Abstract
Purpose
Although the Parks-Bielschowsky three-step test is the cornerstone of cyclovertical strabismus diagnosis, it has not been validated against an external benchmark. We evaluated the test’s sensitivity in clinical diagnosis of superior oblique palsy in patients with unequivocal magnetic resonance imaging (MRI) evidence of superior oblique atrophy.
Methods
A total of 73 strabismic patients were selected from a prospective MRI study because they exhibited superior oblique atrophy indicative of superior oblique denervation and thus confirmatory of superior oblique palsy. Of these, 50 patients who had no confounding factors were included for detailed study. Ocular motility data were evaluated to determine sensitivity of single and combined clinical findings in diagnosis of superior oblique palsy.
Results
Maximum mean ipsilesional superior oblique cross section was reduced to 9.6 ± 0.6 mm2 (mean ± standard error) in superior oblique palsy, representing 52% of the 18.5 ± 0.6 mm2 contralesional superior oblique maximum cross section and 52% of the 18.4 ± 0.4 mm2 control maximum superior oblique cross section (P < 0.001). Of the 50 patients, 35 (70%) with superior oblique atrophy fulfilled the entire three-step test. In 14 (28%) patients two steps were fulfilled; in 1 patient (2%), only one step. Affected superior oblique cross section was similar in orbits that fulfilled the three-step test (9.8 ± 0.9 mm2) and those that did not (9.1 ± 0.7 mm2; P = 0.58).
Conclusions
The complete three-step test fails to detect 30% of cases of superior oblique atrophy. Often only two of three steps are positive in superior oblique palsy.
The Parks-Bielschowsky three-step test, originally applied to the setting of isolated cyclovertical extraocular muscle paresis, is the cornerstone of cyclovertical strabismus, especially for diagnosis of superior oblique palsy.1 The clinical signature of unilateral superior oblique palsy includes: (1) ipsilesional central gaze hypertropia, (2) greater in contralesional than ipsilesional gaze, (3) and greater in ipsilesional than contralesional head tilt.2–4 The three-step test presumes that there is unopposed activity of the palsied superior oblique muscle’s antagonist, the inferior oblique, that increases hypertropia in contralateral gaze.5 The head tilt phenomenon is supposed to result from deficit of the palsied superior oblique’s incycloduction during ocular counterrolling (OCR).3 Deficient incycloduction is theorized to be replaced by the ipsilateral superior rectus, but at cost of hypertropia during ipsilateral head tilt.6 Based on these assumptions, when all three steps are positive, many clinicians diagnose superior oblique weakness, notwithstanding highly individual hypertropia incomitance attributed to secondary changes, including so-called inferior oblique overaction and superior rectus contracture.7,8
Recent advances in magnetic resonance imaging (MRI) have enabled direct study of the functional anatomy of the superior oblique belly. In superior oblique palsy, superior oblique contractility is well correlated with maximum cross-sectional area in central gaze.9 MRI in monkeys with experimental intracranial trochlear neurectomy readily demonstrates superior oblique atrophy.9–11 Therefore, it seems reasonable to regard superior oblique atrophy as a sufficient objective confirmation of superior oblique palsy; atrophy is both signature of trochlear denervation and a biologically plausible correlate of weakness. Although such a stringent criterion might miss some cases of superior oblique weakness, it is seems certain that an atrophic superior oblique could not generate normal oculorotary force.
Recent evidence suggests that the three-step test’s mechanism is not fully understood. Kono and colleauges6 demonstrated absence of relationship between superior oblique size and head tilt–dependent hypertropia in superior oblique palsy. MRI shows that the ipsilesional inferior oblique muscle does not exhibit hypertrophy or supranormal contractility in superior oblique palsy, as implied by the term inferior oblique overaction.11 Kushner has emphasized that multiple etiologies can mimic superior oblique palsy in the three-step test and lead to misattribution of the cause of hypertropia.12 Muscle pulley heterotopy,13 superior oblique tendon anomalies,14 and skew deviation15 can all yield a positive result; these “masqueraders” fulfill the three-step test without superior oblique atrophy.16
No prior studies have validated three-step test sensitivity against any other indicator of superior oblique function. Were the test ideally sensitive, all patients with radiographic evidence of superior oblique palsy should exhibit positive three-step test. This study aimed to evaluate the three-step test sensitivity in diagnosis of superior oblique palsy and to determine whether particular motility findings improve sensitivity in diagnosing superior oblique palsy.
