Abstract
Novel therapies hold promise for high-risk smoldering multiple myeloma (SMM). Recent studies suggest that modern combination approaches can be options for high-risk SMM to obtain deep molecular responses with favorable toxicity profiles. Although pioneering treatment trials based on small numbers of patients suggest progression-free and overall survival benefits, application of the data to real-life practice remains to be validated. Therapeutic modulation of disease tempo, disease burden, clonal evolution, and tumor microenvironment in SMM remains to be understood and calls for reliable biomarkers reflective of disease biology. Here, we review studies that open a new management platform for SMM, address ongoing dilemmas in practice and under investigation, and highlight emerging scientific questions in the era of SMM treatment.
INTRODUCTION
Uniform consensus criteria for smoldering multiple myeloma (SMM) appeared in 2003 that defined SMM to be without end-organ damage and to have either high serum M-protein (≥ 3 g/dL) or a high number of clonal bone marrow plasma cells (≥ 10%) or both.1 SMM is currently considered a precursor state of multiple myeloma (MM), with a 10% annual risk of transformation.2,3 More recently, subsets of SMM were identified as having higher risks of developing MM2,4–6 and having genetic similarity to MM.7 The high-risk SMM accounts for more than a quarter of all patients with SMM and, without intervention, carries a substantial risk of progression into symptomatic disease (70.4% progression with a median follow-up of 29.8 months).8
PATHOGENESIS AND BIOLOGY OF MYELOMA
Myeloma is a clonal proliferation of postgerminal center plasma cells that are phenotypically abnormal.9 Proliferation of myeloma clones occurs over multiple steps of somatic hypermutation. Hyperdiploidy and translocation of immunoglobulin H (IgH) gene are the two primary genetic events that lead to cyclin D dysregulation.10 Secondary genetic events include mutations of MYC, TP53, KRAS, and other genes involved in the nuclear factor kappa B pathway.11–14 With genetic changes, myeloma clones diversify and expand to evolve from precursor states of monoclonal gammopathy of undetermined significance (MGUS) and SMM toward more symptomatic and aggressive disease.
What is the pattern of clonal evolution in myeloma? A conventional belief was that one clone could acquire mutations and diversify along the disease progression (Fig 1B and 1C). Indeed, when four patients who transitioned from high-risk SMM to MM were followed up with whole genome sequencing (WGS), they acquired an average of 433 novel mutations and outgrowth of up to six subclones.7 Surprisingly, the diversity of clones appeared to be determined early, and the acquisition of mutations led to expansion of the preexisting clones (Fig 1A).7,16,17 These findings resonate with sequencing studies of other hematologic malignancies, which also showed expansion of the size of existing clones but not the diversity.15 The molecular mechanism of mutations leading to disease progression remains to be studied. One hypothesis proposed that translocations may lead to the loss of tumor suppressor genes, such as BRCA2 and UNC5D, which were lost in active MM but not in SMM.7 Whole exome sequencing of patients with MM revealed recurrent mutations of genes involved in MAPK, PI3K, MET, or noncanonical nuclear factor kappa B signaling pathways, such as BRAF, KRAS, ROBO1, FAM46C, and PEG3.17 On the basis of reported sequencing experiences, myeloma has clonal heterogeneity and evolves with clonal competitions.16,18,19
Fig 1.
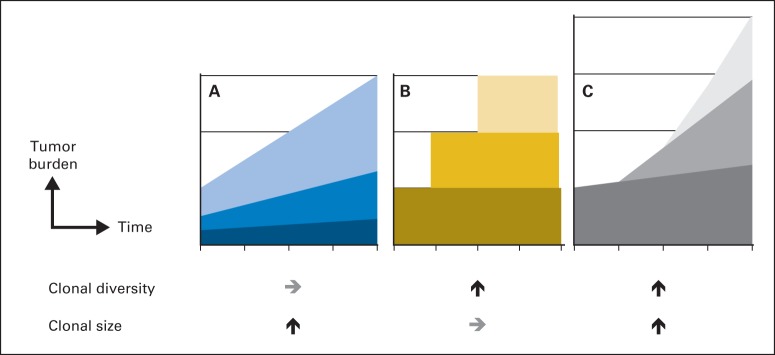
Temporal evolution of myeloma clones. As myeloma progresses, clones can theoretically undergo three different changes. (A) Progression of myeloma can occur as a result of the expansion of clonal size while the diversity of the clones remains the same. (B) Progression of myeloma may be a result of the increase of clonal diversity as additional clones emerge over time, while the size of each clone remains stable. (C) Myeloma clones can increase both in size and diversity at the same time, which potentially projects to a greater tumor burden (greater height). Studies have suggested that the diversification of clonal heterogeneity may occur as an early event as in (B) and (C), whereas the expansion of the size of each clone follows later within the existing library of clones as in (A).7,15
The role of bone marrow microenvironment in the pathogenesis of myeloma is an area of extensive research. In vitro, complex cell trafficking was observed during the adhesion of human myeloma cells to stroma and endothelial cells, which stimulate production of growth factors and cytokines from both parties, including inerleukin-6 (IL-6), vascular endothelial growth factor, insulin-like growth factor 1 (IGF-1), and stromal cell derived factor 1 (SDF-1).20–23 Evidence of increased osteoclast activity and decreased osteoprotegerin were observed in greater degree in MM than in precursor states.24 Gene expression profiling further strengthened the observation by showing methylation of genes that modulate the microenvironment such as GPX3, RBP1, SPARC, and TGFBI.25 A permissive microenvironment promotes the growth of myeloma clones and can stimulate disease progression into an active disease (Fig 2). Thus, targeting the microenvironment is a potential strategy for halting cell trafficking and growth of myeloma clones in bone marrow niches.26
Fig 2.
