Abstract
Background
Renal ischemia-reperfusion (I/R) is a severe clinical complication with no specific treatment. Resveratrol has been shown as a promising experimental agent in renal I/R due to its effect on cellular energy metabolism, oxidative stress, and inflammation. Recently, we identified two biologically active resveratrol analogues (RSVAs), RSVA405 and RSVA314. We hypothesized that both RSAVs would attenuate I/R-induced renal injury.
Methods
Adult male rats were subjected to renal I/R through bilateral renal pedicle clamping for 60 min, followed by reperfusion. RSVA405 (3 mg/kg BW), RSVA314 (3 mg/kg BW), or vehicle (10% DMSO and 33% Solutol in PBS) was administered by intraperitoneal injection 1 h prior to ischemia. Blood and renal tissues were collected 24 h after I/R for evaluation.
Results
Administration of RSVA405 and RSVA314 significantly reduced the serum levels of renal dysfunction and injury markers, including creatinine, blood urea nitrogen, aspartate aminotransferase, and lactate dehydrogenase, compared to vehicle. The protective effect of RSVA405 and RSVA314 was also reflected on histologic evaluation. Both RSVAs reduced the number of apoptotic cells by more than 60% as determined by TUNEL assay, compared to vehicle. The renal ATP levels of the vehicle group was decreased to 52.4% of control, while those of the RSVA405 and RSVA314 groups were restored to 72.3% and 79.6% of control, respectively. Both RSVAs significantly reduced the protein expression of inducible nitric oxide synthase and nitrotyrosine, and the mRNA levels of TNF-α, IL-6 and IL-1β.
Conclusions
RSVA405 and RSVA314 attenuate I/R-induced renal injury through the modulation of energy metabolism, oxidative stress, and inflammation.
Keywords: renal ischemia-reperfusion, resveratrol analogues, energy metabolism, oxidative stress, inflammation
1. Introduction
Renal ischemia-reperfusion (I/R) injury is a severe complication commonly occurring during major vascular surgery and is unavoidable in renal transplantation (1–4). It is the most common cause of acute kidney injury (AKI) in the surgical intensive care unit. Patients with AKI are at an increased risk for chronic kidney disease, end stage renal disease, and mortality (5). Further, the severity of AKI is predictive of the likelihood for progression to chronic kidney disease (6). The treatment of AKI in the clinical setting is limited to reversal of the causal insult and managing the complications of decreased renal function (7). Therefore, the identification of novel therapeutic agents is of priority for this disease.
The pathophysiology of renal I/R can be described as an early energy deficit during ischemia which is followed by a secondary phase of oxidative injury, inflammation, and metabolic dysfunction during reperfusion (8). Resveratrol is a polyphenol which has significant protective effects against ischemia-reperfusion injury of various organs (9–14). Specifically, resveratrol has been shown to be protective in several animal models of renal I/R-induced injury through its anti-oxidant and anti-inflammatory effects (15–20). Interestingly, resveratrol is also an activator of the metabolic enzymes sirtuin 1 (Sirt1) and 5′ adenosine monophosphate-activated protein kinase (AMPK) (21). Pharmacologic activation of these two enzymes has been shown to be protective in renal I/R-induced injury through the enhancement of renal energy metabolism (11, 22–24).
Although the beneficial effects of resveratrol have been demonstrated in vitro and in animal studies, the poor bioavailability of this molecule has been a major concern in human studies (25). To identify alternatives, we have screened several molecules with structural similarity to resveratrol as previously described (26). RSVA405 and RSVA314 were shown to activate AMPK 50 times more potently than resveratrol (26, 27). In addition, RSVA405 and RSVA314 were also shown to have an anti-inflammatory effect through the inhibition of STAT3 function (27, 28). In this study, we hypothesized that administration of these two newly identified resveratrol analogues would be protective against I/R-induced renal injury. To test this hypothesis, we employed a previously established rodent model of bilateral renal I/R injury (29). Animals subjected to renal I/R were pre-treated with RSVA405 or RSVA314. The effects of treatment with these two resveratrol analogues on I/R-induced renal injury were determined by analyzing several markers of renal injury, energy metabolism, oxidative stress, and inflammation.
2. Materials and methods
2.1. Experimental animals
Male Sprague-Dawley rats (300–350 g) were purchased from Charles River Laboratories (Wilmington, MA). Only males were used in this study to eliminate potential gender variability. The rats were housed in a temperature-controlled room, on a 12-h light/dark cycle. Rats were fed a standard Purina rat chow diet and allowed water ad libitum.
