Abstract
Background
We have previously shown that mitochondrial uncoupling protein-2 (UCP-2) is increased in a swine model of hibernating myocardium (HM). Although UCP-2 reduces oxidant stress, it can promote inefficiency of the electron transport chain. In this study, we tested whether UCP-2 remains increased in revascularized HM (RHM) following coronary artery bypass grafting (CABG).
Methods
Seven swine underwent thoracotomy with placement of a constrictor on the left anterior descending artery (LAD). Twelve weeks later, a left internal mammary artery (LIMA) graft was placed on the distal LAD. Four weeks post-CABG, CT angiography documented patent grafts and function. At the terminal study, blood flow to the LAD and remote territories were assessed during high-dose dobutamine and mitochondria isolated from both regions for analysis. Comparisons were made to a group of swine with HM who underwent constrictor placement without bypass grafting (n=4).
Results
During dobutamine infusion, RHM demonstrated lower blood flows (2.44±0.23 versus 3.43±0.30 ml/min/g; P<0.05) and reduced wall thickening (33±9% versus 52±13%; P<0.05) compared with remote regions. RHM had lower respiratory control indices (3.7±0.3 versus 4.3±0.4; P<0.05) with persistently increased UCP-2 content.
Conclusion
Despite patent grafts, RHM demonstrates a submaximal response to dobutamine infusion and increased mitochondrial UCP-2 expression. These data support the notion that recovery of the mitochondria in RHM is delayed early post-CABG and may contribute to impaired oxygen consumption and contractile reserve during catecholamine challenges.
INTRODUCTION
Uncoupling protein (UCP) is a class of proteins expressed within the inner membrane of mitochondria. At least five UCPs have been identified in mammals. UCP-1 is best described in brown adipose tissue (BAT) for thermogenesis in hibernating animals1. UCP-2 has been shown to be ubiquitous in the heart and is transcriptionally expressed and activated by superoxide2. When overexpressed within the heart, it reduces the degree of oxidant damage in the tissue 3, 4 and in fact, prolongs survival in transgenic mice with increased expression compared with mice with genetic disruption of the UCP-2 gene5. We have previously shown that maximal superoxide production is increased in isolated heart mitochondria from UCP-2 knock out compared with wild type mice6 demonstrating UCP-2’s role in reducing oxidant stress. In addition, increased UCP-2 expression in swine hearts that underwent an ischemic preconditioning protocol during the second window of protection (SWOP) is associated with reduced reactive oxygen species7. In a swine model of hibernating myocardium, we have also shown that chronic ischemia leads to an increase in UCP-2 expression and reduced oxidant stress in mitochondria isolated from hibernating heart tissue8. Despite these favorable effects of UCP-2, increased overexpression has been observed in patients with heart failure and may prove maladaptive in regards to maintaining contractile function9–11. Because regional wall function may not recover immediately following revascularization12, 13the goal of the present study was to determine whether UCP-2 expression remains increased following revascularization of hibernating hearts with a left internal mammary artery (LIMA) graft.
MATERIALS AND METHODS
All animal studies were approved by the Institutional Animal Care and Use Committee of the Minneapolis VA Medical Center and conform to current National Institutes of Health and American Physiological Society guidelines for the use and care of laboratory animals.
Operative Technique
For these studies, we used a swine model of chronically ischemic hibernating myocardium that has previously been shown to have decreased regional blood flow and wall thickening in the absence of significant necrosis14. In brief, two survival operations were performed in 7 animals. The first operation created the hibernating myocardium by inducing chronic ischemia and the second operation surgically revascularized the myocardium. The terminal procedure occurred 4 weeks after the 2nd (bypass) operation, as we have previously described15. Chronic hibernation model (8-10 kg pigs). Animals were sedated with telazol (4 mg/kg; IM) and xylazine (2 mg/kg; IM), intubated and anesthetized with isoflurane (2%). Using a left thoracotomy approach, a plastic c-shaped constrictor with an internal diameter of 1.5mm was placed around the LAD just proximal to the first diagonal without occluding the vessel and secured with sutures. The animal recovered for 12 weeks. To confirm hibernating myocardium, we documented proximal LAD stenosis by multi-detector computer tomography (MDCT) and wall motion abnormality by transthoracic echocardiogram (TTE) prior to the second operation. After 12 weeks, animals underwent the second survival operation for revascularization. Revascularization model (50-60 kg pigs). Animals were again sedated, intubated, and anesthetized as noted above. Coronary artery revascularization was performed via a midline sternotomy with the left internal mammary artery (LIMA) dissected free from the chest wall. Lidocaine (1mg/kg) and heparin (100 units/kg) were administered. The (LAD) artery distal to the site of stenosis was then exposed. Using an off-pump technique, a LIMA anastomosis to the LAD was performed. A second MDCT and TTE were done four weeks after revascularization to document graft patency and assess function in the LAD territory. Terminal procedure (70-80 kg pigs). Four weeks after revascularization, a terminal study was performed. Fluorescently labeled color microspheres were injected into the left ventricle prior to and following a five minute infusion of dobutamine (40 µg/kg/min) to determine regional blood flows. A redo sternotomy was performed, the heart excised, and tissue samples obtained for blood flow, proteomic and histologic analysis. A control group (n=4) who underwent a thoracotomy with constrictor placement followed 12 weeks later by median sternotomy and dissection of the LIMA artery but without any bypass procedure were sacrificed 16 weeks after their initial surgery.
