Abstract
Background:
Lateral epicondylitis is a common problem affecting 1-3% of the population. There has been much debate about the best treatment modality for this condition. There is, however, no conclusive evidence in support of any of the proposed treatment modalities. In this trial, we have studied the effect of corticosteroid injection (with or without splinting) with normal saline injection (with or without splinting).
Materials and Methods:
In this double-blind, randomized clinical trial, individuals were randomly assigned to either of four treatment groups and received either 40 mg depomedrol injection alone, 40 mg depomedrol injection with splinting, normal saline injection alone, or normal saline injection with splinting. They were evaluated using the visual analog scale (VAS) at weeks 2, 4 and 24 and with the Oxford elbow scale (OES) at 24 weeks.
Results:
A total of 79 patients were participated in the study. The corticosteroid injection groups had better pain relief as measured by VAS at 2 and 4 weeks compared with the two saline injection groups. Mean VAS difference at week 0 versus week 2 was 4.5 ± 0.9 and 2.8 ± 0.6 in corticosteroid injection groups and saline injection groups respectively (P < 0.01) but at 24 weeks, there was only moderate benefit reported for the group which received steroid injection and splinting (P < 0.01) compared to the saline injection groups. The saline injection groups reported better improvement in OES scores (20.1 ± 3.7) at the end of the trial compared corticosteroid injection groups (16.1 ± 2.9) (P < 0.05).
Conclusion:
Our results indicate that despite the clear pain reduction benefit associated with steroid injection in short term, this benefit in comparison with normal saline injection fades by the 24th week of follow-up.
Keywords: Corticosteroid, injection, lateral epicondylitis, normal saline
INTRODUCTION
Lateral epicondylitis is also known as tennis elbow or tendonitis of the extensor muscles of the forearm and refers to pain and tenderness over the lateral epicondyle of the humerus; this pain is exaggerated by resisted dorsiflexion of the wrist or the middle finger.[1,2] This condition is more common in patients aged between 35 and 55 years.[3] It has been estimated that 1-3% of the population suffer from this condition with equal distribution between men and women. Lateral epicondylitis is seen more commonly in the dominant arm and among Caucasians.[4]
Pathophysiology of lateral epicondylitis most commonly involves osteotendinous part of the extensor muscles of the wrist at their origin (the lateral epicondyle); among these, the tendon of the extensor carpi radialis brevis is more commonly involved. It is believed that the injury involves tears (either microscopic or macroscopic) in the origin of the extensor muscles of the wrist, which leads to an inflammatory response and in the chronic cases granulation and fibrous tissue.[4] Studies have shown that most cases of lateral epicondylitis involve fibrotic tissue and angiogenesis as a result of which some believe that “tendinosis” is a more correct term for the condition than “tendinitis.”[5]
It has been argued that the most common causes of lateral epicondylitis are overuse injuries.[6] Tendons are relatively hypovascular and as a result are prone to injuries which are cause by hypoxia such injuries usually result from occupational and athletic activities.[6,7] These activities include (but are not limited to): Backhand stroke in racquet sports, pitching in baseball, typing on keyboards, repetitive occupational hand movements such as hammering or driving screws and carrying heavy briefcases.[4]
Symptoms of the condition include pain on the lateral aspect of the elbow, reduced grip, increase in pain with activity and reduced strength of the extensor activities of the wrist. Despite the fact that this condition often lacks an inflammatory component, its main manifestation is pain.[5,8] The main sign of the lateral epicondylitis is tenderness of the lateral epicondyle coupled with pain on resisted dorsiflexion of either wrist or middle finger.[5]
There is much debate about the best treatment approaches for lateral epicondylitis. The evidence shows that 95% of patients heal either spontaneously or by conservative measures alone.[9] This has led some practitioners to believe that it is not necessary to treat lateral epicondylitis,[10] their argument is supported by studies such as the study by Smidt et al. which showed a limited benefit for treatment modalities other than expectant management.[11]
One of the treatments which has unequivocal evidence both for and against it, is corticosteroid injection. A meta-analysis by Aspenberg showed that while corticosteroid injection has short term benefits, in the long term it is more likely to cause harm.[12] However, there are others who believe that corticosteroids are a poor treatment choice for lateral epicondylitis.[13]
In order to address treatment concerns for patients with lateral epicondylitis; we did a randomized double-blind clinical trial to test the effectiveness of steroid injection versus placebo and immobilization versus no immobilization in treating patients with lateral epicondylitis.