2.2. Rodent model of renal I/R injury
Rats were randomly assigned into different groups (n=5/group). Initially, rats were intraperitoneally injected with 0.5 ml of 3 mg/kg BW RSVA405, RSVA314, or 10% dimethyl sulfoxide (DMSO) plus 33% Solutol in PBS as vehicle. The dose range and route of drug administration were selected based on previous publications using resveratrol in renal I/R (18, 19, 30). RSVA405 and RSVA314 were obtained from Chembridge (Hit2Lead compounds # 5113025 and 5194489, respectively; San Diego, CA) and their chemical structures are depicted in Fig. 1 (26). An hour after injection, the rats were anesthetized with isoflurane (Butler Schein, Dublin, OH) inhalation. Using a midline abdominal incision, renal I/R injury was induced by bilateral renal pedicle clamping for 60 min. Then the clamp was removed, and reflow (reperfusion) was visually verified. No anti-coagulants were administered at any point during this study. Control animals were euthanized for sample collection and were not subjected to any surgical procedures. At 24 h after reperfusion, animals were anesthetized and plasma and tissue samples were harvested and stored at −80°C until analysis. All experiments were performed in accordance with the guidelines for the use of experimental animals by the National Institutes of Health (Bethesda, MD) and were approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
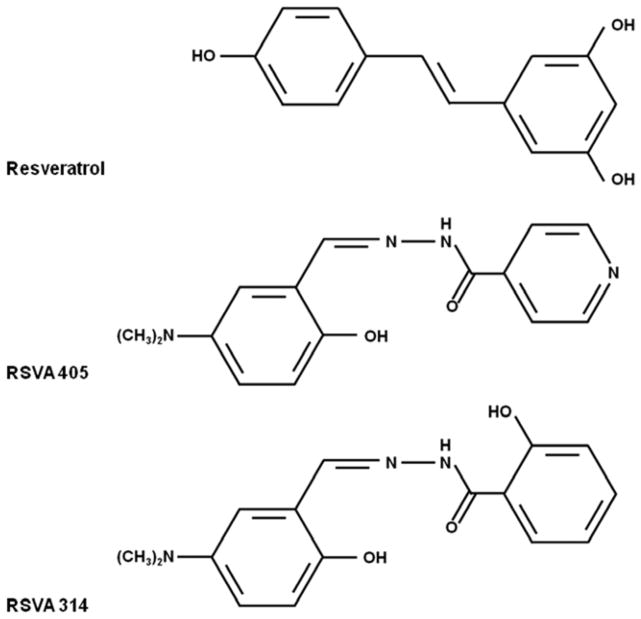
Fig. 1.
The chemical structure of the resveratrol analogues RSVA405 and RSVA314.
2.3. Determination of organ injury variables
Plasma levels of creatinine, blood urea nitrogen (BUN), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) were determined at 24 h after reperfusion by using commercial assay kits according to the manufacturer’s instructions (Pointe Scientific, Lincoln Park, MI).
2.4. Histologic examination
Morphologic alterations in the kidneys at 24 h after reperfusion were examined by light microscopy. The tissue was fixed in 10% formalin and later embedded in paraffin. The tissue paraffin sections (4-μm thick) were stained with hematoxylin and eosin. Histologic injury was assessed and scored by a blinded investigator according to a modified score by Kelly et al (31). Briefly, 10 random high power fields (HPFs) along the corticomedullary junction were examined. Each HPF was evaluated for tubular cell injury (karyorrhexis, karyolysis), tubular cell detachment, loss of brush border, abnormal bowman space, and cast formation. Each of the aforementioned categories was scored using a semi-quantitative scale: 1, <10%; 2, 10–25%; 3, 26–75%; and 4, >75% of damage area in the kidneys. An average score for each sample was then calculated.
2.5. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Paraffin-embedded sections were deparaffinized in xylene and rehydrated in a graded series of ethanol. Fluorescence staining was performed using an In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN). The assay was conducted according to the manufacturer’s instructions. The nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI). Results were expressed as the average number of TUNEL-positive staining nuclei per 10 HPF.
2.6. Determination of adenosine triphosphate (ATP) levels
The right and left kidneys were divided into sagittal sections. Each half of the kidney was immediately frozen in liquid nitrogen. Later, the frozen right and left kidneys were pulverized together in liquid nitrogen. Kidney tissue (100 mg) was homogenized in 300 μl assay buffer and centrifuged at 13,000g for 10 min. The supernatant was deproteinized by perchloric acid precipitation followed by potassium hydroxide neutralization before subjecting to the ATP assay kit from BioVision (Mountain View, CA).
2.8. Western blotting
Kidney tissue (100 mg) was homogenized in 500 μl of lysis buffer (10 mM Tris-HCl pH 7.5, 120 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) containing a protease inhibitor cocktail (Roche Diagnostics) using high frequency sonication. Samples were then centrifuged at 12,000 rpm at 4°C for 15 min and supernatant was collected for further analyses. Protein concentration was determined by a BIO-RAD DC™ protein assay kit (BIO-RAD, Hercules, CA). 50 μg of protein was fractionated on a Bis-Tris gel and transferred to a nitrocellulose membrane. The membranes were blocked by incubation in 0.2× PBS with 0.1% casein and incubated with primary anti-nitrotyrosine, iNOS and β-actin antibodies (Santa Cruz Biotechnology) in 0.2× PBS with 0.1% casein and 0.1% Tween 20. After washing, the blots were incubated subsequently with corresponding fluorescent secondary antibody (LI-COR, Lincoln, NE). Bands were detected using the Odyssey FC Dual-Mode Imaging system 2800 (LI-COR). Protein density was analyzed by NIH ImageJ software and all protein densities presented were normalized to their respective β-actin bands.