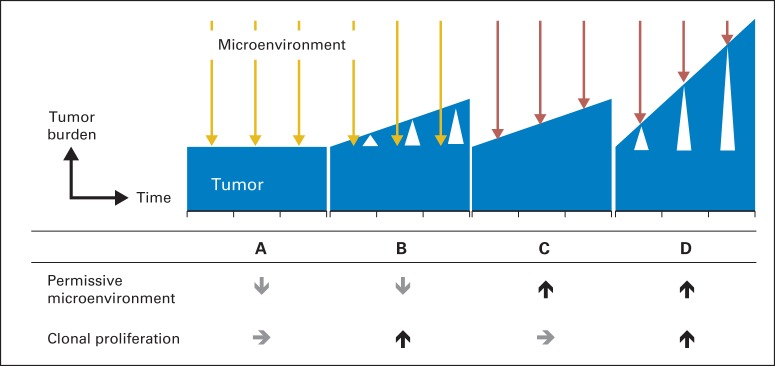
Interaction of tumor and microenvironment. Clone and microenvironment are the two factors that interact throughout the disease course. (A) When a clone is quiescent and microenvironment is nonpermissive (gold arrow) to myeloma growth, tumor burden (blue histogram) remains low and stable. (B) If a clone proliferates at a greater potential (white triangle), it can grow through the nonpermissive environment and elevate the tumor burden. (C) Conversely, microenvironment can be permissive to myeloma growth (red arrow) while proliferative potential remains stable. (D) When both factors are favorable to myeloma growth, tumor burden can increase rapidly.
Next generation sequencing (NextGen Sequencing [NGS]) has added depth to our understanding of high-risk SMM to the level of the genome. For instance, single nucleotide polymorphism array revealed that genetic complexity increased incrementally from precursor states to active MM, with SMM being the intermediate between MGUS and MM.27 As was expected, MM had the highest frequency of copy number aberrations and copy neutral loss of heterozygosity. Numbers of aberrations and also profiles of aberrations were different in SMM. SMM had more deletion 16q (30%) than MM (21%), whereas MM had more loss of 4q and gain of 11q, suggesting that the acquisition or imbalance of certain mutations may change the kinetics of the disease.27 In corroboration with other sequencing results, WGS revealed that the accumulation of genetic abnormalities was more prominent in SMM and the most complex in MM.7 Notably, when WGS reports of patients with high-risk SMM were compared with reports of those with MM, patterns and numbers of mutations were similar, suggesting that the high-risk SMM group represents a subset of asymptomatic patients who are biologically similar to patients with MM. In an effort to use molecular profiling studies in a predictive functional manner, several gene-expression profiling platforms have been developed for active MM to help identify patients with aggressive biology.28–30 Recent data show that a high-risk signature based on the 70-gene model in asymptomatic MGUS may predict progression to clinically symptomatic myeloma.31 On a rational note, these findings suggest that high-risk SMM denotes a distinct group of asymptomatic patients who may benefit from early intervention.
IDENTIFICATION OF PATIENTS WITH SMM AT HIGH RISK OF TRANSFORMATION TO MM
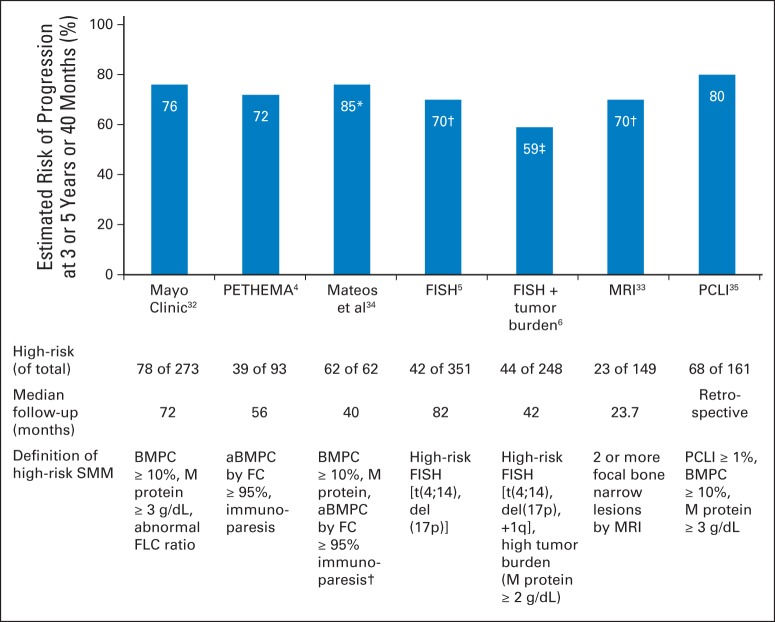
With potential therapies available for SMM, it is vital to identify the correct population to treat (Fig 3). One tool to use for stratifying risk is Mayo Clinic criteria, which defines the high-risk group by high M-protein (≥ 3 g/dL), high bone marrow plasma cell infiltration (≥ 10%), and abnormal serum free light chain ratio (outside the range of 0.125 to 8). In the presence of all three risk factors, 5-year risk of progression to active MM was 76%.32 The Spanish Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHEMA) study group uses different parameters and defines high-risk SMM as the presence of ≥ 95% neoplastic plasma cells by flow cytometry and immunoparesis. By Spanish criteria, 5-year risk of progression was 72% when both risk factors were present.4 In a head-to-head comparison of the two models in 77 patients with SMM, a significant discrepancy between the two existed, demonstrating a concordance rate of only 28.6%.36 For practical reasons, the aforementioned phase III clinical trial by Mateos et al34 (lenalidomide-dexamethasone v observation) used a hybrid method to define high-risk SMM: patients demonstrating ≥ 10% bone marrow plasma cells and a monoclonal component (IgG ≥ 3 g/dL, IgA ≥ 2 g/dL, or urine Bence Jones protein > 1 gram per 24 hours) or one of these criteria and ≥ 95% aberrant plasma cells by flow cytometry. When the hybrid method was applied to the real-world observation study of 2,494 patients, aberrant immunophenotype data for plasma cells were not available because of the lack of feasibility of using a flow cytometry technique. In this study, up to 30% of newly diagnosed patients with SMM or 4.2% of all patients with MM were candidates for treatment.8 High-risk SMM populations may encompass a significant proportion of patients with myeloma who have unmet needs, which calls for a more reliable tool for risk stratification.