MATERIALS AND METHODS
This randomized double-blind clinical trial was conducted in the Kashani University Hospital in Isfahan, Iran during the first 6 months of the 2013. Patients with confirmed lateral epicondylitis, who had not received any treatment prior to enrolment, were entered into the study if they had none of the exclusion criteria and gave informed consent for participation [Figure 1]. Patients were seleted from a pool of individuals who were screened for upper extremity complains, those who were suspected of suffering from lateral epicondylitis were examined by an orthopedic surgeon and if a diagnosis of lateral epicondylitis was confirmed and none of the exclusion criteria were present, then they were asked to participate in an orientation session where the trial was explained to them and information booklets were distributed between them. Those patients who agreed to participate in the trial were asked to give written consents. The inclusion criteria included a history compatible with lateral epicondylitis and positive examination in palpation of the elbow over the lateral epicondyle, with resisted wrist extension, resisted middle finger extension and/or the mills test, and patients with symptoms lasting more than 6 weeks, no history of acute trauma, fracture, and/or surgery within 12 months, patients who had not received corticosteroid injection, physiotherapy, splint or casting during the past 6 months, no bilateral involvement and no history of cervical disk herniation, radiculopathy or abnormal electrophysiologic study.
Figure 1.
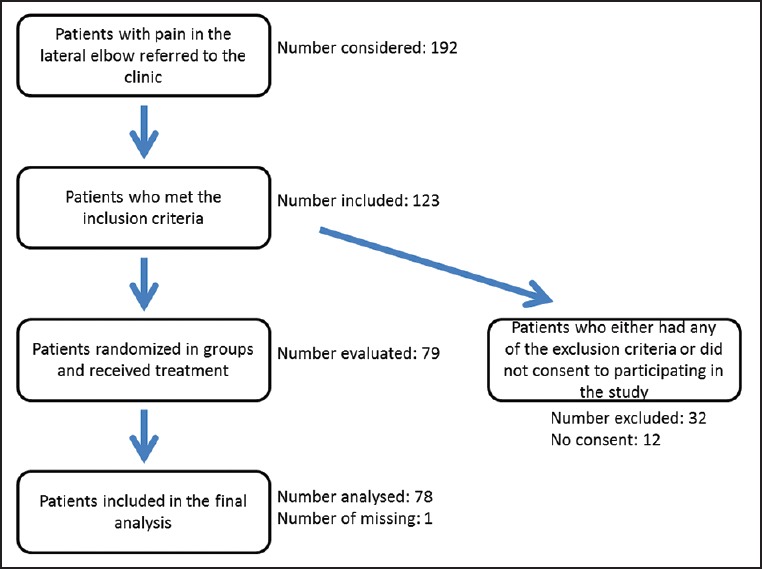
Study design
Random numbers table was used to allocate patients between Groups 1, 2, 3 and 4. These assignments were then put into concealed envelopes and given to the trial clerk. Each of the trial subjects would be given a concealed envelope. The trial pharmacist also prepared a series of similar vials containing either 40 mg of Depomedrol (Aburaihan Comp., Iran) (1 cc) or 1 cc normal saline and coded them either 1, 2, 3 or 4, the group assignments were not decoded until the end of the trial when the final analysis was due to take place. Patients took their envelopes to the trial pharmacist who gave them a coded vial which they took to the orthopedic surgeon who made the injection. Due to the color difference of depomedrol and normal saline, both vials and syringes were covered by stickers in order to conceal the injection solution. Maximum point of tenderness was identified and injection was performed in fanlike fashion in that area. After the injection the trial clerk took the patients to a technician who gave patients in Groups 1 and 3 long arm splints.