Cardiac Imaging
2D Transthoracic ECHO (TTE). We measured regional myocardial function in the LAD and circumflex regions of the swine heart. The circumflex region (remote) was used as an internal control with measurements at standardized locations to allow for comparisons between animals. TTE with and without dobutamine infusion was performed using a Vivid 7 Ultrasound machine (General Electric, New York, NY) during deep sedation without intubation. Heart rate, rhythm and oxygenation were monitored during the procedure. Wall thickening was measured at the right parasternal short-axis view in the posterior (circumflex) and anterior (LAD) walls. Wall thickening was computed as the difference between end-systolic and end-diastolic wall thickness and expressed as a percentage of end-diastolic thickness. Multi-Detector Computer Tomographic Analysis (MDCT). MDCT was used to document the initial LAD stenosis as well as confirm LIMA graft patency at 4 weeks post-CABG. MDCT scanning of the hibernating and revascularized animals was performed using the Brilliance 40 MDCT scanner (Phillips Medical Systems, Cleveland, Ohio) during deep sedation with Telazol without designated breath-hold or beta-blockade. EKG gated image acquisition was used to reconstruct images retrospectively. A volume of 150 ml of contrast media was injected intravenously at a rate of 4 ml/s. Scanning was triggered automatically when contrast enhancement within the descending aorta reached a threshold level of 150 Hounsfield Units (HU). At least ten phases were reconstructed for each study and coronary analysis was done at either 75% phase or end-systolic phase. Image analysis was done with a dedicated workstation (Philips Extended Brilliance Workspace, Cleveland, Ohio).
Tissue Analysis
Myocardial Blood Flows. Myocardial blood flow was quantified with 15 micrometer fluorescently labeled microspheres injected into the left ventricle at rest and during a 5 minute dobutamine (40 µg/kg/min) infusion at the time of the terminal study. Forty thousand fluorescently labeled microspheres (Triton Technology Inc, San Diego CA) per kilogram were injected for each analysis. Following the terminal study, the heart was serially sectioned and samples of fresh tissue were rapidly excised from subendocardial and subepicardial in the LAD and remote territories for regional perfusion analysis. Approximately 1 gram of tissue from each sample was digested with 4M potassium hydroxide with 1% Tween 80 for 72 hours. The digested material was filtered through 10 micrometer isopore membrane filter so as to retain the microsphere sediments upon the filter. The filters were washed in 3 ml diethylene glycol monoethyl ether acetate which dissolves the microspheres and releases the fluorochrome. The fluorescent signal was then measured in a Perkin Elmer LS-55 Luminescence Spectrometer. The fluorescent signal was normalized to the tissue weight and differences analyzed between samples. High-Energy Nucleotides. Just prior to sacrifice, using a modified variable-speed drill, a transmural biopsy (50-100 mg) was obtained from the LAD and Remote regions and transferred to liquid nitrogen-cooled 2-methylbutane within 1 second. High-energy nucleotides were extracted from frozen heart tissue within 24 hours in 7.1% perchloric acid, homogenized and centrifuged. The supernatant was neutralized (pH 7.20) with 2N KOH, 0.4 M imidazole, and 0.4 M KCL and centrifuged to remove potassium perchlorate. Samples were then frozen at −80°C. ATP and phosphocreatine were assayed spectrophotometrically in a two-step coupled enzymatic system with hexokinase and glucose-6-phosphate dehydrogenase with the reduction of NADP at an absorbance of 340 nm7. Histology. Tissue samples were fixed with 4% paraformaldehyde and processed as paraffin-embedded sections. For histochemistry, sections were stained with hematoxylin-eosin and Masson’s trichrome. Triphenyl tetrazolium chloride (TTC) stain was used to confirm viability of the myocardium. Mitochondrial Isolation. Fresh tissue that was rapidly excised from subendocardial and subepicardial samples from the LAD and remote territories were obtained. As previously described, myocardial tissue was placed in ice cold mitochondrial isolation buffer (MIB), pH 7.15, containing 50 mM sucrose, 200 mM mannitol, 1 mM EGTA. 5mM KH2PO25 mM MOPS, nagarse 2 mg/ml and 0.1 % fatty acid free BSA. Myocardium was minced and a 5% homogenate was made6, 16. The supernatant was centrifuged two times, each at 8,000×g for ten minutes. The mitochondria were collected and the protein concentration determined. In general, the mitochondrial preparations contained about 5 mg of protein/ml and the citrate synthase activity amounted to 2.4 U/mg.