2.7. RT-PCR analysis
Total RNA was extracted from tissue samples after 24 h reperfusion using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized from 3.5 μg of total RNA using Oligo (dT)12–18 primer (Invitrogen) and murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA). The expressions of target mRNAs were detected by quantitative real-time PCR (7300 Real Time PCR System, Applied Biosystems) with SYBR Green as detection dye. Primer sequences are TNF-α: F- TGATCGGTCCCAACAAGGA, R- GGGCCATGGAACTGATGAGA; IL-6: F- AGGGAGATCTTGGAAATGAGAAAA, R- CATCATCGCTGTTCATACAATCAG; IL-1β: F- GACCTGTTCTTTGAGGCTGACA, R- AGTCAAGGGCTTGGAAGCA; and β-actin: F- CGTGAAAAGATGACCCAGATCA, R- TGGTACGACCAGAGGCATACAG. Relative expression of each mRNA was calculated using ΔΔ Ct threshold method. The level of β-actin mRNA was used for normalization. Relative expression of mRNA was expressed as fold change in comparison to the control tissues.
2.9. Statistical Analysis
Data are expressed as mean ± standard error and compared by one-way analysis of variance using Student-Newman-Keuls’ test. Differences in values were considered significant at P < 0.05.
3. Results
3.1. Resveratrol analogues attenuate renal injury and protect renal function after I/R
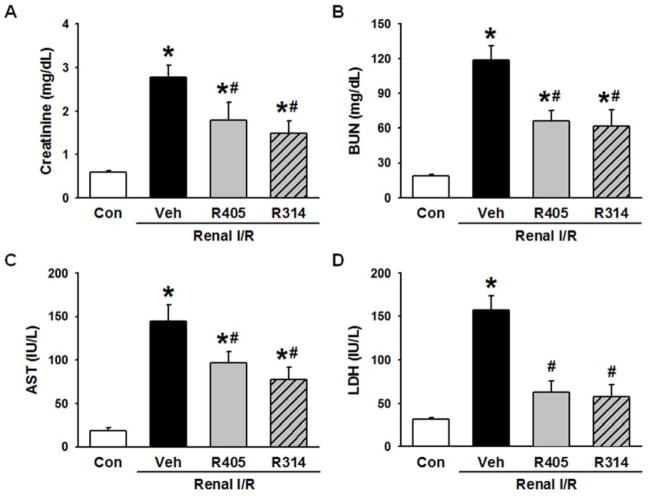
Serum levels of creatinine and BUN were used as indicators of renal dysfunction after I/R. The levels of these markers were significantly increased in the vehicle group in comparison to the control group (Fig. 2A and B), indicating a marked degree of renal dysfunction after I/R in our model. RSVA405 and RSVA314 treatment showed a 35.8% and 46.2% decrease in serum levels of creatinine (Fig. 2A), and a 44.3% and 47.9% decrease in serum levels of BUN (Fig. 2B), respectively, compared to the vehicle group.
Fig. 2.
RSVA405 and RSVA314 attenuate renal injury after renal I/R. Blood of the vehicle-, RSVA405- and RSVA314- treated rats were collected 24 h after reperfusion. Serum (A) creatinine, (B) blood urea nitrogen (BUN), (C) aspartate aminotransferase (AST) and (D) lactate dehydrogenase (LDH) were measured. Data are presented as mean ± standard error (n=4–5/group). *P < 0.05 vs. control; #P < 0.05 vs. vehicle. Con, control; Veh, vehicle; R405, RSVA405; and R314, RSVA314.
Serum levels of AST and LDH were used as indicators of renal injury after I/R (32). The levels of these markers were significantly increased in the vehicle group in comparison to the control group (Fig. 2C and D), indicating a severe degree of renal injury after I/R in our model. RSVA405 and RSVA314 treatment showed a 33.0% and 46.6% decrease in serum AST levels (Fig. 2C), and a 59.8% and 63.2% decrease in serum LDH levels (Fig. 2D), respectively, compared to the vehicle group.
3.2. Resveratrol analogues preserve renal histologic architecture after I/R
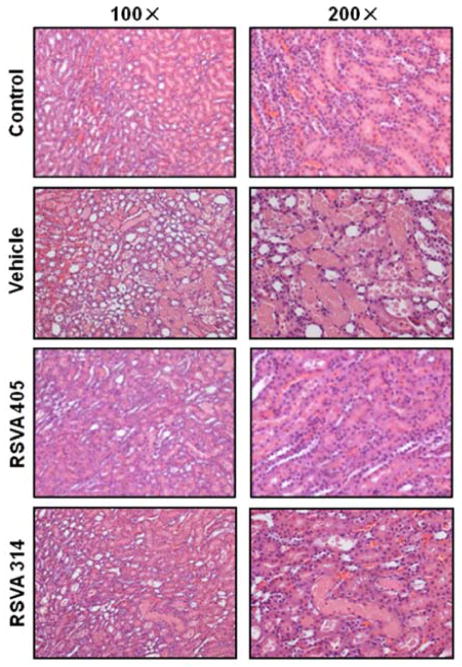
Histologic evaluation of the kidneys from the vehicle group was associated with marked kidney damage as represented by tubular cell injury, tubular epithelial cell sloughing, and cast formation (Fig. 3). The extent of injury was reduced in the treatment groups (Fig. 3). The difference in injury is semi-quantitatively represented in Fig. 4. The scoring method ranged from 0 to 20, the highest number representing maximum injury as described in Materials and Methods. The vehicle group scored 17.9 ± 0.4 in comparison to a score of 2.0 ± 0.4 in the control group (Fig. 4). Treatment with RSVA405 and RSVA314 significantly reduced the histologic injury score to 7.9 ± 0.4 and 10.3 ± 1.6, respectively, compared to the vehicle group (Fig. 4).
