Abstract
Background:
Impairment of intestinal barrier function and increased translocation of bacteria to the systemic blood flow contribute to the emergence of sepsis. Probiotics might be of beneficial effects on critically ill-patients, modulating intestinal barrier function and reducing inflammation. The aim of this trial was to determine the effect of probiotics on inflammatory markers in critically ill-patients in Intensive Care Unit (ICU).
Materials and Methods:
This trial was conducted on 40 critically ill-patients admitted to the ICU. Patients were randomly assigned to receive placebo or probiotic containing Lactobacillus, Bifidobacterium and Streptococcus thermophilus (VSL#3) for 7 days. Acute Physiology and Chronic Health Evaluation (APACHE II) score Sequential Organ Failure Assessment (SOFA) and systemic concentrations of interleukin-6 (IL-6), procalcitonin (PCT) and protein C were measured before initiation of the study and on days 4 and 7.
Results:
A significant difference in IL-6 (P = 0.003), PCT (P = 0.014) and protein C (P < 0.001) levels, and also APACHE II and SOFA scores (P < 0.001) was seen over the treatment period between two groups. Moreover, there was a significant decrease in serum IL-6 levels (from 211.85 ± 112.76 to 71.80 ± 28.41) (P < 0.001) and PCT levels (from 1.67 ± 1.27 to 0.47 ± 0.41) (P < 0.001) and a significant increase in serum protein C levels (from 7.47 ± 3.61 to 12.87 ± 3.63) (P < 0.001) in probiotic group during the study.
Conclusion:
Probiotics could reduce inflammation in critically ill-patients and might be considered as an adjunctive therapy in the treatment of critically ill-patients.
Keywords: Inflammation, probiotics, sepsis
INTRODUCTION
Sepsis which is characterized by a systemic inflammatory response syndrome (SIRS) and the presence of an infection is one of the most serious complications in critically ill patients and it may finally lead to cell death and multi-organ dysfunction syndrome (MODS). Sepsis and its subsequent complications are the most common cause of death in Intensive Care Units (ICUs).[1,2,3] It is estimated to affect 18 million people worldwide annually and kill 1,400 people daily. In the United States alone, 750,000 people develop sepsis each year about 30% of which die.[4] Inflammation plays a key role in the development of sepsis; elevated levels of various inflammatory cytokines could be detected in patients with sepsis.[5] Interleukin 6 (IL-6) is an inflammatory biomarker with a diagnostic and prognostic value in patients with sepsis which is used in the prediction of mortality in patients with severe sepsis.[6,7,8] Procalcitonin (PCT) has been proposed as a specific biomarker for early diagnosis of sepsis in recent years that can be helpful in determining the prognosis of sepsis.[6,9] Protein C, an important anticoagulant and anti-inflammatory factor, is a prognostic indicator in sepsis, and its deficiency is associated with poor outcome.[10,11] Gastrointestinal (GI) tract has been blamed for the pathogenesis of sepsis and MODS due to the impairment of intestinal barrier function and increased translocation of bacteria to systemic blood flow.[12,13,14,15] Intestinal microbes can be a major source of systemic infection in patients of ICU.[13] In contrast, Bifidobacterium and Lactobacillus, Endogenous probiotic bacteria of the gut, play a critical role in maintaining the intestinal mucosal barrier and enhancing immune responses.[16] Despite the introduction of various antibacterial and antifungal drugs, pharmacological interventions have not been so successful in reducing sepsis mortality and have failed to increase the recovery rate.[17]
Probiotics are of beneficial effects in the treatment of a wide range of GI disease such as different types of diarrhea,[18,19] inflammatory bowel diseases,[20] irritable bowel syndrome[21] and pouchitis.[22,23] Since they play a role in reduction or elimination of pathogens and toxins, releasing of nutrients, antioxidants and growth factors to stimulate intestinal motility, and regulation of the immune defence mechanisms by changing the intestinal flora, probiotics seem to have beneficial effects in the improvement of critically ill-patients.[24,25] We have previously studied the effect of probiotics on CRP and oxidative stress factors and shown that they can have an effect on inflammation.[25] Thus, the purpose of this double-blind, placebo-controlled, and randomized clinical trial was to determine the effect of probiotic containing Lactobacillus, Bifidobacterium and Streptococcus thermophilus on inflammatory biomarkers in critically ill-patients in the ICU.
