Abstract
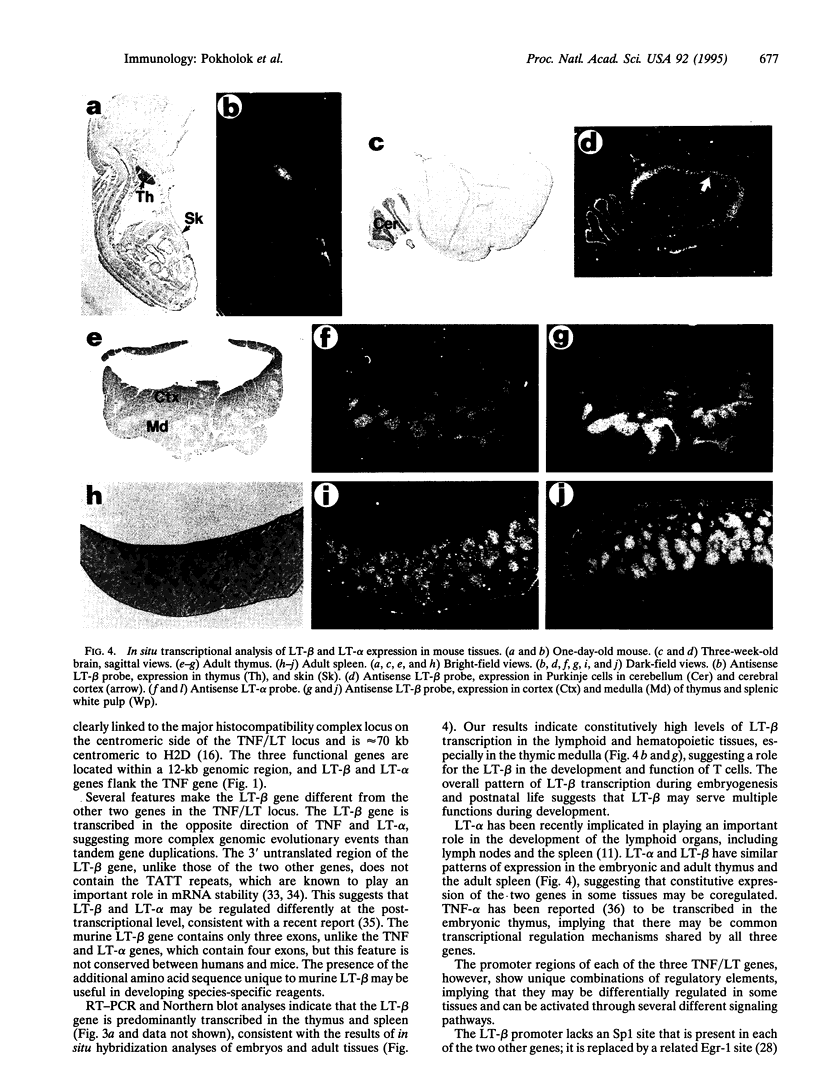
Tumor necrosis factor alpha (TNF-alpha) and soluble lymphotoxin (LT) (also called LT-alpha or TNF-beta) are cytokines with similar biological activities that are encoded by related and closely linked genes. TNF-alpha, a mediator of the inflammatory response, exists in soluble and transmembrane forms. LT-alpha can be secreted or retained at the cell surface by binding to a 33-kDa transmembrane subunit, LT-beta. The recently cloned human LT-beta gene encodes another TNF family member and is linked to the TNF/LT locus within the major histocompatibility complex locus. The cell surface LT is a heterotrimer consisting of LT-alpha and LT-beta, whose physiological function is not yet clearly defined. We now report the sequence analysis of the genomic region and cDNA of murine LT-beta gene, which is closely associated with the TNF-alpha and LT-alpha genes within the murine major histocompatibility complex locus. Unlike the TNF-alpha, LT-alpha, and human LT-beta genes, which contain four exons, the murine LT-beta contains three exons and encodes a 244-amino acid polypeptide with a 66-amino acid insert that is absent from the human homologue. In situ hybridization demonstrates constitutive expression of LT-beta in lymphoid and hematopoietic tissues. LT-beta transcription is maximal in the thymic medulla and in splenic white pulp. LT-beta mRNA is also detected in the skin and in specific regions of the brain. The LT-beta promoter region contains putative Ets-binding sites, suggesting that the expression of LT-beta may be regulated in part by Ets transcription factors whose pattern of lymphoid expression overlaps that of LT-beta.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- Beutler B., van Huffel C. Unraveling function in the TNF ligand and receptor families. Science. 1994 Apr 29;264(5159):667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- Bhat N. K., Komschlies K. L., Fujiwara S., Fisher R. J., Mathieson B. J., Gregorio T. A., Young H. A., Kasik J. W., Ozato K., Papas T. S. Expression of ets genes in mouse thymocyte subsets and T cells. J Immunol. 1989 Jan 15;142(2):672–678. [PubMed] [Google Scholar]
- Bosselut R., Levin J., Adjadj E., Ghysdael J. A single amino-acid substitution in the Ets domain alters core DNA binding specificity of Ets1 to that of the related transcription factors Elf1 and E74. Nucleic Acids Res. 1993 Nov 11;21(22):5184–5191. doi: 10.1093/nar/21.22.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning J. L., Androlewicz M. J., Ware C. F. Lymphotoxin and an associated 33-kDa glycoprotein are expressed on the surface of an activated human T cell hybridoma. J Immunol. 1991 Aug 15;147(4):1230–1237. [PubMed] [Google Scholar]