Fig. 3.
RSVA405 and RSVA314 preserve renal architecture after I/R. Kidney tissues of the vehicle-, RSVA405- and RSVA314-treated rats were collected 24 h after reperfusion. Representative photomicrographs of histological staining with hematoxylin and eosin in the corticomedullary junction at 100× (Left) and 200× magnification (Right).
Fig. 4.
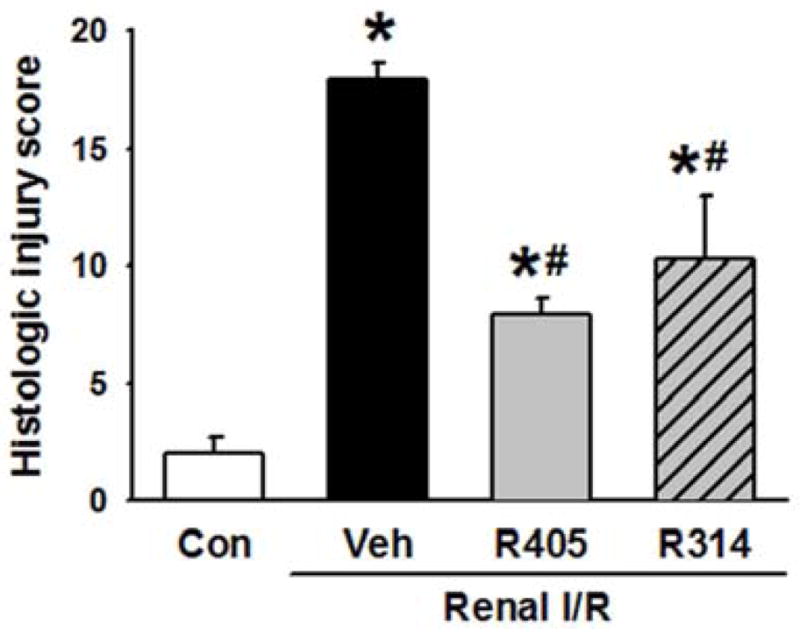
Histologic score of renal I/R injury. The extent of damage at the corticomedullary junction was graded with a modified schema as described in Materials and Methods. The score represents the percentage of morphologic alterations as follows: 1, <10%; 2+, 10–25%; 3+, 26–75%; 4+, >75%. Data are presented as mean ± standard error (n=3–4/group). *P < 0.05 vs. control; #P < 0.05 vs. vehicle. Con, control; Veh, vehicle; R405, RSVA405; and R314, RSVA314.
3.3. Resveratrol analogues decrease apoptosis after renal I/R
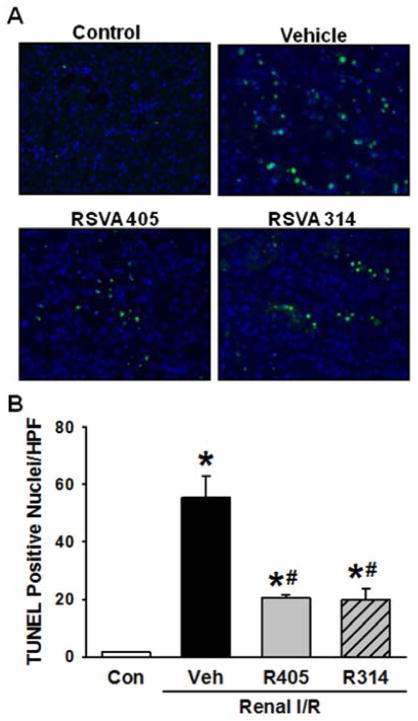
TUNEL assay was performed on renal tissue sections for assessment of apoptosis (Fig. 5A). There was a significant increase in the number of apoptotic cells after renal I/R as represented by a mean of 55.7 ± 7.3 TUNEL-positive nuclei/HPF in the vehicle group in comparison with 1.5 ± 0.2 in the control group (Fig. 5B). RSVA405 and RSVA314 markedly decreased the number of apoptotic cells after I/R by 63.1% and 64.2%, respectively, compared to the vehicle group (Fig. 5B).
Fig. 5.
RSVA405 and RSVA314 decrease apoptosis after renal I/R. Kidney tissues of the vehicle-, RSVA405- and RSVA314-treated rats were collected 24 h after reperfusion. (A) Representative photomicrographs of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL; green) and 4′,6-diamidino-2-phenylindole (DAPI) as counterstain (blue) at 200× magnification. (B) Graph representing the number of TUNEL positive nuclei per high power field as described in Materials and Methods. Data are presented as mean ± standard error (n=3–4/group). *P < 0.05 vs. control; #P < 0.05 vs. vehicle. Con, control; Veh, vehicle; R405, RSVA405; and R314, RSVA314.
3.4. Resveratrol analogues preserve renal ATP levels after I/R
Renal tissue ATP levels were measured to assess for the effects of resveratrol analogues on renal energy metabolism after I/R. Consistent with previous reports (23, 33, 34), ATP levels were 52.4% lower than the control at 24 h after reperfusion (Fig. 6). The ATP levels were significantly improved to 72.3% and 79.6% of the control by RSVA405 and RSVA314, respectively, after renal I/R (Fig. 6).