MATERIALS AND METHODS
Study participants
Patients admitted to the ICU of the Shohada Hospital (Tabriz University of Medical Sciences, Tabriz, Iran) between December 2011 and October 2012 was eligible for the study. Inclusion criteria were as the followings: (1) Critically ill-patients with positive SIRS and Acute Physiology and Chronic Health Evaluation (APACHE II) score of 15-30; (2) receiving enteral nutrition; (3) expected ICU stay of at least 7 days. Exclusion criteria were as the followings: (1) pregnant and lactating women; (2) patients who could not tolerate enteral nutrition; (3) unstable hemodynamics; (4) intestinal obstruction; (5) intestinal ischemia; (6) short bowel syndrome; (7) pancreatitis.
After approval of ethics committee of the Tabriz University of Medical Sciences and obtaining written informed consent from the patients or their legal guardians, 40 patients (20 in probiotic and 20 in the placebo group) were enrolled in this trial. A computer-generated random sequence was kept in a remote secure location and administered by an independent third party who was not involved with the clinical conduct of the study until all study data were collected and verified. Patients and those involved in enrolling participants, administering interventions and assessing outcomes were blind to group assignments. Our clinical trial was registered in Iranian Registry of Clinical Trials with code number of (IRCT201112143320N6).
Treatment
All patients received enteral nutrition with Fresubin original fibre (Fresenius Kabi, Homburg, Germany) throughout the first 24-h of admission via nasogastric tube, which provided 1kcal/ml. They were randomly assigned to two 20-person groups; the first group received standard treatment plus placebo, and the second group received standard treatment plus VSL#3, 2 sachets daily for 7 days. Each sachet of probiotics (VSL#3; VSL Pharmaceuticals, Sigma-Tau Pharmaceuticals Inc. Ft Lauderdale, FL) contained 450 billion viable lyophilized bacteria consisting of 4 strains of Lactobacillus (Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, and Lactobacillus delbrueckii subsp. Bulgaricus), 3 strains of Bifidobacterium (Bifidobacterium longum, Bifidobacterium breve, and Bifidobacterium infantis) and Streptococcus salivarius subsp. Thermophilus.
Study design
This was a single-center double-blind (researcher and patient) and placebo-controlled trial. Enteral feeding was started at 25 ml/h and increased by 25 ml/h every 4 h until the target rate was achieved. When gastric residual volumes exceeded 150 ml, prokinetic agents were initiated, and feeding was advanced until the target rate was achieved. The probiotic group received 2 sachets of probiotics, while the placebo group received 2 sachets of placebo twice daily at 9 a.m. and p.m. The placebo preparation had identical packing and was manufactured by the same company. All patients in the study received concomitant therapy, including antibiotics, as considered appropriate by the attending physician.
Nutritional assessment
Weight and height of the patients were recorded, and body mass index was calculated using the formula weight (kg)/height2 (m). Energy requirements were calculated as 25-30 kcal/kg and protein requirements as 1.2-1.5 g/kg. Daily energy and protein intake were recorded.
Outcome measures
Acute physiology and chronic health evaluation II and sequential organ failure assessment scores
Acute Physiology and Chronic Health Evaluation II[26] and Sequential Organ Failure Assessment (SOFA)[27] scores were calculated for all patients prior to the study and on days 4 and 7.
Biochemical analysis
Five ml of blood was obtained from each patient to evaluate IL-6, PCT and Protein C before initiation of the study and on days 4 and 7.
Statistical analysis
The total sample size of 40 subjects (20 in each group) was calculated based on published levels of IL-6 differences in critically ill-patients[28] at the 5% significance level with the power of 80%. Samples were selected from general ICU of Shohada hospital in Tabriz using convenience sampling method.