Statistical Analysis
Data are expressed as means and standard errors (SEM) and were calculated for the hibernating LAD regions that had undergone bypass surgery. Differences between the LAD and remote regions were tested with Student's paired t-test with significance at the P < 0.05 value.
RESULTS
In Vivo Measurements
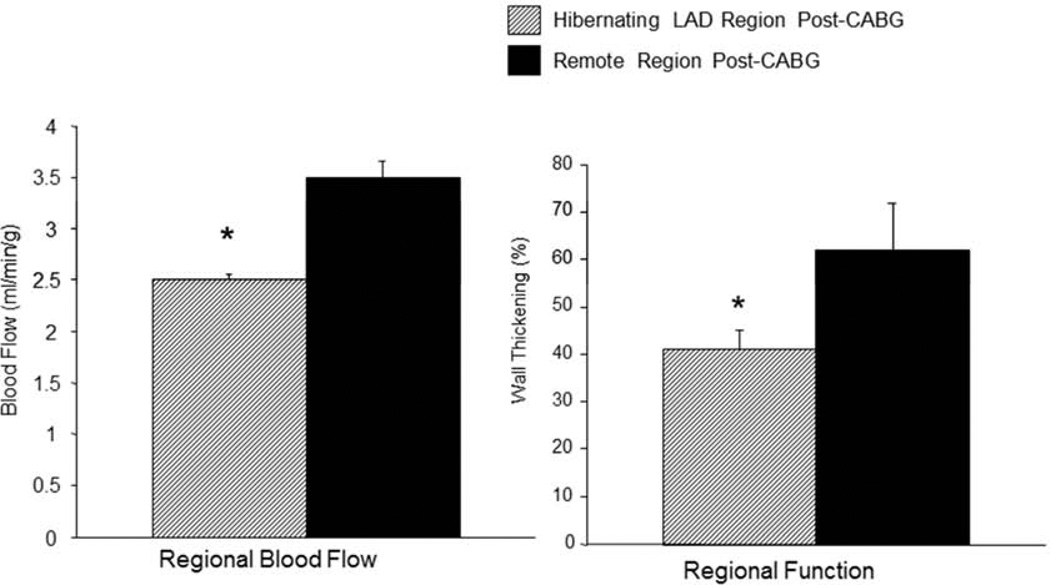
The CT angiogram at 12 weeks following instrumentation showed a severe stenosis in the LAD region of all animals, and in the revascularization group, a follow-up study at 4 weeks following CABG, just prior to the terminal study, showed a patent LIMA graft (Figure 1). At the time of the terminal study and under basal conditions, regional blood flow in the revascularized LAD region was slightly lower than the remote regions (0.56±0.06 and 0.67±0.10 ml/min/g respectively; NS) as was regional wall thickening by ECHO (35±4% and 41±4% respectively; NS). During the high-dose dobutamine infusion however, the differences in regional blood flow became much more substantial (2.44±0.23 and 3.43±0.30 ml/min/g in the LAD and Remote regions respectively; P<0.05) as did the differences in regional wall thickening (33±9% and 52±13% respectively; P<0.05) (Figure 2). The limitation in perfusion during the catecholamine stress was not related to the conduit, which was demonstrated to be widely patent during the CTA study as well as during the terminal study. Tissue biopsies for ATP and Phosphocreatine levels were obtained following the catecholamine stress, just prior to sacrifice, and did not show any regional differences in high-energy nucleotides (Table 1), suggesting that there was no active ischemia at the high work state.