Fig. 6.
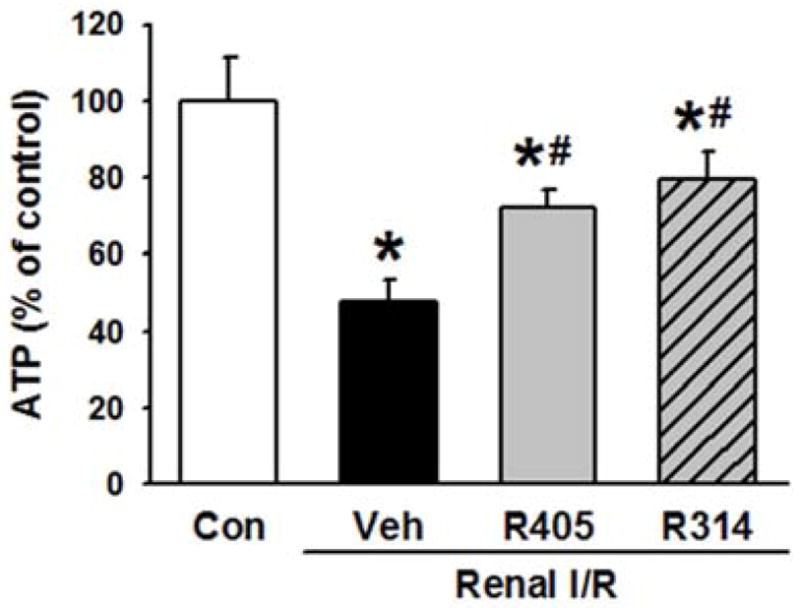
Resveratrol analogues increase ATP levels after renal I/R. (A) Kidney tissues of the vehicle-, RSVA405- and RSVA314-treated rats were collected 24 h after reperfusion for determination of ATP levels. Data are presented as mean ± standard error (n=4–5/group). *P < 0.05 vs. control, #P < 0.05 vs. vehicle. Con, control; Veh, vehicle; R405, RSVA405; and R314, RSVA314.
3.5. Resveratrol analogues decrease oxidative stress after I/R
Inducible nitric oxide synthase (iNOS) and nitrotyrosine protein levels were measured by Western blotting, as markers for oxidative and nitrosative stress (35–39). The expression of iNOS was 1.8-fold higher in the vehicle group than the control (Fig. 7A). This was decreased to the control levels with the administration of RSVA405 and RSVA314 (Fig. 7A). Further, nitrotyrosine protein expression mimicked the pattern shown by iNOS, with a 1.5-fold increase in the vehicle group, which was restored to control levels in the treatment groups (Fig. 7B).
Fig. 7.
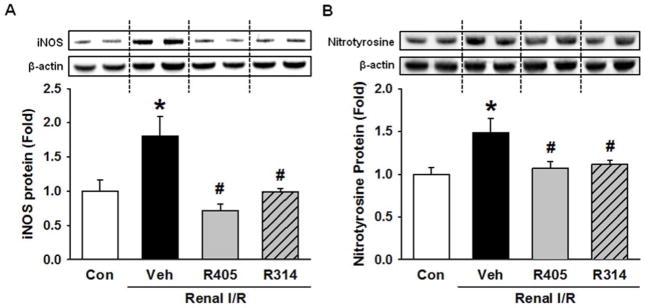
Resveratrol analogues attenuate oxidative stress after renal I/R. Kidney tissues of the vehicle-, RSVA405- and RSVA314-treated rats were collected 24 h after reperfusion for determination of (A) inducible nitric oxide synthase (iNOS) and (B) nitrotyrosine protein expression levels by Western blotting. Data are presented as mean ± standard error (n=3–5/group). *P < 0.05 vs. control; #P < 0.05 vs. vehicle. Con, control; Veh, vehicle; R405, RSVA405; and R314, RSVA314.
3.6. Resveratrol analogues attenuate renal inflammation after I/R
To assess for renal inflammation, mRNA expression of various inflammatory cytokines were measured using RT-PCR (Fig. 8). Expression of TNF-α, IL-6, and IL-1β were significantly increased by 1.50-, 1.62-, and 3.01-fold, respectively, in the vehicle group, compared to the control group (Fig. 8A–C). RSVA405 and RSVA314 significantly reduced the levels of TNF-α by 49.6% and 34.3%, IL-6 by 25.0% and 21.2%, and IL-1β by 58.6% and 50.3%, respectively, compared to the vehicle group (Fig. 8A–C).
Fig. 8.
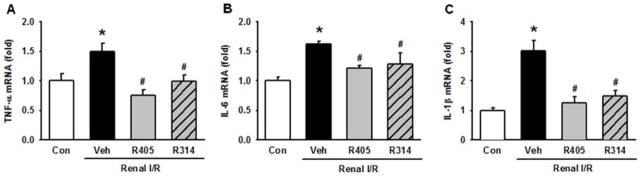
Resveratrol analogues decrease the expression of renal pro-inflammatory cytokines after I/R. Kidney tissues of the vehicle-, RSVA405- and RSVA314-treated rats were collected 24 h after reperfusion. Renal tissue mRNA expression of (A) TNF-α, (B) IL-6 and (C) IL-1β are determined by RT-PCR analysis. Data are presented as mean ± standard error (n=4–5/group). *P < 0.05 vs. control; #P < 0.05 vs. vehicle. Con, control; Veh, vehicle; R405, RSVA405; and R314, RSVA314.
