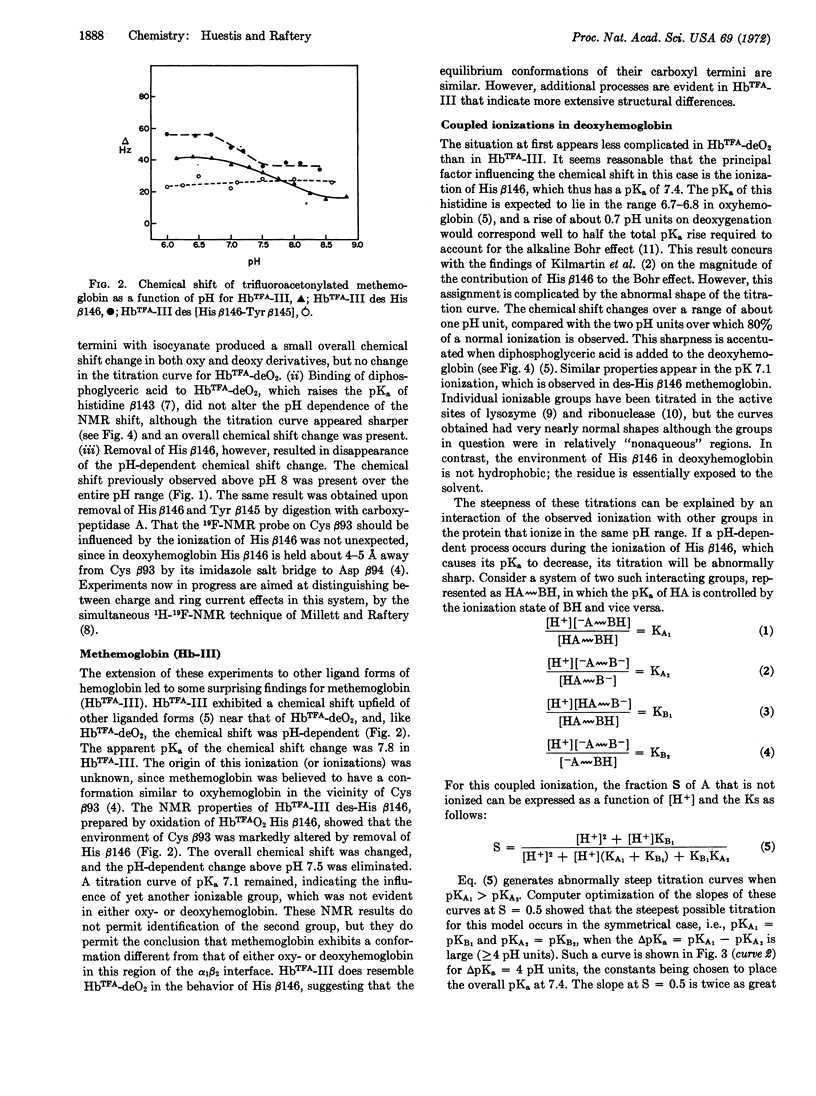
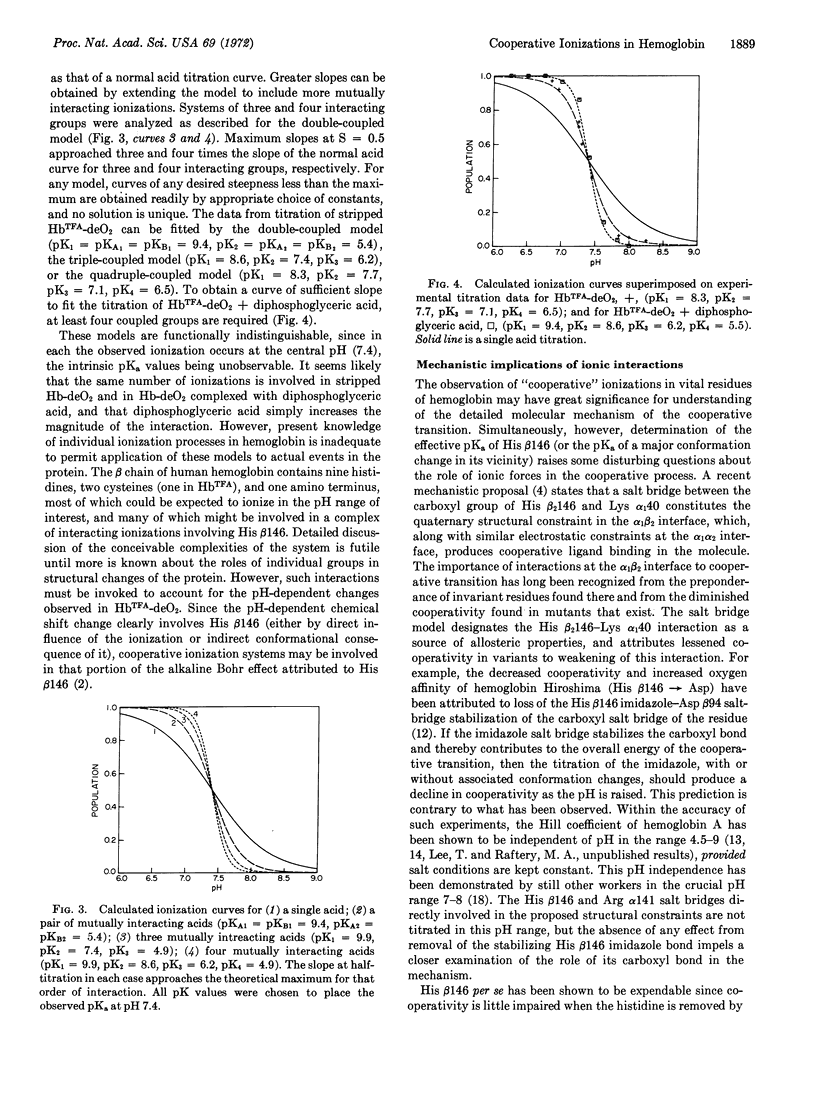
Abstract
19F-Nuclear magnetic resonance studies of specifically fluorinated hemoglobin derivatives have been used to determine the apparent pKa of the histidine β146 imidazole in deoxyhemoglobin. The titration of this residue was found to be abnormally sharp, particularly in the presence of diphosphoglyceric acid. The explanation advanced for this unusual titration curve may have implications for the mechanism of cooperative ligand binding. The possible role of such ionizations is discussed in light of some chemical evidence that the cooperative binding process is governed to a greater extent by internal nonpolar forces than by electrostatic interactions of exposed groups.