Fig 3.
Identification of high-risk smoldering multiple myeloma (SMM) and risk of transformation to multiple myeloma, by published models. (*) Actual rate of progression in an untreated patient with high-risk SMM during the median follow-up of 40 months.34 This study used a hybrid method to defined high-risk SMM that combined Mayo Clinic and Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHEMA) criteria. The high-risk group was defined as patients with ≥ 10% bone marrow plasma cells and monoclonal component (immunoglobulin G ≥ 3 g/dL, immunoglobulin A ≥ 2 g/dL, or urine Bence Jones protein > 1 gram per 24 hours) or one of these criteria and ≥ 95% abnormal bone marrow plasma cells by flow cytometry (FC) with immunoparesis. (†) Three-year estimated risks of progression. Original article reported median time to progression in individual risk groups; 3-year risks of progression were estimated based on Kaplan-Meier survival curves. (‡) Three-year risk of progression was reported. Bars without symbols (*, †, ‡) are for 5-year risk of progression. aBMPC, abnormal bone marrow plasma cells; BMPC, bone marrow plasma cells; FLC, free light chain; FISH, fluorescent in situ hybridization; MRI, magnetic resonance imaging; PCLI, plasma cell labeling index.
Detection of chromosomal abnormalities is an alternative method of identifying high-risk SMM (Fig 1). The prognostic value of fluorescent in situ hybridization (FISH) –detected cytogenetic abnormalities in active MM appears to be applicable to SMM. For instance, t(4;14), deletion 17p, and gain 1q, which are well-known high-risk mutations found in MM,37–39 were also associated with shorter time to progression (TTP) in SMM5 independent of tumor burden.6 If tumor burden was higher in addition to these high-risk mutations, the risk of progression almost doubled (2-year TTP, 59% v 30%).6 On the basis of these findings, cytogenetic analysis may have a prognostic role in SMM in forecasting the risk of disease progression into active MM.
SMM: A DISEASE STATE TO TREAT OR NOT TO TREAT?
Although SMM is currently understood as a precursor disease of MM with the risk of transformation into active MM, on the basis of current guidelines, the standard of care for SMM is not to treat.40 Since 1988, many studies have contributed to establishing this nonintervention strategy in SMM (Table 1).41 A prospective study of thalidomide in 76 patients with SMM demonstrated significant reduction of tumor burden (ie, lower M-protein concentration).42 Furthermore, the addition of bisphosphonates to thalidomide had an added benefit in lowering the M-protein concentration by 25% or more.43 Nevertheless, treating SMM with single-agent thalidomide brought three concerns.42 First, early studies suggested that intervention may put selective pressure on myeloma clones and could potentially give growth advantage to fitter and more aggressive clones (Fig 4). In fact, in the study by Rajkumar et al42 based on 76 patients, those who achieved partial response (PR) with thalidomide had shorter TTP and inferior event-free survival (EFS). As pointed out by the authors, the underlying causes remain to be better understood.42 Second, long-term thalidomide therapy was not as well tolerated as expected and required dose reduction (86%) or discontinuation (50%) in several patients. Third, clinically meaningful response rate was low (PR or better in 42%) and did not impact survival. Variations of alkylating regimens containing melphalan and the addition of bisphosphonates to alkylators did not show any survival benefit.44–47
Table 1.
Selected Clinical Trials for Treatment of SMM
| Reference | Year of Publication | NCT No. | Trial Starting Year and Status | Study Design | Phase | Intervention | No. of Patients and Disease | Outcome/Comment | TTP (months) | ORR (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Alkylating agents | ||||||||||
| Alexanian et al41 | 1988 | Retrospective | Vincristine-doxorubicin-dexamethasone or melphalan-prednisone | 23 SMM; 10 IMM | Median OS: 105 months; deferral of treatment did not impact likelihood of remission or survival | 19 | 64 | |||
| Hjorth et al46 | 1993 | Randomized trial | Melphalan-prednisone; initial v deferred | 25 SMM and IMM | Median OS: 52 months; no significant difference in ORR or OS | 21 | 52-55 | |||
| Bisphosphonates only | ||||||||||
| Musto et al49 | 2008 | Randomized trial | III | Zoledronate v observation | 163 SMM | 1-year zoledronate decreased skeletal events; no significant difference in TTP or PFS | 67 | NA | ||
| Small molecules | ||||||||||
| Rajkumar et al50 | 2001 | Pilot single arm | Thalidomide | 16 SMM and IMM | MR or better: 69%; PR or better: 47.5% | NA | 69 | |||
| Barlogie et al43 | 2008 | Single arm | II | Thalidomide- pamidronate | 76 SMM | 4-year OS: 91%; high rates of dose reduction (86%) and discontinuation (50%) | 84 | 63 | ||
| Witzig et al51 | 2013 | Randomized trial | III | Thalidomide-zoledronate v zoledronate | 68 SMM | Median TTP: 2.4 (thalidomide and zoledronate) v 1.2 years (zoledronate only) (P = .02) | 29 | NR at 71 months | ||
| Mateos et al34 | 2013 | Randomized trial | III | Lenalidomide-dexamethasone v observation | 119 high-risk SMM | 3-year OS: 94% (treatment) v 80% (observation) (P = .03); high rate of discontinuation in patients given maintenance lenalidomide (30%) | NR at 40 months | 79-90 | ||
| NCT01169337 | 2010, ongoing | Randomized trial | III | Lenalidomide v observation | 370* high-risk SMM | End points: ORR, PFS, OS | — | — | ||
| NCT01572480 | 2012, ongoing | Single arm | II | Carfilzomib-lenalidomide-dexamethasone | 30 high-risk SMM | End points: ORR, PFS | — | — | ||
| NCT00983346 | 2009, ongoing | Pilot, single arm | Bortezomib | 20* SMM | End point: bone anabolic effect | — | — | |||
| Monoclonal antibodies or receptor antagonists | ||||||||||
| Lust et al52 | 2009 | Single arm | II | Anakinra | 47 SMM and IMM | Anakinra: IL-1 receptor antagonist; median PFS: 37.5 months; MR (n = 3), PR (n = 5) | 38† | NA | ||
| Korde et al52a | 2014 | Single arm | II | IPH2101 | 9* SMM | IPH2101: antiKIR Ab; MR (n = 1), stable disease (n = 6), progression (n = 2) | NA | 11 | ||
| NCT01222286 | 2010, completed | Randomized trial | II | IPH2101 | 30* SMM | IPH2101: anti-KIR Ab; results not published to date; end point: ORR | — | — | ||
| NCT01302886 | 2011, completed | Single arm | II | BHQ880 | 58* high-risk SMM | BHQ880: anti-DKK1 neutralizing Ab; results not published to date; end point: ORR | — | — | ||
| NCT01484275 | 2011, ongoing | Randomized trial | II | Siltuximab v placebo | 100* high-risk SMM | Siltuximab: anti-IL-6 mAb; end point: 1-year PFS | — | — | ||
| NCT01441973 | 2012, ongoing | Single arm | II | Elotuzumab | 58* high-risk SMM | Elotuzumab: anti-CS1 IgG1 mAb; end point: ORR | — | — | ||
| NCT01838369 | 2013, ongoing | Single arm | II | BI-505 | 10* SMM | BI-505: anti-intercellular adhesion molecule-1 mAb; end point: ORR | — | — |
Abbreviations: Ab, antibody; IgG1, immunoglobulin G1; IL-1, interleukin-1; IMM, indolent multiple myeloma; mAb, monoclonal antibody; MR, minor response (25%-50% decrease in M-protein); NA, not assessed; NR, not reached; ORR, overall response rate, including minor response; OS, overall survival; PFS, progression-free survival; PR, partial response (≥ 50% decrease in M-protein); SMM, smoldering multiple myeloma; TTP, time to progression.