4. Discussion
AKI secondary to renal I/R still remains a major complication without specific therapy due to its complex pathophysiology. I/R injury encompass a number of deleterious cellular processes highlighted by dysregulated energy metabolism, inflammation and oxidative stress, which ultimately results in cellular death and organ dysfunction. Resveratrol has been shown to modulate each of these cellular events in various models of I/R injury, including renal I/R (15); however, its clinical use has been limited (25). We have previously identified that RSVA405 and RSVA314 have higher biological activity than resveratrol in activating AMPK. In this study, we sought to investigate the efficacy of these two novel RSVAs in attenuating I/R-induced renal injury. We have demonstrated that RSVA405 and RSVA314 significantly reduced serum markers of renal dysfunction and injury, including creatinine, BUN, AST, and LDH. This was further supported by the preservation of renal histologic architecture in the treatment groups when compared to the vehicle group. Further, treatment with RSVA405 and RSVA314 were associated with a significant reduction in apoptosis induced by I/R injury.
There is a significant body of literature which supports the role of resveratrol in the activation of the metabolic regulators AMPK and Sirt1 (21, 40, 41). Activation of these two enzymes results in an increase in energy production and mitochondrial biogenesis, which is associated with protection against renal I/R injury (23, 33, 42–46). In line with these studies, we have shown that treatment with RSVA405 and RSVA314 significantly increased ATP levels in the kidneys and decreased the severity of renal injury after I/R. Similarly, our lab has also demonstrated that the combination of AMPK activator 5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide (AICAR) and carnitine can enhance energy metabolism and thereby protect against renal I/R injury (33). We have also recently shown that pharmacologic activation of Sirt1 with SRT1720, a compound structurally unrelated to resveratrol, preserves the mitochondrial mass, increases ATP levels and attenuates I/R-induced renal injury (23). Taken together, stimulating energy metabolism to enhance ATP production may provide another strategy to protect the kidneys from I/R-induced injury.
Oxidative stress is a hallmark of I/R injury (8, 35, 47). Particularly, iNOS has been implicated in the pathogenesis of renal I/R-induced oxidative injury (32, 35, 36, 38). iNOS catalyses the production of nitric oxide which reacts with free oxygen radicals and form peroxynitrite. The generation of peroxynitrite in turn induces tissue injury through lipid peroxidation, apoptosis, necrosis, and neutrophil recruitment by nitration of tyrosine residues on tissue proteins (48). This effect was further confirmed in vivo where mice lacking the iNOS gene were protected from renal I/R injury in comparison to their wild-type counterparts (32, 38). In correspondence, we have observed that treatment with RSVA405 and RSVA314 decreases the iNOS expression in the kidneys and concomitantly decreases peroxynitrite generation, as measured by nitrotyrosine levels (39), after I/R. It has been repeatedly demonstrated that many of the beneficial effects of resveratrol can be attributed to its antioxidant properties, either through direct scavenging properties or through the regulation of enzymes involved in redox balance (19, 49, 50). In a study by Bertilli et al pretreatment with resveratrol prior to bilateral renal ischemia in rats reduced oxidative stress and decreased renal-I/R induced mortality (16). Thus, RSVA405 and RSVA314 possess similar antioxidative effects as resveratrol. Other agents targeting iNOS have been shown to be beneficial in the setting of hypoxia-induced injury. For example, treatment with GW274150 and FR260330, potent iNOS inhibitors, was renoprotective in both mice and rats after bilateral renal I/R (32) as well as in vervet monkeys (38).
Renal I/R is associated with an inflammatory response due to the release of damage associated molecules, which results in the recruitment of inflammatory cells from the circulation into the renal parenchyma (8). This is usually accompanied by the release of inflammatory cytokines. We have demonstrated the capability of RSVA405 and RSVA314 in effectively inhibiting the mRNA expression of the proinflammatory cytokines TNF-α, IL-6, and IL-1β elevated in the kidneys after I/R injury. This observation is in support of our previous work showing an inhibitory effect of RSVA405 and RSVA314 on the inflammatory regulator STAT3 in lipopolysacharide-stimulated RAW cells, ultimately resulting to decreased cytokine release (28). Such anti-inflammatory activity has also been described using resveratrol in multiple disease models (51, 52). In a rat renal I/R model, Sener et al reported a significant decrease in serum TNF-α levels at 6 h after reperfusion in the group which was pretreated with intraperitoneal injection of resveratrol (20). In addition to inhibiting STAT3 phosphorylation (53), resveratrol can also inhibit NF-κB signaling through Sirt1 activation thereby attenuating the production of proinflammatory cytokines (54, 55).
There are a few limitations of this work which will need to be further addressed before these two resveratrol analogues can be considered for clinical use. In this study, only male rats were used. It would be necessary to demonstrate effectiveness of RSVA405 and RSVA314 in both genders in the future. The resveratrol analogues were administered prior to the onset of ischemia in this study, which limits its clinical applicability to planned surgical procedures such as transplantation, cardiac bypass, or vascular clamping. It will be interesting to know the efficacy of these resveratrol analogues when administered after the onset of I/R or later, which will make them applicable to a wider range of clinical settings. Also important, is the determination of the pharmacokinetic and safety profile of these agents, which is necessary for future drug development.