The data were analyzed using the statistical software program Statistical Package for the Social Sciences (SPSS Inc. 233 South Wacker Drive, 11th Floor, Chicago, IL 60606-6412) Version 16.0, SPSS. Kolmogorov–Smirnov test was used to assess the normality of the variables distribution. Independent t-tests were performed on all baseline data between groups. Differences in variables at baseline and after treatment were assessed with a repeated measures analysis of variance that included a time × treatment interaction. Data were further analyzed with a Sidak post-hoc test for multiple comparisons. Independent t-tests were used to assess differences between the treatment groups. P < 0.05 were considered as significant for all statistical tests.
RESULTS
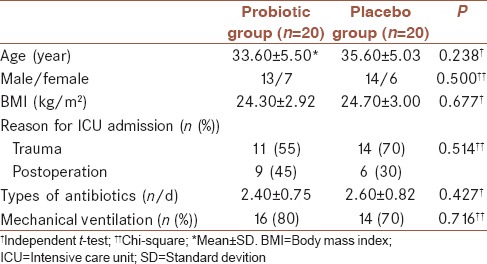
Eligibility was determined for a total of 96 ICU admissions between December 2011 and October 2012. Among eligible patients, 40 were enrolled [Figure 1]. Demographic characteristics of patients are shown in Table 1. No significant differences in age, sex, body mass index and use of antibiotics were observed between two groups.
Figure 1.
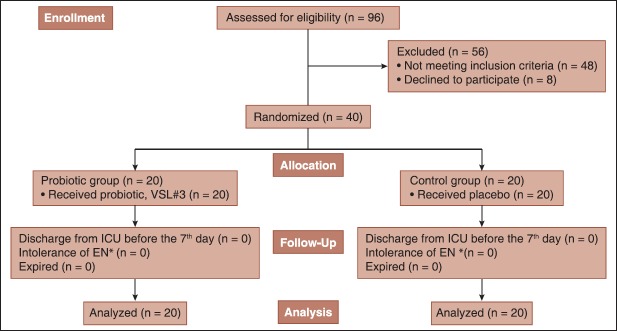
Study overview (*Enteral nutrition)
Table 1.
Demographic and baseline characteristics of patients
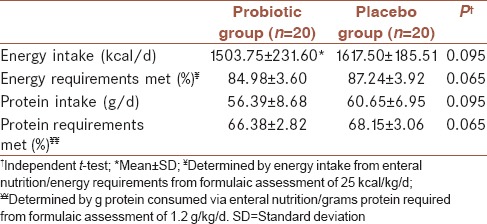
Nutritional variables of patients in each group were assessed and shown in Table 2. Mean daily energy intake was compared with energy requirements derived from formulaic assessment of 25kcal/kg/d. Mean protein intakes were compared with protein requirements calculated by formulaic methods. No significant differences existed between two groups for mean energy and protein intake. The two most common reasons for interrupting enteral nutrition included temporary cessation for medical procedures or increased gastric residuals to more than 200 ml.
Table 2.
Nutritional variables of patients by treatment group
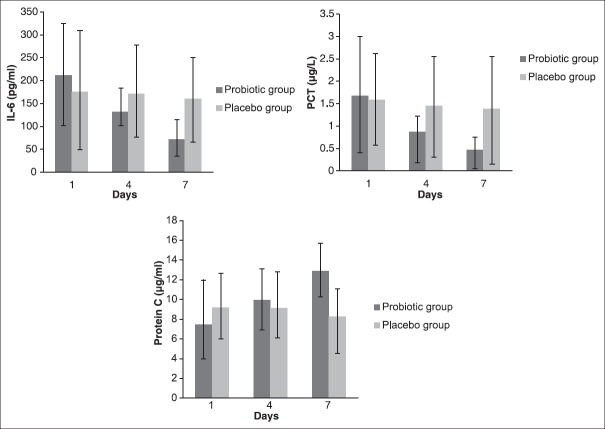
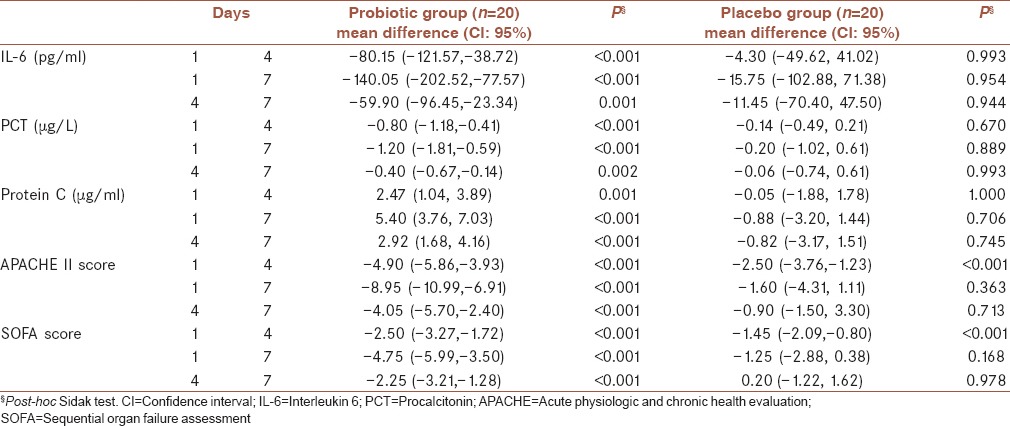