Estimated number of enrolled participants.
Value for median PFS. TTP was not reported.
Fig 4.
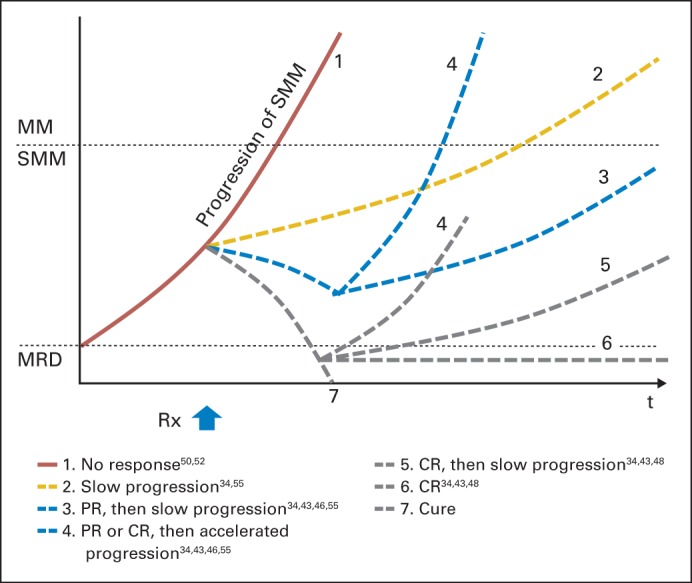
Trajectories of treatment in smoldering multiple myeloma (SMM). Theoretically, treatment response in SMM can branch into seven different scenarios. (1) A group may show no response to treatment and progress, as seen in treatment with interleukin-1 receptor antagonist52 or bisphosphonates.49 (2) Treatment can slow disease progression without meeting criteria for partial response (PR). (3) Treatment can achieve PR followed by slow disease progression (as shown in 3) or stable disease (not shown). As an example, single-agent thalidomide was able to achieve PR in 25%43 and 47.5% of patients50 in two different trials. (4) Some patients who achieved PR or complete response (CR) may undergo selection of fitter clones and later have an accelerated progression. This was an observed phenomenon as shown by a shorter time to progression in the PR group treated with thalidomide.50 (5, 6, 7) Treatments can lead to CR, as seen in 14% of patients with SMM treated with lenalidomide and dexamethasone34 or up to 100% when treated with a three-drug combination.48 Long-term follow-ups are required to monitor the consequence after CR, which may be followed by relapse with slow progression (5), stable disease with a disease burden below the level of detection (shown as minimal residual disease [MRD]; 6), or cure (7). MM, multiple myeloma; Rx, treatment.
A RISING NEW CONCEPT IN SMM
The advent of novel therapies has dismantled the conventional watch-and-wait concept. More tolerable and efficacious therapies have surfaced as potential treatments for SMM. Since the 2013 release of study results showing benefit of treatment with novel agents, a new era has opened for SMM.
The first study that demonstrated survival benefit in SMM is a published randomized phase III prospective study of 119 patients with high-risk SMM.34 In that study, lenalidomide plus dexamethasone delayed TTP and improved 3-year overall survival (OS) rate compared with no treatment (93% v 76% in observation; hazard ratio, 0.31; P = .03). In addition, results of a single-arm pilot study with the addition of carfilzomib to the same two-drug regimen (lenalidomide plus dexamethasone) was presented at the 2013 Annual Meeting of the American Society of Hematology, and it showed higher rates of deep responses.48 Importantly, previous treatment trials for SMM have explored single- or double-drug approaches to avoid excessive toxicity. However, based on larger studies treating newly diagnosed MM, three-drug combination therapy may yield deeper responses without compromising tolerability compared with two-drug approaches.53,54,56,57 Although efficacy and toxicity profiles of combination therapies are subject to the individual drugs that compose the treatment, three-drug regimens (eg, carfilzomib, lenalidomide and dexamethasone) based on preliminary data in high-risk SMM demonstrated augmented overall responses with deep minimal residual disease (MRD) –negative remissions.48 That three-drug combination study48 is ongoing, and the original article is anticipated to be published in the coming year.
Driven by these paradigm-changing studies, SMM has gained heightened attention, thus inspiring the startup of numerous clinical investigations. As of October 2014, 15 prospective trials have been registered at the ClinicalTrials.gov database to investigate the effect of various agents in SMM, including immunomodulatory agents, proteasome inhibitors, monoclonal antibodies, antioxidants, and multidrug combinations (Table 1).