Herein, we describe RSVA405 and RSVA314 as two novel agents with beneficial effects on renal I/R-induced injury. The molecular mechanisms by which these two compounds provide protection from renal I/R remains to be further investigated. Nevertheless, we have demonstrated that RSVA405 and RSVA314 may accomplish their renoprotective effects through decreasing apoptotic cell death, enhancing energy metabolism, and attenuating oxidative stress and inflammation after I/R. Thus, these two resveratrol analogues may be promising agents in the treatment of patients suffering from renal I/R injury.
Footnotes
A.K., W.Y., P.M., and P.W. conception and design of research; A.K., and M.K. data collection; A.K., W.Y., and J.M.P. analyzed data; A.K., W.Y., J.N. and G.F.C. interpreted results of experiments; A.K. drafted manuscript; W.Y., J.M.P, J.N. and G.F.C. critically revised manuscript; P.W. revised the final version of manuscript.
Disclosures
This work was supported in part by the National Institutes of Health grant HL076179 to PW. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kazmers AJL, Perkins A. The Impact of Complications after Vascular Surgery in Veterans Affairs Medical Centers. J Surg Res. 1997;67:62–66. doi: 10.1006/jsre.1996.4946. [DOI] [PubMed] [Google Scholar]
- 2.Aydin Z, van Zonneveld AJ, de Fijter JW, Rabelink TJ. New horizons in prevention and treatment of ischaemic injury to kidney transplants. Nephrol Dial Transplant. 2007;22:342–346. doi: 10.1093/ndt/gfl690. [DOI] [PubMed] [Google Scholar]
- 3.Powell JT, Tsapepas DS, Martin ST, Hardy MA, Ratner LE. Managing renal transplant ischemia reperfusion injury: novel therapies in the pipeline. Clin Transplant. 2013;27:484–491. doi: 10.1111/ctr.12121. [DOI] [PubMed] [Google Scholar]
- 4.Goto R, Issa F, Heidt S, Taggart D, Wood KJ. Ischemia-reperfusion injury accelerates human antibody-mediated transplant vasculopathy. Transplantation. 2013;96:139–145. doi: 10.1097/TP.0b013e318295ee32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(Suppl):1–138. [Google Scholar]
- 8.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 9.Gedik E, Girgin S, Ozturk H, Obay BD, Buyukbayram H. Resveratrol attenuates oxidative stress and histological alterations induced by liver ischemia/reperfusion in rats. World J Gastroenterol. 2008;14:7101–7106. doi: 10.3748/wjg.14.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmali N, Esenkaya I, Karadag N, Tas F. Effects of resveratrol on skeletal muscle in ischemia-reperfusion injury. Ulus Travma Acil Cerrahi Derg. 2007;13:274–280. [PubMed] [Google Scholar]
- 11.Plin C, Tillement JP, Berdeaux A, Morin D. Resveratrol protects against cold ischemia-warm reoxygenation-induced damages to mitochondria and cells in rat liver. Eur J Pharmacol. 2005;528:162–168. doi: 10.1016/j.ejphar.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Lin Y, Chen F, Zhang J, Wang T, Wei X, et al. Neuroprotective effect of resveratrol on ischemia/reperfusion injury in rats through TRPC6/CREB pathways. J Mol Neurosci. 2013;50:504–513. doi: 10.1007/s12031-013-9977-8. [DOI] [PubMed] [Google Scholar]
- 13.Budak B, Seren M, Turan NN, Sakaogullari Z, Ulus AT. The protective effects of resveratrol and L-NAME on visceral organs following aortic clamping. Ann Vasc Surg. 2009;23:675–685. doi: 10.1016/j.avsg.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Dong W, Li F, Pan Z, Liu S, Yu H, et al. Resveratrol ameliorates subacute intestinal ischemia-reperfusion injury. J Surg Res. 2013;185:182–189. doi: 10.1016/j.jss.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Kitada M, Koya D. Renal protective effects of resveratrol. Oxid Med Cell Longev. 2013;2013:568093. doi: 10.1155/2013/568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertelli AA, Migliori M, Panichi V, Origlia N, Filippi C, et al. Resveratrol, a component of wine and grapes, in the prevention of kidney disease. Ann N Y Acad Sci. 2002;957:230–238. doi: 10.1111/j.1749-6632.2002.tb02919.x. [DOI] [PubMed] [Google Scholar]
- 17.Giovannini L, Migliori M, Longoni BM, Das DK, Bertelli AA, et al. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol. 2001;37:262–270. doi: 10.1097/00005344-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Saito M, Satoh S, Kojima N, Tada H, Sato M, et al. Effects of a phenolic compound, resveratrol, on the renal function and costimulatory adhesion molecule CD86 expression in rat kidneys with ischemia/reperfusion injury. Arch Histol Cytol. 2005;68:41–49. doi: 10.1679/aohc.68.41. [DOI] [PubMed] [Google Scholar]
- 19.Chander V, Chopra K. Protective effect of nitric oxide pathway in resveratrol renal ischemia-reperfusion injury in rats. Arch Med Res. 2006;37:19–26. doi: 10.1016/j.arcmed.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Sener G, Tugtepe H, Yuksel M, Cetinel S, Gedik N, et al. Resveratrol improves ischemia/reperfusion-induced oxidative renal injury in rats. Arch Med Res. 2006;37:822–829. doi: 10.1016/j.arcmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Idrovo JP, Yang WL, Matsuda A, Nicastro J, Coppa GF, et al. Post-treatment with the combination of 5-aminoimidazole-4-carboxyamide ribonucleoside and carnitine improves renal function after ischemia/reperfusion injury. Shock. 2012;37:39–46. doi: 10.1097/SHK.0b013e31823185d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khader A, Yang WL, Kuncewitch M, Jacob A, Prince JM, et al. Sirtuin 1 Activation Stimulates Mitochondrial Biogenesis and Attenuates Renal Injury After Ischemia-Reperfusion. Transplantation. 2014 doi: 10.1097/TP.