Subjects and Methods
Patients provided written informed consent for participation according to a protocol approved by the University of California Institutional Review Board and conforming to requirements of the US Health Insurance Portability and Accountability Act of 1996. Strabismic patients who exhibited superior oblique atrophy on coronal plane MRI during a prospective study of 1999 to 2012 were included. No attempt was made to distinguish acquired atrophy from other causes of superior oblique hypoplasia, because it was reasoned that any cause of deficient superior oblique size would be indicative of deficient function. Versions, torsion measured by double Maddox rods, and three-step test were performed by the senior author (JLD) before MRI scans. Patients were excluded for prior strabismus surgery, additional causes of strabismus, or incomplete data. Heterotropia was measured using prism-cover at distance. Versions were graded from 0 to ± 4. To maximize sensitivity, a “step” was liberally defined if the difference in hypertropia between ipsilesional and contralesional gaze or head tilt was ≥1Δ. Control subjects were recruited by advertising and underwent full examinations to verify normal corrected vision and motility and Titmus stereopsis of 40 arcsec. MRI was identical in controls and patients.
High-resolution, T1- or T2-weighted fast spin-echo, surface coil MRI was performed at 1.5-T (Signa; GE Healthcare; Milwaukee, WI), as described elsewhere.11,17 Eighteen to 20 contiguous, quasicoronal planes were obtained in 2 mm slices using a 256 × 256 matrix over an 8 cm field of view. Each monocularly imaged eye fixated for approximately 2.5 minutes on a small afocal target 2 cm distant in central gaze.
MRI images were quantified before clinical data review by an investigator masked to history and laterality of strabismus using ImageJ 1.46R (Wayne Rasband, National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/). The superior oblique muscle was digitally outlined in MRI images, and the maximum cross-sectional area was automatically computed (Figure 1) for comparison by paired and unpaired t tests. Versions were analyzed using the Wilcoxon rank sum test. Sensitivity was analyzed using the χ2 test.
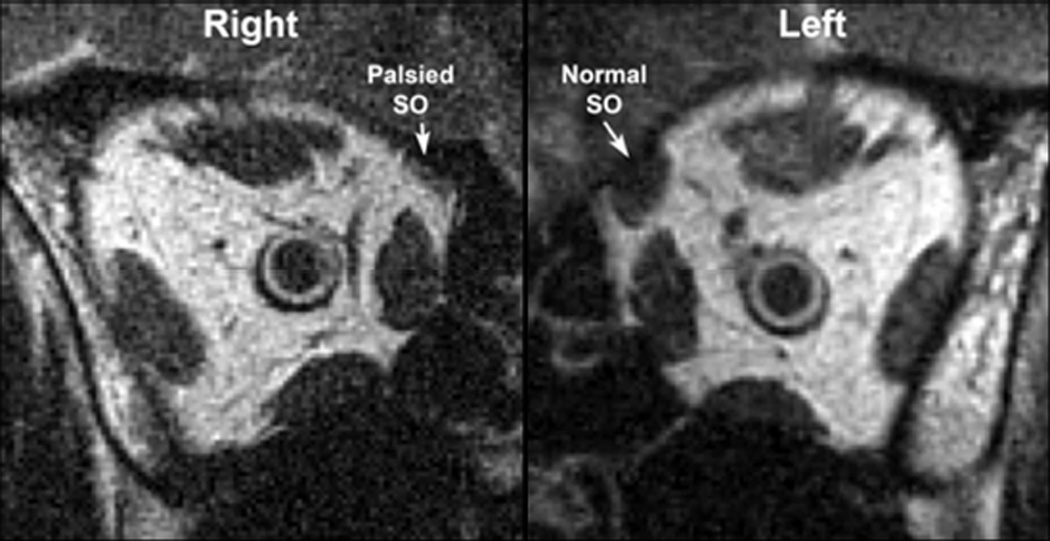
FIG. 1.
MRI of both orbits of patient demonstrating atrophied right and normal left superior oblique muscles.