The trial subjects were then evaluated using Oxford elbow scale (OES) and visual analog scale (VAS)[14] (OES is the gold standard for clinical evaluation of elbow complaints) by the trial clerk. The patients were evaluated at the baseline and before administration of treatment and they were also asked to come to the trial office at 2 weeks, 4 weeks and 24 weeks for follow-up evaluation which was also conducted by administration of OES and VAS by the trial clerk. OES is a patient reported questionnaire consisting of 12 questions that has been shown to have high specificity and sensitivity in assessing the outcomes of interventions on the elbow of the patients.[15,16] These questions assess three domains pertaining to the elbow: Function, pain and social-psychological with four questions for each domain. The questionnaire was translated into Persian by a professional translator and was then translated back into English in order to ascertain accuracy. The translated questionnaire was then validated using a group of 20 random individuals and its reliability was tested by retest of the same individuals (which showed 95% reliability).
After all of the enrolled patients finished the 24 weeks follow-up, the data was entered into an IBM SPSS database (ver. 18) and analyzed. The groups were subsequently decoded so that the interventions used in each group become known. Due to the number of groups and multiplicity of comparisons made, we used Bonferroni correction in order to adjust the P value accordingly.
This study was approved by the Ethics Committee of the Isfahan University of Medical Sciences (Protocol Record 387278) and funds were provided by the University Research Council.
RESULTS
Overall in the 6 months period of recruitment, 91 patients which met the inclusion and exclusion criteria were identified and from this pool of patients, 79 accepted to participate in the study. At the end of the 24 weeks follow-up, 78 patients had finished the study with one patient lost to follow-up. After decoding the groups it became known that Group 1 had received 40 mg depomedrol along with long arm splint, Group 2 had received 4 mg depomedrol alone, Group 3 had received normal saline with splint and finally Group 4 had received normal saline injection only. There were 21, 19, 19 and 20 test subjects in the first, second, third and fourth group, respectively. One of the patients in the second group was lost to the follow-up after the initial treatment and was subsequently excluded from the final analysis. Overall there were 35 males and 43 females in the study. Chi-square test failed to show any significant difference between the groups with regards to gender distribution (P = 0.866). The average age of the participants was 47.39 years (standard deviation [SD]: 6.53, range: 32-65); repeated measure analysis of variance (ANOVA) test did not show any significant difference in the age composition of the subjects of the study groups (P = 0.622). These data are summarized in Table 1.
Table 1.
Distribution of variables between the four groups
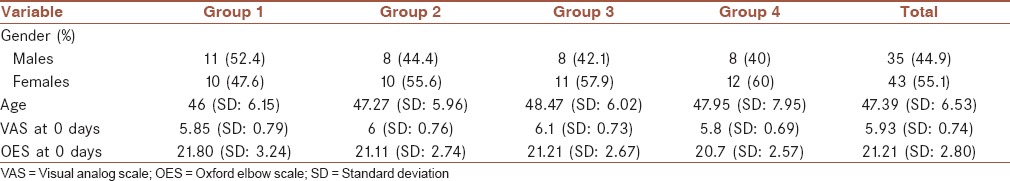
The average VAS score of the participants prior to the administration of the treatment was 5.93 (out of ten, SD: 0.93) and no significant intergroup difference was noticed (P = 0.577). The average Oxford elbow score of the patients prior to receiving treatment was 21.21 (SD: 2.80) again repeated measure ANOVA test failed to show any intergroup difference (P = 0.658) [Table 1].
Before and after tests showed that all groups reported better outcomes at different follow-up intervals and at the end of the trial. In order to understand which treatment was more effective we compared the mean differences of the groups outcomes at different stages of the trial.