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 24.Yousuf S, Atif F, Ahmad M, Hoda N, Ishrat T, et al. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 2009;1250:242–253. doi: 10.1016/j.brainres.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 25.Tome-Carneiro J, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan FA, Garcia-Conesa MT, et al. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des. 2013;19:6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vingtdeux V, Chandakkar P, Zhao H, d’Abramo C, Davies P, et al. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. Faseb j. 2011;25:219–231. doi: 10.1096/fj.10-167361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vingtdeux V, Chandakkar P, Zhao H, Davies P, Marambaud P. Small-molecule activators of AMP-activated protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis. Mol Med. 2011;17:1022–1030. doi: 10.2119/molmed.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capiralla H, Vingtdeux V, Venkatesh J, Dreses-Werringloer U, Zhao H, et al. Identification of potent small-molecule inhibitors of STAT3 with anti-inflammatory properties in RAW 264.7 macrophages. Febs j. 2012;279:3791–3799. doi: 10.1111/j.1742-4658.2012.08739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah KG, Rajan D, Jacob A, Wu R, Krishnasastry K, et al. Attenuation of renal ischemia and reperfusion injury by human adrenomedullin and its binding protein. J Surg Res. 2010;163:110–117. doi: 10.1016/j.jss.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chander V, Chopra K. Protective effect of resveratrol, a polyphenolic phytoalexin on glycerol-induced acute renal failure in rat kidney. Ren Fail. 2006;28:161–169. doi: 10.1080/08860220500531112. [DOI] [PubMed] [Google Scholar]
- 31.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee PK, Patel NS, Sivarajah A, Kvale EO, Dugo L, et al. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63:853–865. doi: 10.1046/j.1523-1755.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 33.Idrovo JP, Yang WL, Matsuda A, Nicastro J, Coppa GF, et al. Post-treatment with the combination of 5-aminoimidazole-4-carboxyamide ribonucleoside and carnitine improves renal function after ischemia/reperfusion injury. Shock. 2012;37:39–46. doi: 10.1097/SHK.0b013e31823185d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saba H, Batinic-Haberle I, Munusamy S, Mitchell T, Lichti C, et al. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic Biol Med. 2007;42:1571–1578. doi: 10.1016/j.freeradbiomed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61:855–861. doi: 10.1046/j.1523-1755.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 36.Ling H, Gengaro PE, Edelstein CL, Martin PY, Wangsiripaisan A, et al. Effect of hypoxia on proximal tubules isolated from nitric oxide synthase knockout mice. Kidney Int. 1998;53:1642–1646. doi: 10.1046/j.1523-1755.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 37.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, et al. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 38.Qi S, Xu D, Ma A, Zhang X, Chida N, et al. Effect of a novel inducible nitric oxide synthase inhibitor, FR260330, in prevention of renal ischemia/reperfusion injury in vervet monkeys. Transplantation. 2006;81:627–631. doi: 10.1097/01.tp.0000199282.05021.0c. [DOI] [PubMed] [Google Scholar]
- 39.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–895. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- 41.Fullerton MD, Steinberg GR. SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes. 2010;59:551–553. doi: 10.2337/db09-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan H, Yang HC, You L, Wang YY, He WJ, et al. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013;83:404–413. doi: 10.1038/ki.2012.394. [DOI] [PubMed] [Google Scholar]
- 43.Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010;333:593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1alpha activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol. 2013;273:345–354. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin A, Sekhon C, Sekhon B, Smith A, Chavin K, et al. Attenuation of ischemia-reperfusion injury in a canine model of autologous renal transplantation. Transplantation. 2004;78:654–659. doi: 10.1097/01.tp.0000131664.18670.17. [DOI] [PubMed] [Google Scholar]
- 46.Seo-Mayer PW, Thulin G, Zhang L, Alves DS, Ardito T, et al. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol Renal Physiol. 2011;301:F1346–1357. doi: 10.1152/ajprenal.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, et al. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:F948–957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 48.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spanier G, Xu H, Xia N, Tobias S, Deng S, et al. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60 (Suppl 4):111–116. [PubMed] [Google Scholar]
- 50.Movahed A, Yu L, Thandapilly SJ, Louis XL, Netticadan T. Resveratrol protects adult cardiomyocytes against oxidative stress mediated cell injury. Arch Biochem Biophys. 2012;527:74–80. doi: 10.1016/j.abb.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Das S, Das DK. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- 52.Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005;78:389–397. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 54.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, et al. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 55.Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]