Results
Of 577 enrolled strabismic patients, 73 exhibited superior oblique atrophy on MRI. Of these, 23 were excluded, leaving 50 for analysis (47 males; mean age, 37.6 [range, 5-83 years]). Mean data on included patients are provided in Table 1, with individual data in e-Supplement 1 (available at jaapos.org). Etiologies of superior oblique palsy included congenital, iatrogenic, traumatic, cerebrovascular, and idiopathic. From 157 normal participants, 40 (80 orbits) age-matched controls were selected.
Table 1.
Superior oblique muscle cross sections in superior oblique palsy
| Ipsilesional maximum superior oblique cross section, mm2 |
Contralesional maximum superior oblique cross section, mm2 |
Age, years | Duration, years | |
|---|---|---|---|---|
| Patients not fulfilling complete three-step test | ||||
| Mean | 9.1 | 18.0 | 41.4 | 4.3 |
| SEM | 0.9 | 1.0 | 4.0 | 1.6 |
| Patients fulfilling complete three-step test | ||||
| Mean | 9.8 | 18.8 | 36.0 | 8.0 |
| SEM | 0.7 | 0.8 | 3.2 | 1.5 |
| P value | 0.58 | 0.61 | 0.34 | 0.15 |
SEM, standard error of the mean.
Superior Oblique Cross-Sectional Area
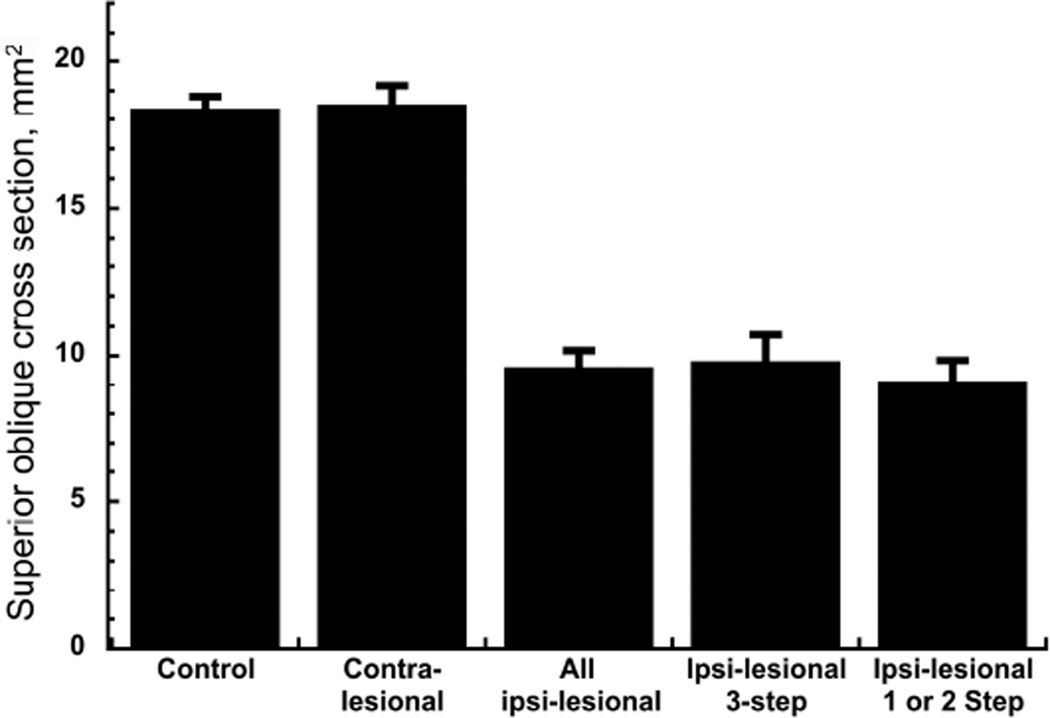
Atrophy of palsied superior oblique muscles was conspicuous on MRI (Figure 1). Maximum mean ipsilesional superior oblique cross-section was reduced to 9.6 ± 0.6 mm2, representing 52% of 18.5 ± 0.6 mm2 contralesional superior oblique cross section, and 52% of the 18.4 ± 0.4 mm2 normal superior oblique cross section (P < 0.001; Figure 2). Mean ipsilesional superior oblique cross section was similar in patients fulfilling the three-step test (9.8 ± 0.9 mm2) and those fulfilling only one or two steps (9.1 ± 0.7 mm2; P = 0.58).