There was a significant difference in serum IL-6, PCT and protein C levels at the end of the study between two groups (P = 0.001, P = 0.005 and P < 0.001, respectively) [Figure 2]. Table 3 shows the levels of IL-6, PCT and protein C and also APACHE II and SOFA scores in various days of the study. There was a significant difference in IL-6 (P = 0.003), PCT (P = 0.014) and protein C (P < 0.001) levels, and also APACHE and SOFA scores (P < 0.001) over the treatment period between two groups. A significant decrease in serum IL-6 and PCT levels (P < 0.001) and a significant increase in serum protein C levels (P < 0.001) in the probiotic group were seen during the study. There was a significant decrease in APACHE II and SOFA scores in both probiotic (P < 0.001) and placebo (P = 0.034 and P = 0.029, respectively) groups during the study; however, the decreases were more in the probiotic group compared with placebo group. Post-hoc Sidak test showed a significant difference for all variables among the different days of the study in the probiotic group [Table 4].
Figure 2.
Serum interleukin-6, procalcitonin and protein C levels during the different days of the study† Independent t-tes
Table 3.
Serum IL-6, PCT and protein C levels and APACHE II and SOFA scores during the intervention period
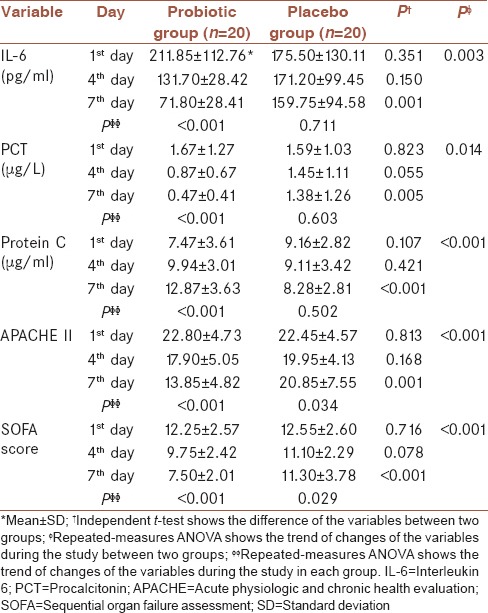
Table 4.
Mean difference in serum IL-6, PCT and protein C levels and APACHE II and SOFA scores over the treatment period in each group
Two patients in the probiotic group and five patients in the placebo group developed sepsis during their ICU stay. Although administration of probiotics decreased the incidence of sepsis, there was not a significant difference between two groups (P = 0.407). The organisms isolated from the blood culture of the patients with sepsis were as followings:
Pseudomonas aeruginosa: One in probiotic and two in the placebo group
Staphylococcus aureus: One in probiotic and two in the placebo group
Acinetobacter: One in the placebo group.
No patients in the probiotic group developed Lactobacillus-induced sepsis.