Although treatment trials bring the hope of improved response, survival, and perhaps a cure for SMM, they also raise fundamental questions regarding our definition of the disease and treatment outcomes. Specifically, what is the definition of response in patients with SMM who are asymptomatic and have no end-organ damage? What would be the long-term impact of treatment in the context of clonal evolution? Answering these questions is not only clinically relevant but also necessary for regulatory purposes to obtain US Food and Drug Administration (FDA) approval of a drug for SMM indication.
ASSESSMENT OF TREATMENT RESPONSE
Outcomes of preventive treatment for SMM have been gauged by the reduction of disease burden and the delay of disease progression, which are represented by serum M-protein,42,43 MRD status,48 TTP,58 and OS.34 Among them, achieving MRD negativity is translated into treatment efficacy and survival in MM59 and may be an important milestone for monitoring disease burden and treatment outcome in SMM. As in MM, MRD testing in SMM is limited because of lack of consensus definitions. Real-time quantitative polymerase chain reaction (RT-PCR) analysis for the rearrangement of the Ig gene is generally used to detect clone-specific MRD, and many variations of this assay are available, ranging from qualitative-PCR and fluorescent-PCR to allele-specific-PCR. Still, sensitivity of PCR is lower than that of flow cytometry (10−4 to 10−5), and the use of PCR is limited when detecting somatically hypermutated tumors. Multicolor flow cytometry can be an alternative method for defining MRD and was recently recognized by the International Myeloma Working Group consensus panel.60 Still, the implication of flow cytometry–defined MRD negativity in SMM is unknown, and there is a paucity of data. Another limitation of flow cytometry is specimen processing, which requires dilution of malignant cells and lowers detection sensitivity. Availability of fresh bone marrow aspirates and multicolor flow panels coupled with frequency of testing limits the widespread use of flow cytometry.
NGS could offer a valuable insight during follow-up assessment of SMM by tracking longitudinal evolution of myeloma with respect to disease biology rather than one-dimensional disease burden. Currently, there is a paucity of data that define the prognostic role of sequencing in a large cohort of patients with SMM. Future studies will need to prospectively assess the role of sequencing with a longitudinal sequencing effort and long-term follow-up.
An important but hard-to-measure outcome is the impact of an intervention on quality of life. When SMM is untreated until hypercalcemia, renal failure, anemia, and bone events occur, it is known to be associated with higher rates of end-organ damage and serious complications, such as renal failure (11% to 13%),58 transfusion,61 and skeletal events (58% to 82%).44,49 Among these, skeletal complications are closely linked to quality of life, and their early detection has a predictive role in SMM. Magnetic resonance imaging and positron emission tomography are promising and sensitive tools for detecting skeletal lesions that can be alternatives for conventional skeletal survey. For instance, magnetic resonance imaging can be useful for initial33,62 and longitudinal63 assessments of SMM and is recommended by International Myeloma Working Group consensus guidelines.40 Early detection of occult lesions and treatment of SMM may provide important benefits for patients by preventing serious complications of the disease, alleviating psychosocial burden, and promoting quality of life. Future trials will need to extrapolate from MM experience64 and incorporate clinically relevant, disease-specific outcomes to end points.49
FUTURE INVESTIGATION
Areas of Future Research in Myeloma Biology
Combinations of novel agents can modify the kinetics of myeloma and achieve deeper responses with MRD negativity. Theoretically, treatment response in SMM can branch into at least seven different scenarios (Fig 4). First, a group may show no response and follow its natural history. Second and third, treatment may slow or halt disease progression with or without meeting response criteria. Fourth, some patients who experience PR or complete response to treatment may undergo selection of fitter clones and later have an accelerated progression, which was an observed phenomenon in a trial with single-agent thalidomide42 and in serial sequencing of four patients with MM.17 Selective treatment pressure on myeloma clone may justify early treatment with an aggressive curative intent. To address these issues, it will be crucial for future treatment trials to incorporate longitudinal sequencing follow-up and capture dynamic changes of dominant clones and subclones during treatment.
The last three groups are the people who achieve complete responses followed by relapse, MRD negativity, or a cure. Modification of disease tempo, depicted as the slope of a curve in Figure 4, appeared to be the most common event in previous treatment trials. At the same time, it is important to note that subgroups of patients may have inherently distinct disease tempo and may show differential responses to treatment. Examples are patients with deletion 17p or t(4;14) who appear to undergo rapid progression of myeloma irrespective of disease burden.5 A population with accelerated disease tempo may need to be approached with separate strategies, including short follow-up intervals and early intervention. The proposed trajectories of outcome must be validated by future studies with long-term follow-up.
Bone marrow microenvironment is an emerging target for SMM treatment. Several clinical trials for patients with high-risk SMM have been launched to study the efficacy of novel drugs that modulate the microenvironment, including anti-CS-1 antibody elotuzumab, anti-IL-6 antibody, anti-IL-1 receptor antagonist,52 and DKK-1 inhibitor. An underlying challenge to these trials is the difficulty in quantifying the permissiveness of the microenvironment. Some studies in patients with SMM reported the use of plasma cell labeling index, high-sensitivity C-reactive protein,52 or direct assays of cytokine production.65,66 Still, there is an unmet need for reliable biomarkers that reflect the tumor microenvironment, which calls for vigilant tissue collection and built-in experimental biomarker studies for future trials.
Suggestions for Current Clinical Practice
Since Kyle and Greipp67 first coined the term SMM in 1980, our understanding in SMM has evolved. We now define high-risk SMM as a subset of asymptomatic patients who carry greater risks of progression into active MM who may benefit from early intervention. Novel therapies hold promise for an improved survival with a deeper response for SMM.34,48 Nevertheless, there is an ongoing dilemma in real-life practice because of the lack of established guidelines for risk stratification and intervention in SMM. It is also unclear which benchmarks need to be achieved in clinical trials to provide compelling evidences to change practice.