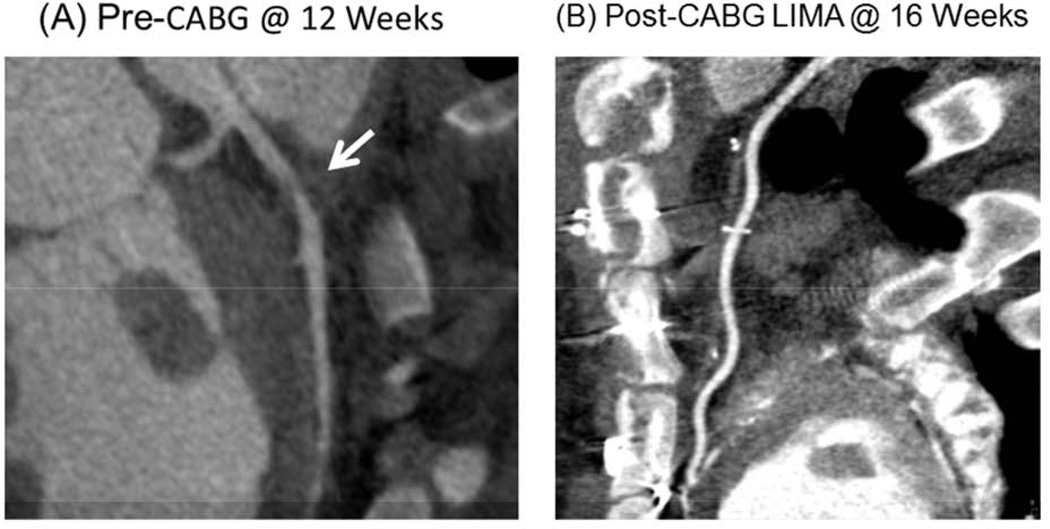
Figure 1.
(A). CT angiography was performed in all pigs 12 weeks following instrumentation and just prior to bypass surgery, to document the presence of a severe stenosis in the LAD region. (B). CT angiography was repeated at 4 weeks following CABG and just prior to the terminal study, to document the presence of a patent LIMA graft. Data are Means±SEM; *P<0.05 versus Remote Region.
Figure 2.
Despite the presence of a widely patent LIMA graft, regional myocardial function and transmural blood flows during the high dose dobutamine infusion were lower in LAD compared with remote regions. Data are Means±SEM; *P<0.05 versus Remote Region.
Table 1.
High-Energy Nucleotides from Revascularized Hibernating Myocardium in a Pig Model
| ATP | Phosphocreatine | Pcr/ATP | |
|---|---|---|---|
| LAD (Revascularized) | 4.53 ± 0.28 | 9.05 ± 0.51 | 2.01 ± 0.09 |
| Remote | 4.89 ± 0.31 | 9.47 ± 0.96 | 1.93 ± 0.11 |
Means ± SEM; Transmural ATP & Phosphocreatine (Pcr) (µmol/gram wet weight)
Mitochondrial Studies
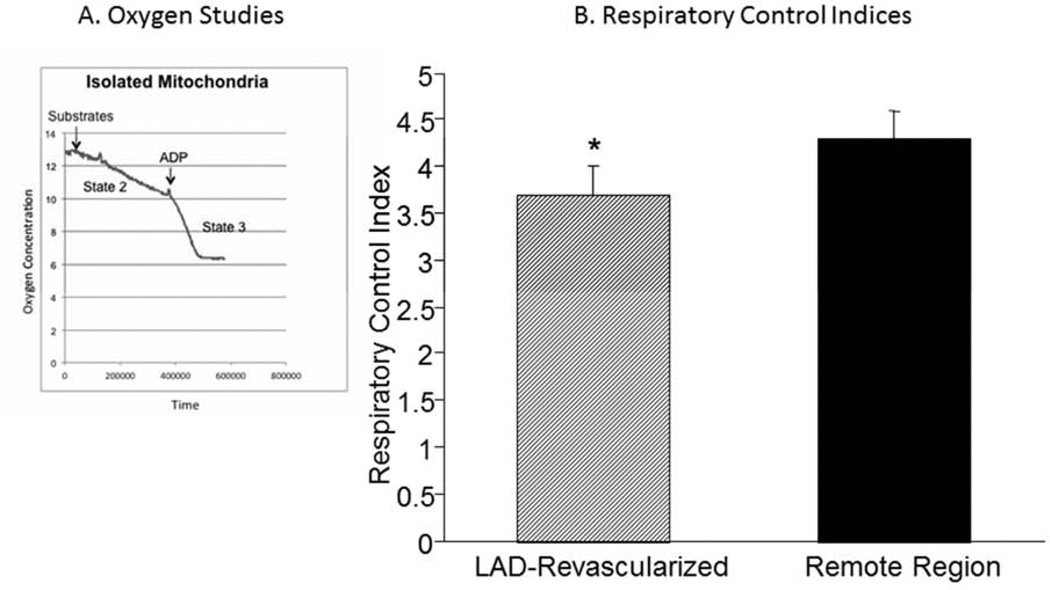