DISCUSSION
The present study used a double-blind, placebo-controlled and randomized design to determine the effects of probiotics on critically ill, eternally fed patients. Overall, the patients who received probiotic showed a greater reduction in inflammation than did the patients who received placebo. Serum levels of IL-6 and PCT have been recommended for early identification of inflammation and sepsis.[6,29] IL-6 is a 21 kDa glycoprotein produced by many cell types including lymphocytes, fibroblasts and monocytes. It has many systemic effects including induction of acute phase protein production in the liver. In clinical studies, IL-6 appears to be a good indicator of activation of the cytokine cascade and predicts subsequent organ dysfunction and mortality.[30] PCT, the precursor to calcitonin, is an 116-amino-acid protein and has been shown to be associated with inflammation and sepsis.[9] In our study, administration of probiotic significantly decreased IL-6 and PCT levels. A similar finding was reported by McNaught et al. who showed that enteral administration of ProViva, an oatmeal-based drink containing L. plantarum 299 v to critically ill patients resulted in significantly lower levels of IL-6 in the probiotic group compared with the controls.[28] In another study performed on critically ill trauma patients receiving a synbiotic formula (Synbiotic 2000Forte) or placebo, there was a statistically significant difference between groups regarding PCT levels with the synbiotic group having the lowest.[31] In a study conducted on pigs, probiotic supplementation including 30% L. acidophilus, 30% Bifidobacterium lactics, 20% Bacillus subtilis and 20% Bacillus natto did not have a significant effect on PCT concentrations of pigs compared with the control group.[32] The positive results of our study may be due to the patient population and the type of the used probiotic. Our trial was performed in a surgical ICU on patients most of whom were traumatic patients; therefore, heterogeneity which is one the most important problems in ICU population was decreased. VSL#3, contains 8 different strains of live lactic acid bacteria specially selected to produce an optimal synergistic composition of bacteria, is a potent probiotic medical food that delivers the highest available concentration of beneficial live bacteria than any other probiotic.[33,34] At the cellular level, VSL#3 positively affects a variety of substances that are involved in gut function. This is important to ensure the correct absorption of nutrients and to maintain barrier functionality.[35] Finally, evidence suggests that VSL#3 can reduce intestinal permeability by tightening the junctions between the cells in the outer layer of the intestine that in turns reduces the likelihood of translocation of pathogens from the intestine into the blood.[36]
Plasma levels of acute phase proteins involved in coagulation and fibrinolysis are elevated during inflammation while natural anticoagulant mechanisms are depressed. Protein C is a natural component of the anticoagulant system. In sepsis, activated protein C attempts to achieve homeostasis by decreasing inflammation and coagulation.[10,37] In our study, administration of probiotic significantly increased protein C levels. To the best of our knowledge, no study has been performed regarding the effect of probiotic administration on protein C.
Acute Physiology and Chronic Health Evaluation II score is a tool to measure the disease severity. An increasing score is closely correlated with the subsequent risk of many common diseases and hospital death.[25,38] SOFA score is a simple and objective score that allows calculation of both the number and the severity of organs’ dysfunctions and during the first few days of ICU admission is a proper indicator of prognosis.[27] Independent of the initial score, an increase in SOFA score during the first 48 h in the ICU predicts a mortality rate of at least 50%.[39] In our study, both APACHE and SOFA scores improved significantly by adding probiotic to the patients. However, in contrast to our results, in a study performed on severe traumatic brain-injured (TBI) patients, APACHE II and SOFA scores were not significantly affected by probiotic treatment.[40] These negative results might be due to the selected strain or dose of bacteria, or the type of patients studied (patients with severe TBI and Glasgow Coma Scale scores between 5 and 8).
Our results showed that there was not a significant difference in the levels of the biomarkers on the 4th day between two groups highlighting the fact that probiotics show their efficacy in the critically ill patients if used for the duration of more than 4 days. Therefore, it seems that the long-duration therapy might be the best method of probiotics administration in ICU.
Limitation of the study
Our study was a single-center study with 40 patients included. Therefore, future multi-center trials with larger sample sizes of critically ill patients are required to achieve more solid results. In addition, our study was performed on surgical patients; hence, for routine administration of probiotics in critically ill-patients, they should be examined in medical or mixed type ICUs. Furthermore, further trials are needed to define the best dosage and optimal duration of therapy in these patients.