FIG. 2.
Superior oblique maximum cross-sectional areas. Error bars indicate standard errors. Contralesional superior oblique cross-sectional areas did not differ significantly from control. All palsied superior oblique muscles exhibited highly significant reduction in maximum cross section (P < 0.001) irrespective of the number of positive step of the three-step test.
Clinical Findings
The entire three-step test was fulfilled by 35 patients (70%) with superior oblique atrophy. Mean motility data are summarized in Table 2, with individual patient data supplied in e-Supplement 2 (available at jaapos.org). Patients fulfilling the three-step criteria had 8.7Δ ± 2.3Δ (mean ± standard error) ipsilesional central hypertropia, similar to those who did not (12.8Δ ± 1.6Δ; P = 0.16). As expected, contralesional gaze hypertropia was significantly greater in patients fulfilling all three steps than in those who did not (22.0Δ ± 2.2Δ vs 8.7Δ ± 1.9Δ; P < 0.05); ipsilesional head tilt hypertropia was also greater in those fulfilling all three steps (22.4Δ ± 2.5Δ vs 13.1Δ ± 2.8Δ; P < 0.05).
Table 2.
Versions and strabismus in superior oblique palsy
| Excyclotorsion, degrees |
Elevation in adduction | Central hypertropia, PD |
Lateral gaze hypertropia, PD |
Head tilt hypertropia, PD |
||||
|---|---|---|---|---|---|---|---|---|
| Supraduction Infraduction | Ipsilateral | Contralateral | Ipsilateral | Contralateral | ||||
| Patients not fulfilling the complete three-step test | ||||||||
| Mean | 7.8 | 0.3 | −1.4 | 8.7 | 8.7 | 8.7 | 13.1 | 5.1 |
| SEM | 1.2 | 0.5 | 0.3 | 2.3 | 2.4 | 1.9 | 2.8 | 1.9 |
| Patients fulfilling the complete three-step test | ||||||||
| Mean | 7.9 | 1.9 | −1.7 | 12.8 | 6.1 | 22.0 | 22.4 | 3.3 |
| SEM | 1.0 | 0.2 | 0.2 | 1.6 | 1.5 | 2.2 | 2.5 | 0.8 |
| P value | 0.96 | <0.05 | 0.44 | 0.16 | 0.33 | <0.05 | <0.05 | 0.30 |
PD, prism diopter; SEM, standard error of the mean.
Age did not differ significantly between patients with superior oblique atrophy fulfilling and those not fulfilling the three-step test (36.0 ± 3.3 vs 41.4 ± 4.0 years; P = 0.34). Likewise, excyclotorsion did not differ between the two cohorts (7.9° ± 1.0° vs 7.8° ± 1.2°; P = 0.96). Infraduction in adduction was similar between cohorts (−1.7 ± 0.2 vs −1.4 ± 0.3; P = 0.44). However, patients fulfilling the three-step test had significantly more supraduction in adduction than those who did not (1.9 ± 0.2 vs 0.3 ± 0.5; P < 0.01) and had an insignificant trend toward longer symptom duration than those who did not (8.0 ± 1.5 vs 4.3 ± 1.6 years; P = 0.15).