For current clinical practice, we strongly recommend that patients with SMM who meet prospectively validated high-risk categories be considered for clinical trials.2,4–6,40 In our opinion, patients with SMM should not be treated outside clinical trials until data from treatment trials mature to give more information. The ongoing dilemmas in clinical practice call for new approaches in drug development and trial design.
Drug Development in High-Risk SMM
Because SMM is an asymptomatic disease state, it imposes new challenges for drug development. For instance, a randomized study comparing two active treatment strategies in SMM with OS as the primary end point would require extremely long follow-up and/or a large sample size to provide sufficient statistical power. In our opinion, traditional end points, such as progression-free survival and OS, are quite cost prohibitive, and they delay regulatory approval of drugs for high-risk SMM. Importantly, per FDA guidelines, more sensitive assays can be implemented to serve as surrogate biomarkers of survival.68 One such parameter is MRD status, which has become increasingly important in response assessment and follow-up in MM.68 Indeed, a growing body of literature in MM has proved a strong correlation between MRD negativity and progression-free survival as well as OS benefits.69–73 On the basis of the aforementioned evidence, the 2014 FDA-National Cancer Institute Roundtable Symposium concluded that flow cytometry–based MRD testing can serve as a molecular end point and a surrogate biomarker for survival in MM.74 As suggested by our experience with MM, we argue that MRD negativity may be an acceptable end point in future treatment trials in high-risk SMM. Because MRD status not only defines the depth of response but can also be followed longitudinally to capture evolving clones, we believe that optimal treatment and monitoring strategies for high-risk SMM have the potential to help develop a cure for this disease. For example, future treatment trials in high-risk SMM can set MRD negativity as a primary end point to demonstrate the depth of response achieved with a study drug and prospectively explore the duration of MRD-negative status as a secondary end point to assess the impact of treatment in preventing clonal evolution. There is also a need for future studies to define the role of repeated MRD testing, including the duration of MRD negativity and the interval of testing and its impact on clinical outcome. Therapeutic modulation of disease tempo, disease burden, clonal evolution, and tumor microenvironment will be emerging scientific issues in the era of SMM treatment that will need to be addressed in model systems and in clinical trials accompanied by molecular-based surrogate biomarkers.
Hence, we propose future randomized trials in high-risk SMM to demonstrate deep and durable responses defined by MRD negativity. Patients with high-risk SMM who are participating in treatment trials need to be prospectively monitored for clonal evolution and investigated for other factors that can hamper the correlation between the biomarker and the anticipated clinical benefit.
Glossary Terms
- clonal evolution (of tumor):
accumulation of mutations in cancer cells guided by selective forces. The genomic content of cells within a single tumor can be heterogeneous as a result of differences in the mutation history of the lineage of individual cells. A growth (dis)advantage as a result of the mostly random mutations creates selective pressure. Cells with the relatively best genotype eventually dominate in the tumor.
- fluorescent in situ hybridization (FISH):
in situ hydridization is a sensitive method generally used to detect specific gene sequences in tissue sections or cell preparations by hybridizing the complementary strand of a nucleotide probe to the sequence of interest. FISH uses a fluorescent probe to increase the sensitivity of in situ hybridization.
- gene expression profiling:
identifying the expression of a set of genes in a biologic sample (eg, blood, tissue) using microarray technology.
- microenvironment:
the unique complex of tumor cells, stromal, and immune infiltrate that can promote or reject tumors as well as shape their phenotype through contact-dependent or soluble mediators.
- minimal residual disease (MRD):
the low level of tumor cells (eg, after chemotherapy) that can only be detected with highly sensitive molecular methods (eg, polymerase chain reaction) or to molecularly defined relapse after long-term remission.
- NextGen Sequencing (NGS):
a non-Sanger rapid DNA sequencing method that can be done with greater speed, developed after the first methodologic articles describing relatively rapid DNA sequencing produced by Sanger et al (1977).
Footnotes
Supported by the Intramural Program of the National Cancer Institute, National Institutes of Health.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Inhye E. Ahn, Sham Mailankody, Ola Landgren
Financial support: Ola Landgren
Administrative support: Ola Landgren
Collection and assembly of data: Inhye E. Ahn, Ola Landgren
Data analysis and interpretation: Inhye E. Ahn, Sham Mailankody, Neha Korde, Ola Landgren
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dilemmas in Treating Smoldering Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Inhye E. Ahn
No relationship to disclose
Sham Mailankody
No relationship to disclose
Neha Korde
No relationship to disclose
Ola Landgren
Honoraria: Onyx Pharmaceuticals, Millennium Pharmaceuticals
REFERENCES
- 1.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 2.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 3.Landgren O, Waxman AJ. Multiple myeloma precursor disease. JAMA. 2010;304:2397–2404. doi: 10.1001/jama.2010.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez-Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–2592. doi: 10.1182/blood-2007-05-088443. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Gupta V, Fonseca R, et al. Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia. 2013;27:1738–1744. doi: 10.1038/leu.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neben K, Jauch A, Hielscher T, et al. Progression in smoldering myeloma is independently determined by the chromosomal abnormalities del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. J Clin Oncol. 2013;31:4325–4332. doi: 10.1200/JCO.2012.48.4923. [DOI] [PubMed] [Google Scholar]