CONCLUSION
The results of this trial are encouraging and suggest that administration of probiotics in critically ill patients is associated with clinical benefits in relation to matched placebo-treated patients: They significantly reduce the levels of inflammatory biomarkers as well as APACHE and SOFA scores. Furthermore, significant increase in protein C levels could be detected. Therefore, probiotics could reduce inflammation in critically ill-patients and might be considered as an adjunctive therapy in the treatment of critically ill-patients. However, further studies with larger sample size are required to clarify their usefulness in this group of patients.
AUTHORS’ CONTRIBUTION
SS contributed in the conception of the work, conducting the study, collecting the samples, preparing the manuscript draft, approval of the final version of the manuscript, statistical analysis, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. ME contributed in the design of the work, statistical analysis, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. HH contributed in sample analyzing via commercially available enzyme-linked immunosorbent assay kit, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MM contributed in the design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. AM contributed in the conception of the work, conducting the study, collecting the samples, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Footnotes
Source of Support: Tabriz University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control (CDC). Increase in National Hospital Discharge Survey rates for septicemia – United States, 1979-1987. MMWR Morb Mortal Wkly Rep. 1990;39:31–4. [PubMed] [Google Scholar]
- 3.Eslami K, Mahmoodpoor A, Ahmadi A, Abdollahi M, Kamali K, Mousavi S, et al. Positive effect of septimeb™ on mortality rate in severe sepsis: A novel non antibiotic strategy. Daru. 2012;20:40. doi: 10.1186/2008-2231-20-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Takala A, Nupponen I, Kylänpää-Bäck ML, Repo H. Markers of inflammation in sepsis. Ann Med. 2002;34:614–23. doi: 10.1080/078538902321117841. [DOI] [PubMed] [Google Scholar]
- 6.Haasper C, Kalmbach M, Dikos GD, Meller R, Müller C, Krettek C, et al. Prognostic value of procalcitonin (PCT) and/or interleukin-6 (IL-6) plasma levels after multiple trauma for the development of multi organ dysfunction syndrome (MODS) or sepsis. Technol Health Care. 2010;18:89–100. doi: 10.3233/THC-2010-0571. [DOI] [PubMed] [Google Scholar]
- 7.Hamishehkar H, Beigmohammadi MT, Abdollahi M, Ahmadi A, Mahmoodpour A, Mirjalili MR, et al. Identification of enhanced cytokine generation following sepsis. Dream of magic bullet for mortality prediction and therapeutic evaluation. Daru. 2010;18:155–62. [PMC free article] [PubMed] [Google Scholar]
- 8.Farhoudi M, Najafi-Nesheli M, Hashemilar M, Mahmoodpoor A, Sharifipour E, Baradaran B, et al. Effect of IMOD™ on the inflammatory process after acute ischemic stroke: A randomized clinical trial. Daru. 2013;21:26. doi: 10.1186/2008-2231-21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balc IC, Sungurtekin H, Gürses E, Sungurtekin U, Kaptanoglu B. Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Crit Care. 2003;7:85–90. doi: 10.1186/cc1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esmon CT. Molecular events that control the protein C anticoagulant pathway. Thromb Haemost. 1993;70:29–35. [PubMed] [Google Scholar]
- 11.Fisher CJ, Jr, Yan SB. Protein C levels as a prognostic indicator of outcome in sepsis and related diseases. Crit Care Med. 2000;28:S49–56. doi: 10.1097/00003246-200009001-00011. [DOI] [PubMed] [Google Scholar]
- 12.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: The role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 13.MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: A prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–8. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacFie J. Current status of bacterial translocation as a cause of surgical sepsis. Br Med Bull. 2004;71:1–11. doi: 10.1093/bmb/ldh029. [DOI] [PubMed] [Google Scholar]
- 15.Murono K, Hirano Y, Koyano S, Ito K, Fujieda K. Molecular comparison of bacterial isolates from blood with strains colonizing pharynx and intestine in immunocompromised patients with sepsis. J Med Microbiol. 2003;52:527–30. doi: 10.1099/jmm.0.05076-0. [DOI] [PubMed] [Google Scholar]