We measured the difference between the mean VAS score at different stages of the trial [Figure 2] and then compared these means’ differences between the four groups. The analysis showed that at the 2nd week follow-up, both of the corticosteroid injection groups were similar (P = 1) but the patients in these two groups had a significantly larger reduction in the VAS score compared with the two groups which received normal saline injection (P < 0.001). In the groups which received normal saline injection the patients in Group 4 who received long arm splints in addition to normal saline injection fared better (mean difference: 0.79, P = 0.005). At 4 weeks follow-up, again the corticosteroid injection groups were similar but significantly better than the normal saline injection groups (P < 0.001) but by this time the two normal saline injection groups had become similar (P = 0.768). By the 24th week, a reversal of results was observed as the groups who received saline injection reported better results; at 24 weeks, the two corticosteroid injection groups were similar (P = 0.372). The group who received depomedrol alone at 24 weeks reported worse scores than the normal saline only injection group (P = 0.044) but they did not report significantly different outcomes compared to the normal saline and splint group (P = 0.847). The group that received both corticosteroid injection and long arm splint, however, were significantly worse than both of the groups that received normal saline injection (P < 0.01).
Figure 2.
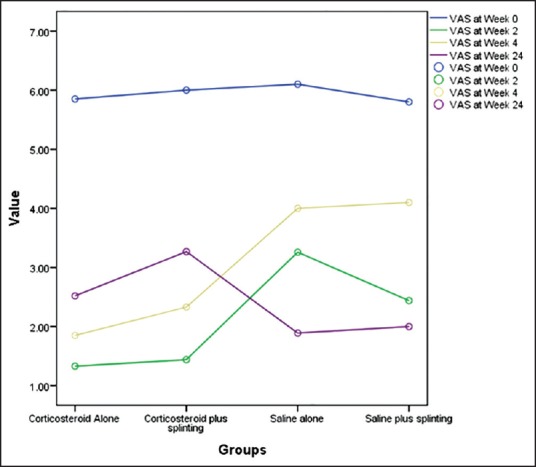
Mean visual analog scale of the four groups during the 24 weeks of study
We measured the difference between the mean OES score at the beginning and the end of the trial [Figure 3] and then compared these means’ differences between the four groups. The depomedrol only group reported similar scores to that of the depomedrol and splint group (P = 1) but they reported lower scores compared to the both normal saline injection groups (P < 0.05). The depomedrol and splint group reported similar scores to the normal saline and splint group (P = 0.157) but reported worse scores compared to the normal saline only group (P = 0.025). The two groups that received normal saline injection reported similar scores (P = 1).
Figure 3.
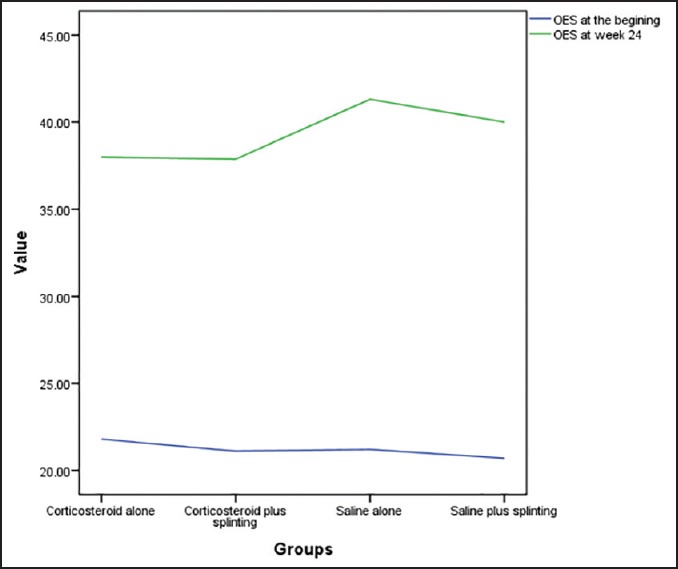
Mean Oxford elbow scale of the four groups at beginning and at week 24
None of the patients were retreated or sought further treatment in the 6 months period of follow-up and as the results showed regardless of the intervention used the patients all reported a degree of improvement compared to their baseline status.