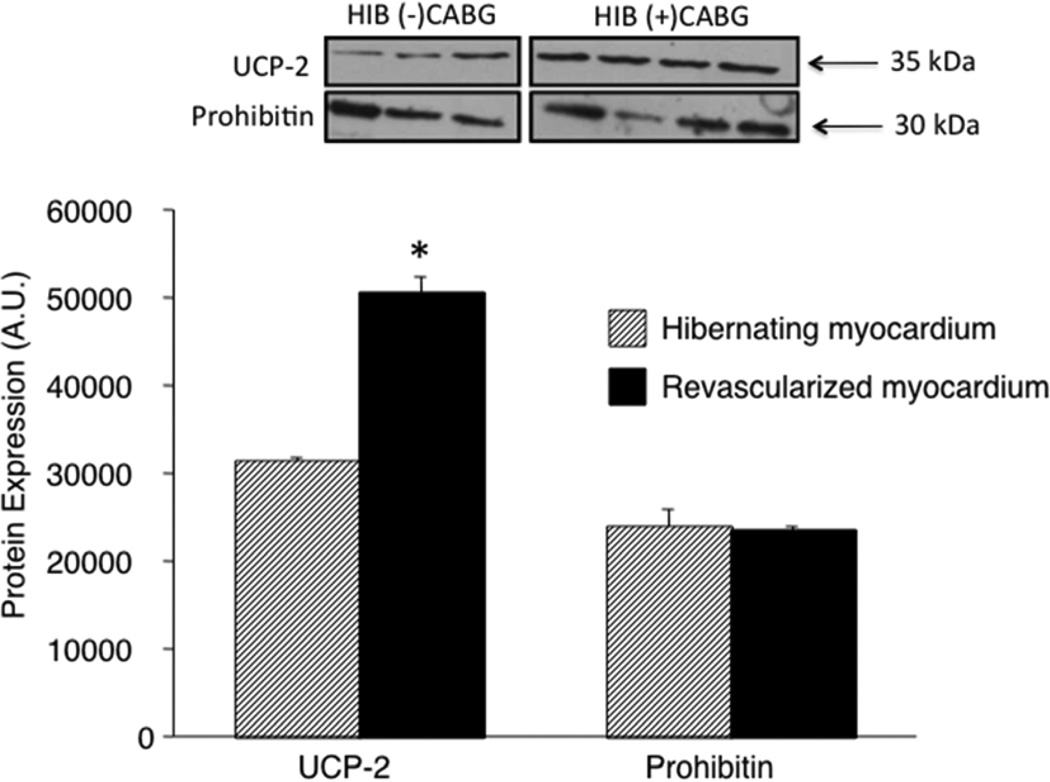
Mitochondria were isolated from each region and studied with an oxygen electrode (Figure 3A). Respiration under State 2 conditions were slightly higher in the LAD compared with remote region (Table 2) while the calculated respiration control index (RCI) (State 3/State2) was slightly lower suggesting a mild degree of uncoupling (Figure 3B). To determine whether UCP-2 was increased in the LAD region of the revascularized hibernating myocardium, we compared the content from the LAD region in revascularized animals (N=3) to those with hibernating myocardium (N=4). As shown by Figure 4, UCP-2 content, when normalized to prohibitin, remained high in the LAD region of animals that had undergone CABG.
Figure 3.
(A) Respiration of isolated mitochondria were studied in an oxygen electrode during State 2 and State 3 conditions. (B) The respiratory control index (State 3/State 2) was slightly but significantly lower in the LAD compared with the Remote region, suggesting the presence of a mild degree of uncoupling. Data are Means±SEM; *P<0.05 versus Remote Region.