Sensitivity of Test Components
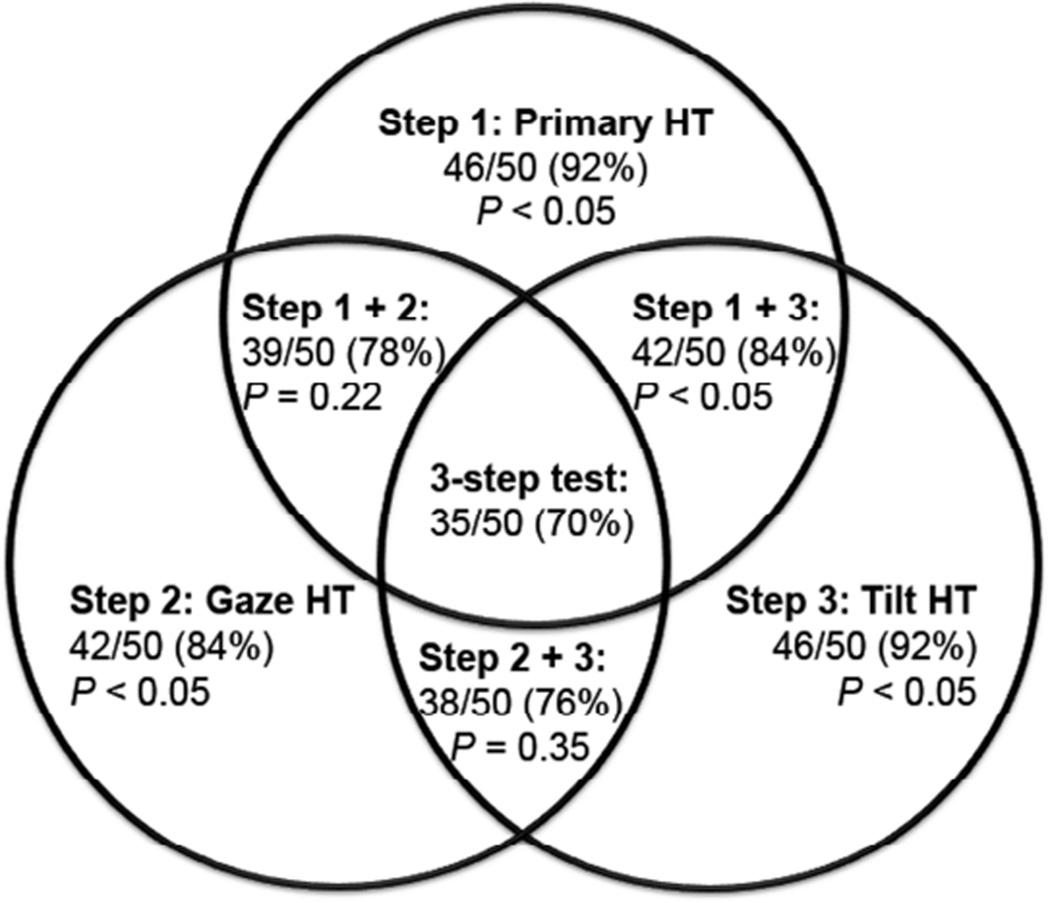
At least one step of the three-step test was fulfilled in all patients. Only two steps were fulfilled in 14 patients (28%), and only one step in 1 patient (2%). Figure 3 summarizes the sensitivity of individual and combined steps. Although acceptance of any step would obviously be more sensitive than would requirement of all three steps (Figure 3; P < 0.05), a single step would reduce specificity.
FIG. 3.
Venn diagram illustrating sensitivities of combinations of individual test steps in diagnosis of superior oblique palsy. Any one step individually is more sensitive than the three-step test (P < 0.05). The combination of steps 1 and 3 is more sensitive than the three-step test (P < 0.05), but no other combination of steps had sensitivity exceeding the complete three-step test. HT, hypertropia.
Ipsilesional central hypertropia (step 1) was present in 46 of 50 patients (92%) with superior oblique palsy. The 4 patients (patients 1, 9, 10, 11) who did not fulfill step 1 were similar in age (39.0 ± 3.3 years; P = 0.32) to patients who fulfilled all steps. Three of these (patients 1, 9, 10) had normal supraduction in adduction, and all had excyclotorsion (5.8° ± 2.5°; P = 0.48) similar to patients fulfilling all 3 steps. There was an insignificant trend for the 4 patients not fulfilling step 1 to have shorter symptom duration than those fulfilling all steps (1.2 ± 0.3 vs 8.0 ± 1.5 years; P = 0.13).
In 42 patients (84%) contralesional gaze hypertropia exceeded ipsilesional gaze hypertropia (step 2). The 8 (patients 1-8) not fulfilling step 2 had the same age (40.1 ± 7.3 years; P = 0.60) and symptom duration (7.6 ± 2.4 years; P = 0.92) as patients fulfilling all steps. Supraduction on adduction in patients not fulfilling step 2 ranged from −3 to +2; excyclotorsion (7.2° ± 1.8°; P = 0.77) was similar to those fulfilling the complete three-step test.