DISCUSSION
Lateral epicondylitis is believed to have three distinctive phases: The initial phase, the subacute phase and the chronic phase.[9] In the first two stages management options include icing, rest, physical therapy and bracing with 95% of patients healing spontaneously with minimal intervention.[9] There is, however, no consent on management of chronic epicondylitis. Because of high rate of spontaneous healing, watchful waiting has been proposed as an initial approach[17] with some even arguing that this may be the best approach overall to lateral epicondylitis.[10]
Non-surgical treatment of lateral epicondylitis is a highly debated issue; over the years many treatment modalities have been proposed but there has been no conclusive evidence to show that either of them works best for this condition. A systematic review by Bisset et al. showed that while enough evidence does not exist; there may be a short term benefit for physical joint manipulation and exercise but evidence for both of these was limited and lacked long term follow-up. Ultrasound, ionization and acupuncture were also shown to be beneficial up to 3 months, but after a year of follow-up their benefit disappeared. Their results also showed that laser therapy and extracorporeal shockwave therapy have no beneficial effect either in short term or long term.[18] There are, however, more recent trials that have shown some effectiveness for radial shock wave therapy in cases where other modalities have failed.[19] Topical non-steroidal anti-inflammatory drugs may also be beneficial in reducing pain in patients.[20]
While over the years, corticosteroid injection was debated as one of the choices for treatment of lateral epicondylitis,[4] in the recent years, the evidence has pointed that corticosteroids, at least in the long run, have limited benefit or are even harmful and cause recurrences. The evidence shows short term (1 month) benefit for either corticosteroid injection or physiotherapy alone (not both) but this benefit fades after the early months.[3,13,21,22] Recent meta-analysis of the available evidence has confirmed the short term benefits of corticosteroid injection, but in the long term, the adverse effects have been shown to far outweigh the short term benefits of this treatment modality.[12] Another systematic review by Coombes et al. again showed that corticosteroid injection in the short term causes pain alleviation but this effect reverses over long term. They also showed that there may be short term benefit for the patients who are treated with Sodium hyaluronate injection, botulinum toxin injection or prolotherapy.[23] Jindal et al. compared steroid injection with injection of autologous blood for treatment of patients with lateral epicondylitis; both treatment modalities were equally effective at 2 weeks but at 6 weeks it was shown that autologous blood injection has a significantly larger effect than steroid injection[24] but more studies are needed for evaluation of this treatment modality. Overall, more recent reviews of injection modalities have shown effectiveness for platelet rich plasma, botulinum toxin, hyaluronic acid and prolotherapy but the trials that reported clear benefits for these modalities were at high risk of bias with the exception of prolotherapy. Corticosteroids were again shown to be beneficial in reducing pain in the short term.[25,26] More recent studies have also shown benefit associated with prolotherapy in treatment of lateral epicondylitis.[27]
In a more recent study by Krogh et al., it was shown that steroid is more effective in pain reduction at 1 month compared to either saline injection or platelet rich plasma injection, but in 3 months no clear advantage for either method was noticed. Furthermore, it was shown that corticosteroid injection leads to reduced color Doppler activity and tendon thickness compared to the other modalities which puts the patients at a higher risk for adverse effects.[28] The high risk of recurrence with corticosteroid injection was again documented in a trial by Mardani-Kivi et al. where they compared corticosteroid injection with procaine injection.[29]
Corticosteroid injection also has many documented adverse effects the most serious of which is tendon rupture. These adverse effects have been reported in most of the clinical trials that evaluated the effectiveness of corticosteroid injection. There seems to be adverse effects for all injection treatment modalities with the exception of sclerosant and platelet-rich plasma injections, which on the other hand have shown no clear beneficial effect either.[30]
Our results have shown that when measuring the pain of the patients using the visual analog scale, they report a clear short term benefit when treated with corticosteroid injection; namely injection of 40 mg depomedrol, but this benefit fades by the 24th week of follow-up. There is, also, no obvious pain relief advantage when splinting is added to the treatment modality. The advantage of corticosteroid injection is lost, however, when the more comprehensive OES is used; OES combines questions about pain, with those inquiring about function and psychosocial aspects. Our results show that when comparing OES scores at 24 weeks, patients who had received normal saline injection reported better scores compared to the group which received corticosteroid injection, which might be related to the rebound effect of the corticosteroid injection. Again, there was no clear benefit for splinting. Our results are consistent with other similar studies in that they show a clear pain relief benefit for corticosteroid injection in the short term, we have shown that this pain relief benefit over placebo lasts through the 6 month follow-up, the advantage is, however, reduced over time and by the 24th week the difference between the groups becomes minimal. On the other hand, the superiority of saline injection at 6 months compared to corticosteroid injection when the OES scale is used shows that while patients who receive steroid injection may have greater pain relief; it seems that in other aspects, the possible unwanted effects of corticosteroids (such as reduced tendon thickness[27]) causes them to have a lower OES score. We, however, did not observe any clinically significant adverse effect in the patients.