Table 2.
Mitochondrial Respiration from Revascularized Hibernating Myocardium in a Pig Model
Means ± SEM;
P = 0.05 vs Remote; State 2 and 3 Respiration (nmol oxygen/min/mg of protein); Respiratory Control Index (RCI) (State 3/State 2).
Figure 4.
The abundance of uncoupling protein (UCP)-2 was determined by Western blot analysis and normalized to prohibitin in the LAD region from the revascularized hibernating pig hearts (N=4) and compared with tissue from the LAD region of hibernating pig hearts (N=3). The Data are expressed as Means±SEM. **P<0.01.
DISCUSSION
The principal finding of this study is that the expression of UCP-2, in isolated mitochondria from hibernating hearts that had undergone successful bypass surgery, remained increased, and in fact were higher than animals with hibernating myocardium who had not undergone CABG, 4 weeks following successful placement of a LIMA graft. These findings support our previous findings from a proteomic analysis of mitochondria using iTRAQ, which demonstrated that the expression of several key electron transport proteins, including complex V, had not returned to normal despite successful revascularization15. The findings are important in light of a growing awareness that revascularization with bypass surgery may not necessarily lead to complete recovery of global and regional function and may not reduce the risk of adverse cardiovascular outcomes12, 13, 17.
Mitochondrial Adaptations Within Chronic Hibernating Myocardium
A landmark observation involving patients with advanced coronary artery disease and left ventricular dysfunction showed that viable heart tissue can be maintained, despite the presence of a severe coronary artery stenosis with reduced regional blood flow and function18. Termed hibernating myocardium, the entity became well-described with the advent of dual tracers and positron emission tomography imaging, highlighting the notion that some patients may demonstrate improved regional blood flow and function at a later stage following CABG19. To test this in an animal model, we developed a swine model of hibernating myocardium, which evolves over 12 weeks and is characterized by a flow-metabolism mismatch, demonstrated via PET imaging, within viable heart tissue14, 20. During the evolution of this condition, a number of changes occur within the mitochondria including increased expression of UCP-28. Functionally, mitochondria that demonstrate a mild degree of uncoupling may be an identifier of a protective phenotype because of the observed inverse relationship between membrane potential (ΔΨm) and superoxide production20. In support of this relationship, we demonstrated UCP-2 expression from isolated mitochondria in wild type mouse hearts reduces the inner membrane potential and lowers maximal superoxide accumulation compared with mitochondria from the hearts of a UCP-2 knock-out mouse model6. At least five UCPs have been identified in mammals with UCP-1 having been most widely described in brown fat as a potential means of limiting energy production and maximizing substrate oxidation with heat production1. During the late phase of ischemic preconditioning, referred to as Second Window of Protection (SWOP), UCP-2 expression is also increased and protects against necrosis, presumably by reducing the degree of oxidant damage4. In a swine model of SWOP, we have also shown that UCP-2 is increased in isolated mitochondria from the post-ischemic heart tissue and reduces superoxide production7. Overall, a universal observation in multiple organ systems is that UCP expression reduces ROS accumulation22.
Mitochondrial Uncoupling Proteins and Heart Failure
Although increased expression of UCP-2 may function as a regulator of oxidant stress, we do not completely understand the mechanism of its regulation within hibernating heart tissue. Indeed, a limitation of our current study is that we remain unable to fully explain the role of UCP-2 in chronically ischemic heart tissue and a direct, causal relationship between UCP-2 and the changes observed in hibernating myocardium has yet to be proven. However, we have previously demonstrated a correlation between UCP-2 upregulation and chronically ischemic myocardium in swine. With the knowledge that proton leaks produced by the oxidation of metabolic substrates result in the dissipation of heat rather than ATP formation and can account for 20-25% of the basal metabolic rate22it is certainly conceivable that increased UCP-2 limits available energy for optimal contraction during high work states. With the observation that UCP expression is increased in patients with heart failure, it is possible that UCP further exacerbates the failing heart by a metabolic pathway9, 11. In a murine model of diabetic cardiomyopathy, a blunted contractile response to beta-adrenergic stimulation was observed and associated with depressed levels of phosphorylated AMPK and a markedly increased content of UCP-323. Although the potential exists that UCP could promote a metabolic cause of depressed contractile function with exacerbation of heart failure, we did not see depressed ATP levels in the LAD region compared with the remote region. Increased UCP-2 expression may also have a detrimental effect on the contractile function of hearts, independent of altered metabolic pathways. In neonatal myocytes, enhanced expression of UCP-2 caused a marked reduction in mitochondrial calcium uptake and had a deleterious effect on excitation-contraction coupling, independent of changes in total ATP level10. These data would further support the notion that UCP-2 content in heart tissue could be responsible for promoting left ventricular dysfunction by a direct effect on the contractile apparatus.