In 46 patients (92%) ipsilesional exceeded contralesional head tilt hypertropia (step 3). The 4 (patients 12-15) not fulfilling step 3 did not differ significantly in age (46.8 ± 1.8 years; P = 0.28) or excyclotorsion (7.5° ± 3.8°; P = 0.91) from patients fulfilling the complete three-step test. Supraduction in adduction ranged from −2 to +4. Similar to step 1, there was an insignificant trend for the 4 patients not fulfilling step 3 to have shorter symptom duration (1.7 ± 0.9 years; P = 0.15) than those fulfilling all steps.
Steps 1 and 2 were fulfilled in 39 patients (78%). This two-step criterion was not statistically more sensitive than the complete three-step test (P = 0.22). Steps 1 and 3 were fulfilled in 42 patients (84%), which was more sensitive than the three-step test (P < 0.05). Finally, steps 2 and 3 were fulfilled in 38 patients (76%), not statistically more sensitive than the three-step test (P = 0.35). Etiology was not correlated with any particular steps (e-Supplement 1, available at jaapos.org).
At least two of the three steps were fulfilled in 49 patients (98%), but this may reduce specificity. The single patient not fulfilling at least two steps (patient 1) had a significantly smaller ipsilesional (8.4 mm2) than contralesional superior oblique cross section (19.1 mm2) yet only 3Δ hyperphoria on ipsilesional head tilt (and orthotropia in all other gazes).
Discussion
This study included 50 patients exhibiting grossly evident unilateral superior oblique atrophy on MRI, including various etiologies and durations of superior oblique palsy. The three-step test was negative in 30% of cases. Patient age, maximum superior oblique cross section, and palsy etiology did not differ significantly with respect to fulfillment of the three-step test. Patients with superior oblique palsy not fulfilling the complete three-step test had significantly less elevation in adduction (often loosely termed inferior oblique overaction) than those fulfilling the three-step test. Less overelevation in adduction may further reduce likelihood of superior oblique palsy diagnosis by clinicians anticipating that inferior oblique overaction is the usual consequence of superior oblique weakness.
This study included only older children and adults with superior oblique atrophy cooperative for motility examinations; although exclusion of very young children may limit our conclusions to this group, it is doubtful that three-step test sensitivity would be greater in toddlers.
Why do only 70% of patients with superior oblique atrophy fulfill the three-step test? Biomechanical simulation demonstrates that superior oblique weakness alone does not explain the large hypertropia.18 In monkeys, superior oblique palsy does not immediately create large hypertropia; this typical clinical sign emerges only later.19 Strabismus associated with superior oblique palsy is not simply due to superior oblique weakness, or even due to changes in the antagonist inferior oblique; it is influenced by additional mechanical and innervational changes.
Kono and colleagues11 reported that in superior oblique palsy, the ipsilesional inferior oblique was not hypertrophic and did not demonstrate supranormal contractility. Size and contractility of the contralesional inferior rectus increase in superior oblique palsy.20 When it occurs, contralesional superior rectus hypertrophy may be a compensatory mechanism in response to vertical misalignment.21 Superior oblique palsy is also accompanied by shifts in the medial rectus, lateral rectus, and inferior rectus pulleys.22,23 Finally, there is MRI evidence that differential compartmental contraction in the lateral rectus and medial rectus muscles elicits cyclovertical actions that might modulate alignment in superior oblique palsy.24,25
We defined a positive “step” as a difference between ipsilesional and contralesional gaze or head tilt of ≥1Δ. This liberal threshold maximized sensitivity, although perhaps not clinically practical. A higher hypertropia difference threshold would have reduced the number of patients fulfilling the complete three-step test, thus reducing sensitivity.
Might any components of the three-step test be more sensitive than others? The combination of ipsilesional central hypertropia (step 1) and ipsilesional exceeding contralesional head tilt hypertropia (step 3) was more sensitive than the complete three-step test. Using only these two steps missed 8 patients (16%) with superior oblique palsy. However, relaxing diagnostic rigor to increase sensitivity may make an already poorly specific test even less specific.16 For instance, a recent study showed that the three-step test was only about 50% specific, with 10 of 22 patients fulfilling the three-step test lacking superior oblique atrophy on MRI.22 The present study challenges the sensitivity of the three-step test, which missed 30% of cases with demonstrated superior oblique atrophy.