- 7.Walker BA, Wardell CP, Melchor L, et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia. 2014;28:384–390. doi: 10.1038/leu.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristinsson SY, Holmberg E, Blimark C. Treatment for high-risk smoldering myeloma. N Engl J Med. 2013;369:1762–1763. doi: 10.1056/NEJMc1310911. [DOI] [PubMed] [Google Scholar]
- 9.Raja KR, Kovarova L, Hajek R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br J Haematol. 2010;149:334–351. doi: 10.1111/j.1365-2141.2010.08121.x. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca R, Blood E, Rue M, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101:4569–4575. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- 11.Chng WJ, Gonzalez-Paz N, Price-Troska T, et al. Clinical and biological significance of RAS mutations in multiple myeloma. Leukemia. 2008;22:2280–2284. doi: 10.1038/leu.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodé L, Eveillard M, Trichet V, et al. Mutations in TP53 are exclusively associated with del(17p) in multiple myeloma. Haematologica. 2010;95:1973–1976. doi: 10.3324/haematol.2010.023697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avet-Loiseau H, Gerson F, Magrangeas F, et al. Rearrangements of the c-myc oncogene are present in 15% of primary human multiple myeloma tumors. Blood. 2001;98:3082–3086. doi: 10.1182/blood.v98.10.3082. [DOI] [PubMed] [Google Scholar]
- 15.Landau DA, Carter SL, Stojanov P, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao S, Choi M, Heuck C, et al. Serial exome analysis of disease progression in premalignant gammopathies. Leukemia. 2014;28:1548–1552. doi: 10.1038/leu.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolli N, Avet-Loiseau H, Wedge DC, et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun. 2014;5:2997. doi: 10.1038/ncomms3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohr JG, Stojanov P, Carter SL, et al. Widespread genetic heterogeneity in multiple myeloma: Implications for targeted therapy. Cancer Cell. 2014;25:91–101. doi: 10.1016/j.ccr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12:335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- 20.Uchiyama H, Barut BA, Mohrbacher AF, et al. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82:3712–3720. [PubMed] [Google Scholar]
- 21.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23:10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghobrial IM. Myeloma as a model for the process of metastasis: Implications for therapy. Blood. 2012;120:20–30. doi: 10.1182/blood-2012-01-379024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birmann BM, Neuhouser ML, Rosner B, et al. Prediagnosis biomarkers of insulin-like growth factor-1, insulin, and interleukin-6 dysregulation and multiple myeloma risk in the Multiple Myeloma Cohort Consortium. Blood. 2012;120:4929–4937. doi: 10.1182/blood-2012-03-417253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Politou M, Terpos E, Anagnostopoulos A, et al. Role of receptor activator of nuclear factor-kappa B ligand (RANKL), osteoprotegerin and macrophage protein 1-alpha (MIP-1a) in monoclonal gammopathy of undetermined significance (MGUS) Br J Haematol. 2004;126:686–689. doi: 10.1111/j.1365-2141.2004.05092.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser MF, Johnson DC, Wu P, et al. Global methylation analysis identifies prognostically important epigenetically inactivated tumor suppressor genes in multiple myeloma. Blood. 2013;122:219–226. doi: 10.1182/blood-2013-03-487884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Runnels JM, Carlson AL, Pitsillides C, et al. Optical techniques for tracking multiple myeloma engraftment, growth, and response to therapy. J Biomed Opt. 2011;16:011006. doi: 10.1117/1.3520571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Corral L, Sarasquete ME, Beà S, et al. SNP-based mapping arrays reveal high genomic complexity in monoclonal gammopathies, from MGUS to myeloma status. Leukemia. 2012;26:2521–2529. doi: 10.1038/leu.2012.128. [DOI] [PubMed] [Google Scholar]
- 28.Chen T, Berno T, Zangari M. Low-risk identification in multiple myeloma using a new 14-gene model. Eur J Haematol. 2012;89:28–36. doi: 10.1111/j.1600-0609.2012.01792.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhan F, Barlogie B, Arzoumanian V, et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood. 2007;109:1692–1700. doi: 10.1182/blood-2006-07-037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuiper R, Broyl A, de Knegt Y, et al. A gene expression signature for high-risk multiple myeloma. Leukemia. 2012;26:2406–2413. doi: 10.1038/leu.2012.127. [DOI] [PubMed] [Google Scholar]
- 31.Dhodapkar MV, Sexton R, Waheed S, et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120) Blood. 2014;123:78–85. doi: 10.1182/blood-2013-07-515239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785–789. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillengass J, Fechtner K, Weber MA, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28:1606–1610. doi: 10.1200/JCO.2009.25.5356. [DOI] [PubMed] [Google Scholar]
- 34.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369:438–447. doi: 10.1056/NEJMoa1300439. [DOI] [PubMed] [Google Scholar]
- 35.Madan S, Kyle RA, Greipp PR. Plasma cell labeling index in the evaluation of smoldering (asymptomatic) multiple myeloma. Mayo Clin Proc. 2010;85:300. doi: 10.4065/mcp.2009.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cherry BM, Korde N, Kwok M, et al. Modeling progression risk for smoldering multiple myeloma: Results from a prospective clinical study. Leuk Lymphoma. 2013;54:2215–2218. doi: 10.3109/10428194.2013.764419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: The experience of the Intergroupe Francophone du Myélome. Blood. 2007;109:3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 38.Avet-Loiseau H, Li C, Magrangeas F, et al. Prognostic significance of copy-number alterations in multiple myeloma. J Clin Oncol. 2009;27:4585–4590. doi: 10.1200/JCO.2008.20.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreau P, Cavo M, Sonneveld P, et al. Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol. 2014;32:2173–2180. doi: 10.1200/JCO.2013.53.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kyle RA, Durie BG, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexanian R, Barlogie B, Dixon D. Prognosis of asymptomatic multiple myeloma. Arch Intern Med. 1988;148:1963–1965. [PubMed] [Google Scholar]
- 42.Rajkumar SV, Gertz MA, Lacy MQ, et al. Thalidomide as initial therapy for early-stage myeloma. Leukemia. 2003;17:775–779. doi: 10.1038/sj.leu.2402866. [DOI] [PubMed] [Google Scholar]