- Browning J. L., Ngam-ek A., Lawton P., DeMarinis J., Tizard R., Chow E. P., Hession C., O'Brine-Greco B., Foley S. F., Ware C. F. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993 Mar 26;72(6):847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco D., Ryseck R. P., Bravo R. Expression of relB transcripts during lymphoid organ development: specific expression in dendritic antigen-presenting cells. Development. 1993 Aug;118(4):1221–1231. doi: 10.1242/dev.118.4.1221. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Katzman P., Alicot E. M., Koller B. H., Geraghty D. E., Orr H. T., Strominger J. L., Spies T. Linkage map of the human major histocompatibility complex including the tumor necrosis factor genes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8535–8539. doi: 10.1073/pnas.84.23.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. W., Mathieson B. J., Gandino L., Blasi E., Radzioch D., Varesio L. Heterogeneity of hematopoietic cells immortalized by v-myc/v-raf recombinant retrovirus infection of bone marrow or fetal liver. J Natl Cancer Inst. 1989 Oct 4;81(19):1492–1496. doi: 10.1093/jnci/81.19.1492. [DOI] [PubMed] [Google Scholar]
- Crowe P. D., VanArsdale T. L., Walter B. N., Ware C. F., Hession C., Ehrenfels B., Browning J. L., Din W. S., Goodwin R. G., Smith C. A. A lymphotoxin-beta-specific receptor. Science. 1994 Apr 29;264(5159):707–710. doi: 10.1126/science.8171323. [DOI] [PubMed] [Google Scholar]
- De Togni P., Goellner J., Ruddle N. H., Streeter P. R., Fick A., Mariathasan S., Smith S. C., Carlson R., Shornick L. P., Strauss-Schoenberger J. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994 Apr 29;264(5159):703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Dunham I., Sargent C. A., Trowsdale J., Campbell R. D. Molecular mapping of the human major histocompatibility complex by pulsed-field gel electrophoresis. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7237–7241. doi: 10.1073/pnas.84.20.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroir B. P., Brown T., Beutler B. Constitutive synthesis of tumor necrosis factor in the thymus. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4864–4868. doi: 10.1073/pnas.89.11.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger G. A., Williams T. W. Lymphocyte cytotoxicity in vitro: activation and release of a cytotoxic factor. Nature. 1968 Jun 29;218(5148):1253–1254. doi: 10.1038/2181253a0. [DOI] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Gene regulation by Ets proteins. Biochim Biophys Acta. 1993 Dec 23;1155(3):346–356. doi: 10.1016/0304-419x(93)90014-4. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Madden S. L., Rauscher F. J., 3rd Positive and negative regulation of transcription and cell growth mediated by the EGR family of zinc-finger gene products. Ann N Y Acad Sci. 1993 Jun 11;684:75–84. doi: 10.1111/j.1749-6632.1993.tb32272.x. [DOI] [PubMed] [Google Scholar]
- Maroulakou I. G., Papas T. S., Green J. E. Differential expression of ets-1 and ets-2 proto-oncogenes during murine embryogenesis. Oncogene. 1994 Jun;9(6):1551–1565. [PubMed] [Google Scholar]
- Millet I., Ruddle N. H. Differential regulation of lymphotoxin (LT), lymphotoxin-beta (LT-beta), and TNF-alpha in murine T cell clones activated through the TCR. J Immunol. 1994 May 1;152(9):4336–4346. [PubMed] [Google Scholar]
- Müller U., Jongeneel C. V., Nedospasov S. A., Lindahl K. F., Steinmetz M. Tumour necrosis factor and lymphotoxin genes map close to H-2D in the mouse major histocompatibility complex. Nature. 1987 Jan 15;325(6101):265–267. doi: 10.1038/325265a0. [DOI] [PubMed] [Google Scholar]