High-resolution MRI readily demonstrates superior oblique contractility in normal subjects as well as superior oblique atrophy and decreased contractility in superior oblique palsy.9 Superior oblique cross-sectional area correlates with contractility.11 It seems reasonable to interpret significantly decreased superior oblique cross-sectional area on MRI as objective confirmation of superior oblique palsy. Our finding that ipsilesional superior oblique cross section in presumed superior oblique palsy was 52% of the contralesional and control superior oblique muscles is consistent with previous studies of superior oblique palsy.6,11,21,22,26
The present study demonstrates that the three-step test is not particularly sensitive to superior oblique palsy. Earlier studies have demonstrated that the three-step test is not highly specific to superior oblique palsy either. Kono and colleagues6 demonstrated absence of relationship between superior oblique size and the Bielschowsky head tilt phenomenon in clinically diagnosed superior oblique palsy. In another study by our group, only about half the subjects with a positive three-step test exhibited abnormal superior oblique size, suggesting that the three-step test may have only about 50% specificity.27 In fact, no clinical features of any kind provided useful clues to the presence of superior oblique atrophy in patients with hypertropia that depended upon head tilt.27 Taken together with the present study, the cumulative evidence argues that it may not be possible to reliably diagnose superior oblique palsy on clinical grounds.
Because diplopia may be the first symptom of neurological disease, and because superior oblique palsy treatment is often surgical,4 diagnostic confirmation can be important. The extensive differential diagnosis of alternative causes of incomitant hypertropia necessitates a sensitive and specific test for superior oblique palsy diagnosis. In the setting of incomitant hypertropia with a negative three-step test, clinicians may entertain diagnoses other than superior oblique palsy and may miss up to 30% of cases. If the situation requires etiologic diagnosis of hypertropia, imaging may be helpful to evaluate superior oblique size. If clinical considerations do not warrant imaging, use of only steps 1 and 3 would increase sensitivity to 84%, albeit with unknown effect on specificity.
Supplementary Material
Acknowledgments
This study was supported by U.S. Public Health Service, National Eye Institute Grant EY08313. The senior author is also supported by an unrestricted from Research to Prevent Blindness (New York, NY). A. Manchandia is Leonard Apt and Klara Spinks Fleming Fellow. J. Demer holds the Leonard Apt Endowed Chair of Pediatric Ophthalmology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 39th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, Boston, Massachusetts, April 3-7, 2013.
FDA disclosure: investigational surface coils were used in MRI imaging.
References
- 1.Plager DA. Superior oblique palsy and superior oblique myokymia. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management Principles and Surgical Techniques. Philadelphia, PA: W.B. Saunders Company; 1999. pp. 222–223. [Google Scholar]
- 2.Bielschowsky A. Lectures on motor anomalies. XI. Etiology, prognosis, and treatment of ocular paralysis. Am J Ophthalmol. 1939;22:723–734. [Google Scholar]
- 3.Parks MM. Isolated cyclovertical muscle palsy. AMA Arch Ophthalmol. 1958;60:1027–1035. doi: 10.1001/archopht.1958.00940081047008. [DOI] [PubMed] [Google Scholar]
- 4.von Noorden GK, Murray E, Wong SY. Superior oblique paralysis: a review of 270 cases. Arch Ophthalmol. 1986;104:1771–1776. doi: 10.1001/archopht.1986.01050240045037. [DOI] [PubMed] [Google Scholar]
- 5.Scott WE, Kraft SP. Classification and surgical treatment of superior oblique palsies: I. Unilateral superior oblique palsies. Trans New Orleans Acad Ophthalmol. 1986;34:15–38. [PubMed] [Google Scholar]