- 43.Barlogie B, van Rhee F, Shaughnessy JD, Jr, et al. Seven-year median time to progression with thalidomide for smoldering myeloma: Partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood. 2008;112:3122–3125. doi: 10.1182/blood-2008-06-164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musto P, Falcone A, Sanpaolo G, et al. Pamidronate reduces skeletal events but does not improve progression-free survival in early-stage untreated myeloma: Results of a randomized trial. Leuk Lymphoma. 2003;44:1545–1548. doi: 10.3109/10428190309178778. [DOI] [PubMed] [Google Scholar]
- 45.D'Arena G, Gobbi PG, Broglia C, et al. Pamidronate versus observation in asymptomatic myeloma: Final results with long-term follow-up of a randomized study. Leuk Lymphoma. 2011;52:771–775. doi: 10.3109/10428194.2011.553000. [DOI] [PubMed] [Google Scholar]
- 46.Hjorth M, Hellquist L, Holmberg E, et al. Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I: A randomized study—Myeloma Group of Western Sweden. Eur J Haematol. 1993;50:95–102. doi: 10.1111/j.1600-0609.1993.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 47.Riccardi A, Mora O, Tinelli C, et al. Long-term survival of stage I multiple myeloma given chemotherapy just after diagnosis or at progression of the disease: A multicentre randomized study—Cooperative Group of Study and Treatment of Multiple Myeloma. Br J Cancer. 2000;82:1254–1260. doi: 10.1054/bjoc.1999.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landgren O, Mailankody S, Kwok M, et al. Clinical and correlative pilot study of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended dosing (CRd-R) in high risk smoldering multiple myeloma patients. Blood. 2013;122 (abstr 1939) [Google Scholar]
- 49.Musto P, Petrucci MT, Bringhen S, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–1595. doi: 10.1002/cncr.23783. [DOI] [PubMed] [Google Scholar]
- 50.Rajkumar SV, Dispenzieri A, Fonseca R, et al. Thalidomide for previously untreated indolent or smoldering multiple myeloma. Leukemia. 2001;15:1274–1276. doi: 10.1038/sj.leu.2402183. [DOI] [PubMed] [Google Scholar]
- 51.Witzig TE, Laumann KM, Lacy MQ, et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia. 2013;27:220–225. doi: 10.1038/leu.2012.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52a.Korde N, Carlsten M, Lee MJ, et al. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica. 2014;99:e81–e83. doi: 10.3324/haematol.2013.103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120:9–19. doi: 10.1182/blood-2012-02-408898. [DOI] [PubMed] [Google Scholar]
- 54.San Miguel JF, Schlag R, Khuageva NK, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol. 2013;31:448–455. doi: 10.1200/JCO.2012.41.6180. [DOI] [PubMed] [Google Scholar]
- 55.Musto P, D'Auria F, Pietrantuono G, et al. Role of thalidomide in previously untreated patients with multiple myeloma. Expert Rev Anticancer Ther. 2008;8:1569–1580. doi: 10.1586/14737140.8.10.1569. [DOI] [PubMed] [Google Scholar]
- 56.Rosiñol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: A randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 57.Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752–5758. doi: 10.1182/blood-2011-05-355081. [DOI] [PubMed] [Google Scholar]
- 58.Wisløff F, Andersen P, Andersson TR, et al. Incidence and follow-up of asymptomatic multiple myeloma: The myeloma project of health region I in Norway—II. Eur J Haematol. 1991;47:338–341. doi: 10.1111/j.1600-0609.1991.tb01857.x. [DOI] [PubMed] [Google Scholar]
- 59.Ludwig H, Miguel JS, Dimopoulos MA, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia. 2014;28:981–992. doi: 10.1038/leu.2013.293. [DOI] [PubMed] [Google Scholar]
- 60.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rago A, Grammatico S, Za T, et al. Prognostic factors associated with progression of smoldering multiple myeloma to symptomatic form. Cancer. 2012;118:5544–5549. doi: 10.1002/cncr.27657. [DOI] [PubMed] [Google Scholar]
- 62.Moulopoulos LA, Dimopoulos MA, Smith TL, et al. Prognostic significance of magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 1995;13:251–256. doi: 10.1200/JCO.1995.13.1.251. [DOI] [PubMed] [Google Scholar]
- 63.Merz M, Hielscher T, Wagner B, et al. Predictive value of longitudinal whole-body magnetic resonance imaging in patients with smoldering multiple myeloma. Leukemia. 2014;28:1902–1908. doi: 10.1038/leu.2014.75. [DOI] [PubMed] [Google Scholar]
- 64.Anderson KC, Kyle RA, Rajkumar SV, et al. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22:231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 65.Bataille R, Jourdan M, Zhang XG, et al. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989;84:2008–2011. doi: 10.1172/JCI114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong Y, Donovan KA, Kline MP, et al. Identification of two groups of smoldering multiple myeloma patients who are either high or low producers of interleukin-1. J Interferon Cytokine Res. 2006;26:83–95. doi: 10.1089/jir.2006.26.83. [DOI] [PubMed] [Google Scholar]
- 67.Kyle RA, Greipp PR. Smoldering multiple myeloma. N Engl J Med. 1980;302:1347–1349. doi: 10.1056/NEJM198006123022405. [DOI] [PubMed] [Google Scholar]
- 68.Munshi NC, Anderson KC. Minimal residual disease in multiple myeloma. J Clin Oncol. 2013;31:2523–2526. doi: 10.1200/JCO.2013.49.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123:3073–3079. doi: 10.1182/blood-2014-01-550020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paiva B, Vidriales MB, Cerveró J, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112:4017–4023. doi: 10.1182/blood-2008-05-159624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rawstron AC, Child JA, de Tute RM, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: Impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31:2540–2547. doi: 10.1200/JCO.2012.46.2119. [DOI] [PubMed] [Google Scholar]
- 72.Rawstron AC, Davies FE, DasGupta R, et al. Flow cytometric disease monitoring in multiple myeloma: The relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood. 2002;100:3095–3100. doi: 10.1182/blood-2001-12-0297. [DOI] [PubMed] [Google Scholar]
- 73.Sarasquete ME, García-Sanz R, González D, et al. Minimal residual disease monitoring in multiple myeloma: A comparison between allelic-specific oligonucleotide real-time quantitative polymerase chain reaction and flow cytometry. Haematologica. 2005;90:1365–1372. [PubMed] [Google Scholar]
- 74.Landgren O, Gormley N, Turley D, et al. Flow cytometry detection of minimal residual disease in multiple myeloma: Lessons learned at FDA-NCI Roundtable Symposium. Am J Hematol. doi: 10.1002/ajh.23831. [epub ahead of print on August 16, 2014] [DOI] [PubMed] [Google Scholar]