CONCLUSION
Our results indicate that despite the clear pain reduction benefit associated with steroid injection in short term, this benefit in comparison with normal saline injection fades by the 24th week of follow-up. Our results also show that splinting has no significant benefit for patients.
AUTHORS’ CONTRIBUTIONS
All authors have contributed in designing and conducting the study. All authors have assisted in preparation of the first draft of the manuscript or revising it critically for important intellectual content. All authors have read and approved the content of the manuscript and confirmed the accuracy or integrity of any part of the work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Assendelft W, Green S, Buchbinder R, Struijs P, Smidt N. Tennis elbow. BMJ. 2003;327:329. doi: 10.1136/bmj.327.7410.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assendelft W, Green S, Buchbinder R, Struijs P, Smidt N. Tennis elbow. Clin Evid. 2004;11:1633–44. [PubMed] [Google Scholar]
- 3.Ahmad Z, Siddiqui N, Malik SS, Abdus-Samee M, Tytherleigh-Strong G, Rushton N. Lateral epicondylitis: A review of pathology and management. Bone Joint J. 2013;95-B:1158–64. doi: 10.1302/0301-620X.95B9.29285. [DOI] [PubMed] [Google Scholar]
- 4.Geoffroy P, Yaffe MJ, Rohan I. Diagnosing and treating lateral epicondylitis. Can Fam Physician. 1994;40:73–8. [PMC free article] [PubMed] [Google Scholar]
- 5.De Smedt T, de Jong A, Van Leemput W, Lieven D, Van Glabbeek F. Lateral epicondylitis in tennis: Update on aetiology, biomechanics and treatment. Br J Sports Med. 2007;41:816–9. doi: 10.1136/bjsm.2007.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson JJ, Best TM. Common overuse tendon problems: A review and recommendations for treatment. Am Fam Physician. 2005;72:811–8. [PubMed] [Google Scholar]
- 7.Tosti R, Jennings J, Sewards JM. Lateral epicondylitis of the elbow. Am J Med. 2013;126:357.e1–6. doi: 10.1016/j.amjmed.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Waseem M, Nuhmani S, Ram CS, Sachin Y. Lateral epicondylitis: A review of the literature. J Back Musculoskelet Rehabil. 2012;25:131–42. doi: 10.3233/bmr-2012-0328. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki K. Current concepts of elbow-joint disorders and their treatment. J Orthop Sci. 2013;18:1–7. doi: 10.1007/s00776-012-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf JM. Do we need to treat tennis elbow? Orthopedics. 2012;35:921–2. doi: 10.3928/01477447-20121023-03. [DOI] [PubMed] [Google Scholar]
- 11.Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: A randomised controlled trial. Lancet. 2002;359:657–62. doi: 10.1016/S0140-6736(02)07811-X. [DOI] [PubMed] [Google Scholar]
- 12.Aspenberg P. Local corticosteroid injections for tennis elbow is a Döbeln medicine. A meta analysis shows short-term benefits, but adverse effects in the long run. Lakartidningen. 2012;109:2203–4. [PubMed] [Google Scholar]
- 13.Corticosteroid injections are a poor treatment for tennis elbow. BMJ. 2013;346:f748. doi: 10.1136/bmj.f748. [DOI] [PubMed] [Google Scholar]
- 14.The B, Reininga IH, El Moumni M, Eygendaal D. Elbow-specific clinical rating systems: Extent of established validity, reliability, and responsiveness. J Shoulder Elbow Surg. 2013;22:1380–94. doi: 10.1016/j.jse.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Dawson J, Doll H, Boller I, Fitzpatrick R, Little C, Rees J, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90:466–73. doi: 10.1302/0301-620X.90B4.20290. [DOI] [PubMed] [Google Scholar]