Restoring the Mitochondrial Proteome Within Hibernating Hearts
Within hibernating heart tissue, chronic myocardial ischemia leads to a reduction in the expression of several electron transport proteins including ATPase within complex V16, 24. At 4 weeks following successful revascularization, we previously showed that the expression of key proteins within the electron transport chain, as determined by iTRAQ, remains depressed in hibernating hearts15. The observation that UCP-2 fails to normalize 4 weeks following CABG, in conjunction with the mild uncoupling seen in mitochondrial respiration studies in the LAD regions, in hibernating swine hearts further demonstrates the lack of complete mitochondrial recovery. Tissue from the LAD territory in hibernating myocardium that was not revascularized was not available for an external control to offer a direct comparison of mitochondrial respiration between hibernating myocardium and revascularized hibernating myocardium. However, using remote myocardium that was not instrumented, to serve as normal, internal control tissue, was relevant for animals that had undergone two survival surgeries. The persistent upregulation of UCP-2 in revascularized hibernating swine hearts indicates ongoing mitochondrial dysfunction. Clearly additional studies are needed, to determine whether more time is needed to induce mitochondrial biogenesis and improve mitochondrial protein expression, and/or reduce the expression of UCP-2, which might facilitate maximal oxygen consumption during high work states.
Conclusions
In summary, we have shown that revascularization of hibernating hearts does not lead to normalization of regional blood flow and function during a high dose catecholamine challenge at 4 weeks following placement of a LIMA graft. Future studies should address whether a longer time for recovery and/or additional interventions are needed, to restore the mitochondrial proteome and return expression of UCP-2 to normal.
Acknowledgments
This work was supported in part by NIH (EM), and VA Merit Review Grants (RK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: All authors contributed to animal surgeries, physiology studies, tissue analysis, and biochemical experiments. Holley, Long, McFalls and Kelly all contributed to the writing and editing of this manuscript. McFalls and Kelly were responsible for study design.
Presented at the 9th Annual Academic Surgical Congress in San Diego, CA, February 4-6, 2014
REFERENCES
- 1.Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, SIUCP and AtUCP. Biochem J. 2000;345:161–179. [PMC free article] [PubMed] [Google Scholar]
- 2.Echtay K, Roussel D, St-Pieere J, Jekabsons M, Cardenas S, Stuart J, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 3.Teshima Y, Akao M, Jones S, Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res. 2003;93:192–200. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- 4.McLeod C, Aziz A, Hoyt R, McCoy P, Sack M. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J Biol Chem. 2005;280:33470–33476. doi: 10.1074/jbc.M505258200. [DOI] [PubMed] [Google Scholar]
- 5.Andrews Z, Horvath T. Uncoupling protein-2 regulates lifespan in mice. Am J Physiol. 2009;296:E621–E627. doi: 10.1152/ajpendo.90903.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrera JA, Ziemba EA, Colbert R, Kelly RF, Kuskowski M, Arriaga EA, et al. Uncoupling protein-2 expression and effects on mitochondrial membrane potential and oxidant stress in heart tissue. Translational research : the journal of laboratory and clinical medicine. 2012 May;159(5):383–390. doi: 10.1016/j.trsl.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabrera J, Ziemba E, Colbert R, Anderson L, Sluiter W, Duncker D, et al. Altered expression of mitochondrial electron transport proteins and improved myocardial energetic state during late ischemic preconditioning. Am J Physiol. 2012;302:H1974–H1982. doi: 10.1152/ajpheart.00372.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFalls E, Sluiter W, Schoonderwoerd K, Manintveld O, Lamers J, Bezstarosti B, et al. Mitochondrial adaptations within chronically ischemic swine myocardium. J Mol Cell Cardiol. 2006;41:980–988. doi: 10.1016/j.yjmcc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Murray A, Anderson R, Watson G, Radda G, Clarke K. Uncoupling proteins in human heart. Lancet. 2004;364:1786–1788. doi: 10.1016/S0140-6736(04)17402-3. [DOI] [PubMed] [Google Scholar]
