65–70. doi: 10.1016/j.earlhumdev.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misri S, Oberlander TF, Fairbrother N, Carter D, Ryan D, Kuan AJ, et al. Relation between prenatal maternal mood and anxiety and neonatal health. Can J Psychiatry. 2004;49:684–9. doi: 10.1177/070674370404901006. [DOI] [PubMed] [Google Scholar]
- 15.Pajulo M, Savonlahti E, Sourander A, Helenius H, Piha J. Antenatal depression, substance dependency and social support. J Affect Disord. 2001;65:9–17. doi: 10.1016/s0165-0327(00)00265-2. [DOI] [PubMed] [Google Scholar]
- 16.Andersson L, Sundström-Poromaa I, Wulff M, Aström M, Bixo M. Neonatal outcome following maternal antenatal depression and anxiety: A population-based study. Am J Epidemiol. 2004;159:872–81. doi: 10.1093/aje/kwh122. [DOI] [PubMed] [Google Scholar]
- 17.Perkin MR, Bland JM, Peacock JL, Anderson HR. The effect of anxiety and depression during pregnancy on obstetric complications. Br J Obstet Gynaecol. 1993;100:629–34. doi: 10.1111/j.1471-0528.1993.tb14228.x. [DOI] [PubMed] [Google Scholar]
- 18.Suri R, Altshuler L, Hendrick V, Rasgon N, Lee E, Mintz J. The impact of depression and fluoxetine treatment on obstetrical outcome. Arch Womens Ment Health. 2004;7:193–200. doi: 10.1007/s00737-004-0057-5. [DOI] [PubMed] [Google Scholar]
- 19.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 20.Boyd RC, Le HN, Somberg R. Review of screening instruments for postpartum depression. Arch Womens Ment Health. 2005;8:141–53. doi: 10.1007/s00737-005-0096-6. [DOI] [PubMed] [Google Scholar]
- 21.Felice E, Saliba J, Grech V, Cox J. Validation of the Maltese version of the Edinburgh Postnatal Depression Scale. Arch Womens Ment Health. 2006;9:75–80. doi: 10.1007/s00737-005-0099-3. [DOI] [PubMed] [Google Scholar]
- 22.Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen H, Bunevicius R, van Baar A, et al. Validation of the edinburgh depression scale during pregnancy. J Psychosom Res. 2011;70:385–9. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet. 2002;360:235. doi: 10.1016/S0140-6736(02)09512-0. [DOI] [PubMed] [Google Scholar]
- 24.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 26.Senturk V, Abas M, Berksun O, Stewart R. Social support and antenatal depression in extended and nuclear family environments in Turkey: A cross-sectional survey. BMC Psychiatry. 2011;11:48. doi: 10.1186/1471-244X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung TK, Lau TK, Yip AS, Chiu HF, Lee DT. Antepartum depressive symptomatology is associated with adverse obstetric and neonatal outcomes. Psychosom Med. 2001;63:830–4. doi: 10.1097/00006842-200109000-00017. [DOI] [PubMed] [Google Scholar]