- 16.Dawson J, Doll H, Boller I, Fitzpatrick R, Little C, Rees J, et al. Specificity and responsiveness of patient-reported and clinician-rated outcome measures in the context of elbow surgery, comparing patients with and without rheumatoid arthritis. Orthop Traumatol Surg Res. 2012;98:652–8. doi: 10.1016/j.otsr.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Johnson GW, Cadwallader K, Scheffel SB, Epperly TD. Treatment of lateral epicondylitis. Am Fam Physician. 2007;76:843–8. [PubMed] [Google Scholar]
- 18.Bisset L, Paungmali A, Vicenzino B, Beller E. A systematic review and meta-analysis of clinical trials on physical interventions for lateral epicondylalgia. Br J Sports Med. 2005;39:411–22. doi: 10.1136/bjsm.2004.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilieva EM, Minchev RM, Petrova NS. Radial shock wave therapy in patients with lateral epicondylitis. Folia Med (Plovdiv) 2012;54:35–41. doi: 10.2478/v10153-011-0095-5. [DOI] [PubMed] [Google Scholar]
- 20.Pattanittum P, Turner T, Green S, Buchbinder R. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating lateral elbow pain in adults. Cochrane Database Syst Rev. 2013;5:CD003686. doi: 10.1002/14651858.CD003686.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coombes BK, Bisset L, Brooks P, Khan A, Vicenzino B. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: A randomized controlled trial. JAMA. 2013;309:461–9. doi: 10.1001/jama.2013.129. [DOI] [PubMed] [Google Scholar]
- 22.Coombes BK, Bisset L, Connelly LB, Brooks P, Vicenzino B. Optimising corticosteroid injection for lateral epicondylalgia with the addition of physiotherapy: A protocol for a randomised control trial with placebo comparison. BMC Musculoskelet Disord. 2009;10:76. doi: 10.1186/1471-2474-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: A systematic review of randomised controlled trials. Lancet. 2010;376:1751–67. doi: 10.1016/S0140-6736(10)61160-9. [DOI] [PubMed] [Google Scholar]
- 24.Jindal N, Gaury Y, Banshiwal RC, Lamoria R, Bachhal V. Comparison of short term results of single injection of autologous blood and steroid injection in tennis elbow: A prospective study. J Orthop Surg Res. 2013;8:10. doi: 10.1186/1749-799X-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Judson CH, Wolf JM. Lateral epicondylitis: Review of injection therapies. Orthop Clin North Am. 2013;44:615–23. doi: 10.1016/j.ocl.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Krogh TP, Bartels EM, Ellingsen T, Stengaard-Pedersen K, Buchbinder R, Fredberg U, et al. Comparative effectiveness of injection therapies in lateral epicondylitis: A systematic review and network meta-analysis of randomized controlled trials. Am J Sports Med. 2013;41:1435–46. doi: 10.1177/0363546512458237. [DOI] [PubMed] [Google Scholar]
- 27.Rabago D, Lee KS, Ryan M, Chourasia AO, Sesto ME, Zgierska A, et al. Hypertonic dextrose and morrhuate sodium injections (prolotherapy) for lateral epicondylosis (tennis elbow): Results of a single-blind, pilot-level, randomized controlled trial. Am J Phys Med Rehabil. 2013;92:587–96. doi: 10.1097/PHM.0b013e31827d695f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: A randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625–35. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 29.Mardani-Kivi M, Karimi-Mobarakeh M, Karimi A, Akhoondzadeh N, Saheb-Ekhtiari K, Hashemi-Motlagh K, et al. The effects of corticosteroid injection versus local anesthetic injection in the treatment of lateral epicondylitis: A randomized single-blinded clinical trial. Arch Orthop Trauma Surg. 2013;133:757–63. doi: 10.1007/s00402-013-1721-x. [DOI] [PubMed] [Google Scholar]
- 30.Hart L. Corticosteroid and other injections in the management of tendinopathies: A review. Clin J Sport Med. 2011;21:540–1. doi: 10.1097/01.jsm.0000407929.35973.b9. [DOI] [PubMed] [Google Scholar]


