Abstract
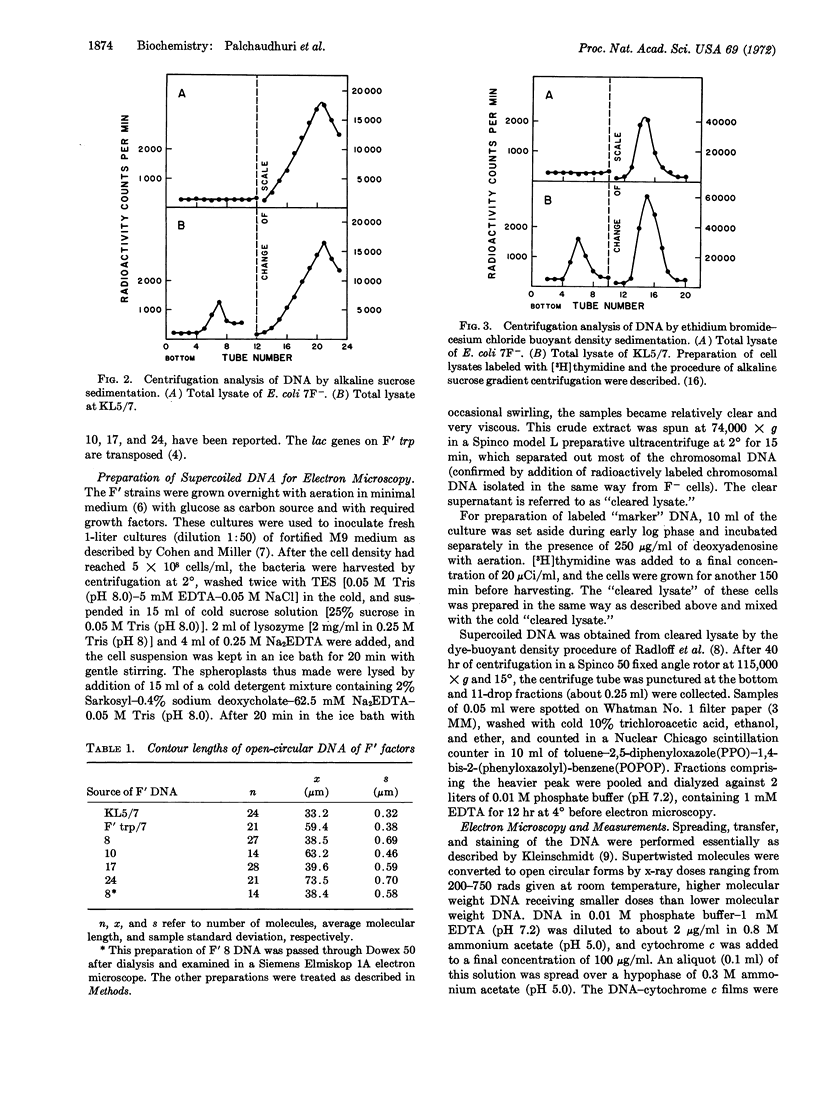
During previous attempts to find mutants in which incompatibility between two F-prime (F′) factors of E. coli is abolished, we found that two F′ factors harbored in the same cell occasionally recombined to form a single genetic unit. Thus, a fused F′trp+arg+ factor was obtained from a mating of a strain carrying recA F′trp+ with a strain carrying recA F′arg+. In the present paper physical fusion of the two F′ factors, inferred from genetic studies, is confirmed by electron microscopy. F DNA was extracted, after detergent lysis, from four fused F′ strains: the parental F′trp+ and F′arg+ strains and three F+ strains. This supercoiled DNA was separated on ethidium bromide-cesium chloride gradients and converted to the open circular from before electron microscopy. The contour lengths in μm of the various DNA preparations were: F′trp+, 59.4; F′arg+, 33.2; the four F′trp+arg+ factors, 38.5, 39.6, 63.2, and 73.5: the three F factors, 31.1, 31.1, and 31.0. Thus, different fused F′ factors formed from the same parental F′ factors vary in length, but are shorter than the sum of the parental F′ factors.
Keywords: episomes, bacterial genetics, contour lengths
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau E., Maas W. K. Inhibition of replication of an F'lac episome in Hfr cells of Escherichia coli. J Bacteriol. 1968 Feb;95(2):531–539. doi: 10.1128/jb.95.2.531-539.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame R., Bishop J. O. The number of sex-factors per chromosome in Escherichia coli. Biochem J. 1971 Jan;121(1):93–103. doi: 10.1042/bj1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Electron microscopic study of the ethidium bromide-DNA complex. J Mol Biol. 1971 Sep 14;60(2):401–403. doi: 10.1016/0022-2836(71)90303-2. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Studies with Escherichia coli sex factors. Cold Spring Harb Symp Quant Biol. 1968;33:425–434. doi: 10.1101/sqb.1968.033.01.049. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Helinski D. R. F 1 sex factor of Escherichia coli. Size and purification in the form of a strand-specific relaxation complex of supercoiled deoxyribonucleic acid and protein. Biochemistry. 1971 Dec 21;10(26):4975–4980. doi: 10.1021/bi00802a022. [DOI] [PubMed] [Google Scholar]
- Maas W. K., Goldschmidt A. D. A mutant of Escherichia coli permitting replication of two F factors. Proc Natl Acad Sci U S A. 1969 Mar;62(3):873–880. doi: 10.1073/pnas.62.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchoudhury S. R., Iyer V. N. Compatibility between two F' factors in an Escherichia coli strain bearing a chromosomal mutation affecting DNA synthesis. J Mol Biol. 1971 Apr 28;57(2):319–333. doi: 10.1016/0022-2836(71)90349-4. [DOI] [PubMed] [Google Scholar]
- Press R., Glansdorff N., Miner P., De Vries J., Kadner R., Maas W. K. Isolation of transducing particles of phi-80 bacteriophage that carry different regions of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1971 Apr;68(4):795–798. doi: 10.1073/pnas.68.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Asymmetric segregation of the complementary sex-factor DNA strands during conjugation in Escherichia coli. J Mol Biol. 1970 Nov 14;53(3):287–303. doi: 10.1016/0022-2836(70)90066-5. [DOI] [PubMed] [Google Scholar]
- Willetts N., Bastarrachea F. Genetic and physicochemical characterization of Escherichia coli strains carrying fused F' elements derived from KLF1 and F57. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1481–1485. doi: 10.1073/pnas.69.6.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]