- Nedospasov S. A., Hirt B., Shakhov A. N., Dobrynin V. N., Kawashima E., Accolla R. S., Jongeneel C. V. The genes for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) are tandemly arranged on chromosome 17 of the mouse. Nucleic Acids Res. 1986 Oct 10;14(19):7713–7725. doi: 10.1093/nar/14.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedospasov S. A., Shakhov A. N., Turetskaya R. L., Mett V. A., Azizov M. M., Georgiev G. P., Korobko V. G., Dobrynin V. N., Filippov S. A., Bystrov N. S. Tandem arrangement of genes coding for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) in the human genome. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):611–624. doi: 10.1101/sqb.1986.051.01.073. [DOI] [PubMed] [Google Scholar]
- Nedospasov S. A., Turetskaia R. L., Mett V. A., Shakhov A. N. Kartirovanie genomnogo lokusa, soderzhashchego geny faktorov nekroza opukholei myshi, metodom gibridizatsionnoi kontsevoi metki s ispol'zovaniem sinteticheskikh oligonukleotidov. Bioorg Khim. 1989 Jul;15(7):990–993. [PubMed] [Google Scholar]
- Nedwin G. E., Naylor S. L., Sakaguchi A. Y., Smith D., Jarrett-Nedwin J., Pennica D., Goeddel D. V., Gray P. W. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res. 1985 Sep 11;13(17):6361–6373. doi: 10.1093/nar/13.17.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul N. L., Lenardo M. J., Novak K. D., Sarr T., Tang W. L., Ruddle N. H. Lymphotoxin activation by human T-cell leukemia virus type I-infected cell lines: role for NF-kappa B. J Virol. 1990 Nov;64(11):5412–5419. doi: 10.1128/jvi.64.11.5412-5419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K., Matsuyama T., Kündig T. M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P. S., Krönke M., Mak T. W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993 May 7;73(3):457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Rothe J., Lesslauer W., Lötscher H., Lang Y., Koebel P., Köntgen F., Althage A., Zinkernagel R., Steinmetz M., Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993 Aug 26;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Waksman B. H. Cytotoxic effect of lymphocyte-antigen interaction in delayed hypersensitivity. Science. 1967 Sep 1;157(3792):1060–1062. doi: 10.1126/science.157.3792.1060. [DOI] [PubMed] [Google Scholar]
- Semon D., Kawashima E., Jongeneel C. V., Shakhov A. N., Nedospasov S. A. Nucleotide sequence of the murine TNF locus, including the TNF-alpha (tumor necrosis factor) and TNF-beta (lymphotoxin) genes. Nucleic Acids Res. 1987 Nov 11;15(21):9083–9084. doi: 10.1093/nar/15.21.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Farrah T., Goodwin R. G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994 Mar 25;76(6):959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Spies T., Morton C. C., Nedospasov S. A., Fiers W., Pious D., Strominger J. L. Genes for the tumor necrosis factors alpha and beta are linked to the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8699–8702. doi: 10.1073/pnas.83.22.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993 Dec 31;75(7):1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]