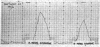Abstract
Dopamine-β-hydroxylase activity is present in mouse neuroblastoma C-1300 tumors. The activity is proportional to the weight of the tumor. Serum activity is markedly increased in mice that bear the tumors. Treatment of mice with 5-bromodeoxyuridine causes marked inhibition of tumor growth and decrease of dopamine-β-hydroxylase activity in the serum. The histochemical studies reveal that 1-5% of the cells in mouse C-1300 neuroblastoma tumors contain catecholamines and that catecholamine-containing processes terminate mainly around blood vessels of the tumor. Dopamine-β-hydroxylase is present in clonal neuroblastoma cell lines. The cell line with the greater tendency to form axon-like processes has a higher activity of this enzyme.
Keywords: 5-bromodeoxyuridine, catecholamines, norepinephrine
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSH I. E. Methods of paper chromatography of steroids applicable to the study of steroids in mammalian blood and tissues. Biochem J. 1952 Jan;50(3):370–378. doi: 10.1042/bj0500370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J Cell Biol. 1970 Jan;44(1):134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. S., Ohuchi T., Goldstein M., Axelrod F., Fish I., Dancis J. Changes in human serum dopamine- -hydroxylase activity with age. Nature. 1972 Apr 7;236(5345):310–311. doi: 10.1038/236310a0. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN M., FRIEDHOFF A. J., POMERANTZ S., CONTRERA J. F. The formation of 3, 4-dihydroxyphenylethanol and 3-methoxy-4-hydroxyphenylethanol from 3, 4-dihydroxy-phenylethylamine in the rat. J Biol Chem. 1961 Jun;236:1816–1821. [PubMed] [Google Scholar]
- Goldstein M., Freedman L. S., Bohuon A. C., Guerinot F. Serum dopamine-beta-hydroxylase activity in neuroblastoma. N Engl J Med. 1972 May 25;286(21):1123–1125. doi: 10.1056/NEJM197205252862102. [DOI] [PubMed] [Google Scholar]
- Goldstein M., Freedman L. S., Bonnay M. An assay for dopamine-beta-hydroxylase activity in tissues and serum. Experientia. 1971 Jun;27(6):632–633. doi: 10.1007/BF02136929. [DOI] [PubMed] [Google Scholar]
- KIRSHNER N. Pathway of noradrenaline formation from DOPA. J Biol Chem. 1957 Jun;226(2):821–825. [PubMed] [Google Scholar]
- Molinoff P. B., Weinshilboum R., Axelrod J. A sensitive enzymatic assay for dopamine- -hydroxylase. J Pharmacol Exp Ther. 1971 Sep;178(3):425–431. [PubMed] [Google Scholar]
- POTTER L. T., AXELROD J. SUBCELLULAR LOCALIZATION OF CATECHOLAMINES IN TISSUES OF THE RAT. J Pharmacol Exp Ther. 1963 Dec;142:291–298. [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Jacob F. 5-bromodeoxyuridine-induced differentiation of a neuroblastoma. Proc Natl Acad Sci U S A. 1970 Sep;67(1):247–254. doi: 10.1073/pnas.67.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum R., Axelrod J. Serum dopamine- -hydroxylase: decrease after chemical sympathectomy. Science. 1971 Sep 3;173(4000):931–934. doi: 10.1126/science.173.4000.931. [DOI] [PubMed] [Google Scholar]