- 16.Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, et al. Effects of probiotic therapy in critically ill patients: A randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85:816–23. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 17.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 18.Yan F, Polk DB. Probiotics as functional food in the treatment of diarrhea. Curr Opin Clin Nutr Metab Care. 2006;9:717–21. doi: 10.1097/01.mco.0000247477.02650.51. [DOI] [PubMed] [Google Scholar]
- 19.Delia P, Sansotta G, Donato V, Messina G, Frosina P, Pergolizzi S, et al. Prevention of radiation-induced diarrhea with the use of VSL#3, a new high-potency probiotic preparation. Am J Gastroenterol. 2002;97:2150–2. doi: 10.1111/j.1572-0241.2002.05946.x. [DOI] [PubMed] [Google Scholar]
- 20.Hedin C, Whelan K, Lindsay JO. Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: A review of clinical trials. Proc Nutr Soc. 2007;66:307–15. doi: 10.1017/S0029665107005563. [DOI] [PubMed] [Google Scholar]
- 21.Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: A multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 22.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–9. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 23.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, et al. Prophylaxis of pouchitis onset with probiotic therapy: A double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–9. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 24.Morrow LE. Probiotics in the intensive care unit. Curr Opin Crit Care. 2009;15:144–8. doi: 10.1097/MCC.0b013e3283252d2d. [DOI] [PubMed] [Google Scholar]
- 25.Ebrahimi-Mameghani M, Sanaie S, Mahmoodpoor A, Hamishehkar H. Effect of a probiotic preparation (VSL#3) in critically ill patients: A randomized, double-blind, placebo-controlled trial (Pilot Study) Pak J Med Sci. 2013;29:490–4. doi: 10.12669/pjms.292.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 27.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 28.McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211–9. doi: 10.1016/j.clnu.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Tabeefar H, Beigmohammadi MT, Javadi MR, Abdollahi M, Mahmoodpoor A, Ahmadi A, et al. Effects of Pantoprazole on Systemic and Gastric Pro-and Anti-inflammatory Cytokines in Critically Ill Patients. Iran J Pharm Res. 2012;11:1051–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwell TS, Christman JW. Sepsis and cytokines: Current status. Br J Anaesth. 1996;77:110–7. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 31.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: Early results of a randomized controlled trial. World J Surg. 2006;30:1848–55. doi: 10.1007/s00268-005-0653-1. [DOI] [PubMed] [Google Scholar]
- 32.Kunavue N, Lien TF. Effects of fulvic acid and probiotic on growth performance, nutrient digestibility, blood parameters and immunity of pigs. J Anim Sci Adv. 2012;2:711–21. [Google Scholar]
- 33.Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol. 2001;152:735–41. doi: 10.1016/s0923-2508(01)01254-2. [DOI] [PubMed] [Google Scholar]
- 34.Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, et al. Impact on the composition of the faecal flora by a new probiotic preparation: Preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1103–8. doi: 10.1046/j.1365-2036.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 35.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A. 2010;107:454–9. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Carbo A, Shaykhutdinov R, et al. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR?. To suppress colitis. PLoS One. 2012;7:e31238. doi: 10.1371/journal.pone.0031238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Najafi A, Mojtahedzadeh M, Mahmoodpoor A, Aghamohammadi M, Ahmadi A, Nahreini Sh, et al. Effect of N-acetylcysteine on microalbuminuria in patients with acute respiratory distress syndrome. Arch Med Sci. 2009;3:408–14. [Google Scholar]
- 38.Mahmoodpoor A, Eslami K, Mojtahedzadeh M, Najafi A, Ahmadi A, Dehnadi-Moghadam A, et al. Examination of Setarud (IMOD™) in the management of patients with severe sepsis. Daru. 2010;18:23–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 40.Tan M, Zhu JC, Du J, Zhang LM, Yin HH. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: A prospective randomized pilot study. Crit Care. 2011;15:R290. doi: 10.1186/cc10579. [DOI] [PMC free article] [PubMed] [Google Scholar]