- 10.Turner J, Gaspers L, Wang G, Thomas A. Uncoupling protein-2 modulates myocardial excitation-contraction coupling. Circ Res. 2010;106:730–738. doi: 10.1161/CIRCRESAHA.109.206631. [DOI] [PubMed] [Google Scholar]
- 11.Murray A, Cole M, CA L, Carr C, Stuckey D, Little S, et al. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol. 2008;44:694–700. doi: 10.1016/j.yjmcc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 12.McFalls E, Baldwin D, Kuskowsk M, Liow J, Chesler E, Ward H. Utility of positron emission tomography in predicting improved left ventricular ejection fraction after coronary artery bypss grafting among patients with ischemic cardiomyopathy. Cardiology. 2000;93:105–112. doi: 10.1159/000007010. [DOI] [PubMed] [Google Scholar]
- 13.Vanoverschelde J, Depre C, Gerber B, Borgers M, Wijns W, Robert A, et al. Time course of functional recovery after CABG surgery in patients with chronic left ventricular ischemic dysfunction. Am J Cardiol. 2000;85:1432–1439. doi: 10.1016/s0002-9149(00)00790-6. [DOI] [PubMed] [Google Scholar]
- 14.McFalls EO, Baldwin D, Palmer B, Marx D, Jaimes D, Ward HB. Regional glucose uptake within hypoperfused swine myocardium as measured by positron emission tomography. Am J Physiol. 1997 Jan;272(1 Pt 2):H343–H349. doi: 10.1152/ajpheart.1997.272.1.H343. [DOI] [PubMed] [Google Scholar]
- 15.Kelly R, Cabrera J, Ziemba E, Crampton M, Anderson L, McFalls E, et al. Continued depression of maximal oxygen consumption and mitochondrial proteomic expression despite successful coronary artery bypasss grafting in a swine model of hibernation. J Thorac Cardiovasc Surg. 2011;141:261–268. doi: 10.1016/j.jtcvs.2010.08.061. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera JA, Butterick TA, Long EK, Ziemba EA, Anderson LB, Duffy CM, et al. Reduced expression of mitochondrial electron transport chain proteins from hibernating hearts relative to ischemic preconditioned hearts in the second window of protection. J Mol Cell Cardiol. 2013 Jul;60:90–96. doi: 10.1016/j.yjmcc.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Velazquez E, Lee K, Deja M, Jain A, Sopko G, Marchenko A, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. New Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimtoola S. The hibernating myocardium. Am Heart J. 1989;117:211–221. doi: 10.1016/0002-8703(89)90685-6. [DOI] [PubMed] [Google Scholar]
- 19.Tillisch J, Brunken R, Marshall R, Schwaiger M, Mandelkern M, Phelps M, et al. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. New Engl J Med. 1986;314:884–888. doi: 10.1056/NEJM198604033141405. [DOI] [PubMed] [Google Scholar]
- 20.McFalls E, Kelly R, Hu Q, Mansoor A, Lee J, Kuskowski M, et al. The energetic state within hibernating myocardium is normal during dobutamine despite inhibition of ATP dependent potassium channel opening with glibenclamide. Am J Physiol. 2007;293:H2945–H2951. doi: 10.1152/ajpheart.00012.2007. [DOI] [PubMed] [Google Scholar]
- 21.Korshunov S, Skulachev V, Starkov A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Letters. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 22.Echtay K. Mitochondrial uncoupling proteins - What is their physiological role? Free Radic Biol Med. 2007;43:1351–1371. doi: 10.1016/j.freeradbiomed.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Daniels A, van Bilsen M, Janssen B, Brouns A, Cleutjens J, Roemen T, et al. Impaired cardiac functional reserve in type 2 diabetic db/db mice is associated with metabolic, but not structural, remodelling. Acta Physiol. 2010;200:11–22. doi: 10.1111/j.1748-1716.2010.02102.x. [DOI] [PubMed] [Google Scholar]
- 24.Page B, Young R, Iyer V, Suzuki G, Lis M, Lioubov K, et al. Persistent regional downregulation in mitochondrial enzymes and upregulation of stress proteins in swine with chronic hibernating myocardium. Circ Res. 2008;102:1–11. doi: 10.1161/CIRCRESAHA.107.155895. [DOI] [PubMed] [Google Scholar]