- 6.Kono R, Okanobu H, Ohtsuki H, Demer JL. Absence of relationship between oblique muscle size and Bielschowsky head tilt phenomenon in clinically diagnosed superior oblique palsy. Invest Ophthalmol Vis Sci. 2009;50:175–179. doi: 10.1167/iovs.08-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khawam E, Scott AB, Jampolsky A. Acquired superior oblique palsy: diagnosis and management. Arch Ophthalmol. 1967;77:761–768. doi: 10.1001/archopht.1967.00980020763009. [DOI] [PubMed] [Google Scholar]
- 8.Knapp P. Classification and treatment of superior oblique palsy. Am Orthopt J. 1974;24:18–22. [PubMed] [Google Scholar]
- 9.Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36:906–913. [PubMed] [Google Scholar]
- 10.Demer JL, Poukens V, Ying H, et al. Effects of intracranial trochlear neurectomy on the structure of the primate superior oblique muscle. Invest Ophthalmol Vis Sci. 2010;51:3485–3493. doi: 10.1167/iovs.09-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kono R, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmology. 2003;110:1219–1229. doi: 10.1016/S0161-6420(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 12.Kushner BJ. Errors in the three-step test in the diagnosis of vertical strabismus. Ophthalmology. 1989;96:127–132. doi: 10.1016/s0161-6420(89)32933-2. [DOI] [PubMed] [Google Scholar]
- 13.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 14.Sato M, Iwata EA, Takai Y, Hikoya A, Koide YM. Superior oblique palsy with class III tendon anomaly. Am J Ophthalmol. 2008;146:385–394. doi: 10.1016/j.ajo.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky MC, Donahue SP, Vaphiades M, Brandt T. Skew deviation revisited. Surv Ophthalmol. 2006;51:105–128. doi: 10.1016/j.survophthal.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Demer J, Miller MJ, Koo EYE, Rosenbuam AL, Bateman JB. True versus masqureading superior oblique palsies: muscle muchanisms revealed by magnetic resonance imaging. In: Lennerstrand G, editor. Update on Strabismus and Pediatric Ophthalmology. Boca Raton, FL: CRC Press; 1995. [Google Scholar]
- 17.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 18.Miller JM, Robinson DA. A model of the mechanics of binocular alignment. Comput Biomed Res. 1984;17:436–470. doi: 10.1016/0010-4809(84)90012-0. [DOI] [PubMed] [Google Scholar]
- 19.Quaia C, Shan X, Tian J, et al. Acute superior oblique palsy in the monkey: effects of viewing conditions on ocular alignment and modelling of the ocular motor plant. Prog Brain Res. 2008;171:47–52. doi: 10.1016/S0079-6123(08)00607-9. [DOI] [PubMed] [Google Scholar]
- 20.Jiang L, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior rectus muscle in superior oblique muscle palsy. Ophthalmology. 2008;115:2079–2086. doi: 10.1016/j.ophtha.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark RA, Demer JL. Enhanced vertical rectus contractility by magnetic resonance imaging in superior oblique palsy. Arch Ophthalmol. 2011;129:904–908. doi: 10.1001/archophthalmol.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demer JL, Kung J, Clark RA. Functional imaging of human extraocular muscles in head tilt dependent hypertropia. Invest Ophthalmol Vis Sci. 2011;52:3023–3031. doi: 10.1167/iovs.10-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci. 1998;39:207–212. [PubMed] [Google Scholar]
- 24.Demer JL, Clark RA, da Silva Costa RM, Kung J, Yoo L. Expanding repertoire in the oculomotor periphery: selective compartmental function in rectus extraocular muscles. Ann N Y Acad Sci. 2011;1233:8–16. doi: 10.1111/j.1749-6632.2011.06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark RA, Demer JL. Differential lateral rectus compartmental contraction during ocular counter-rolling. Invest Ophthalmol Vis Sci. 2012;53:2887–2896. doi: 10.1167/iovs.11-7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozkan SB, Aribal ME, Sener EC, Sanaç AS, Gürcan F. Magnetic resonance imaging in evaluation of congenital and acquired superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1997;34:29–34. doi: 10.3928/0191-3913-19970101-07. [DOI] [PubMed] [Google Scholar]
- 27.Demer JL, Clark RA, Kung J. Functional imaging of human extraocular muscles in head tilt dependent hypertropia. Invest Ophtalmol Vis Sci. 2011;52:3023–3031. doi: 10.1167/iovs.10-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.