Keywords: 19F-NMR, specifically labeled proteins, molecular regulation mechanisms, hydrophobic interactions
Full text
PDF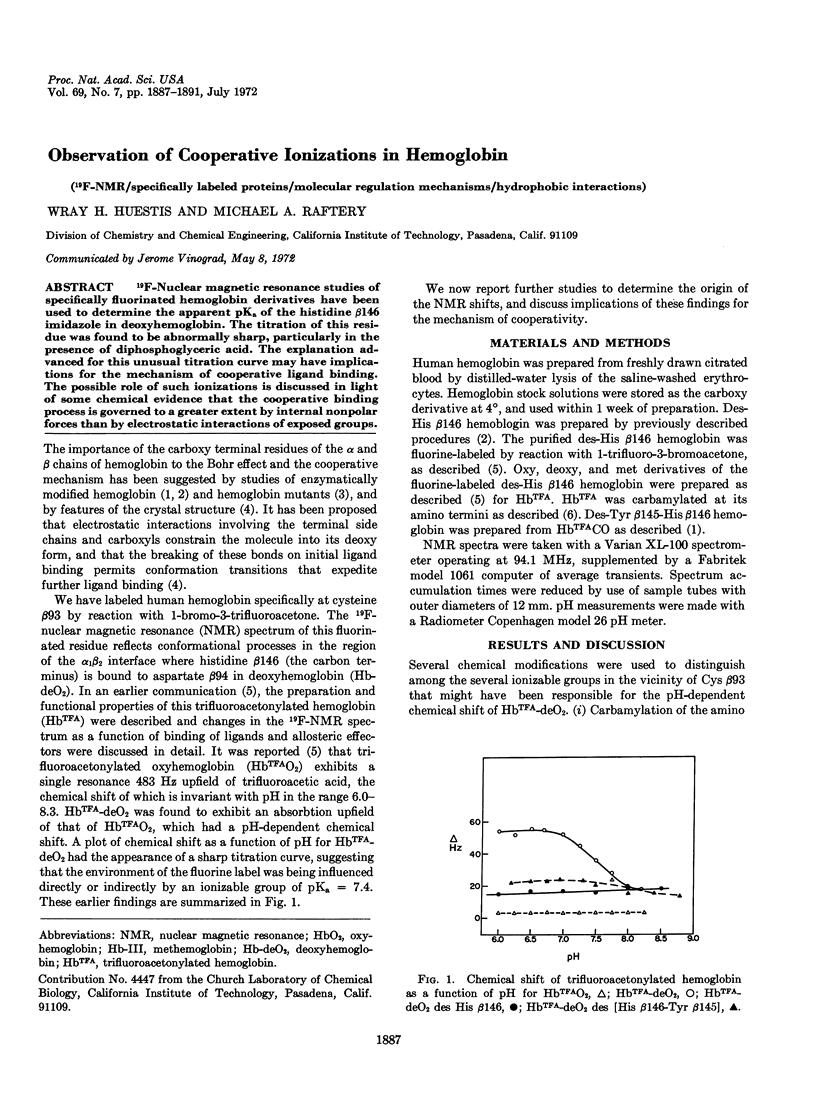


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., Jr, ROSSI-FANELLI A., CAPUTO A. Studies on the relations between molecular and functional properties of hemoglobin. J Biol Chem. 1962 Sep;237:2773–2777. [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- Greer J., Perutz M. F. Three dimensional structure of haemoglobin Rainier. Nat New Biol. 1971 Apr 28;230(17):261–264. doi: 10.1038/newbio230261a0. [DOI] [PubMed] [Google Scholar]
- Hayashi A., Stamatoyannopoulos G. Role of penultimate tyrosine in haemoglobin subunit. Nat New Biol. 1972 Jan 19;235(55):70–72. doi: 10.1038/newbio235070a0. [DOI] [PubMed] [Google Scholar]
- Hewitt J. A., Kilmartin J. V., Eyck L. F., Perutz M. F. Noncooperativity of the dimer in the reaction of hemoglobin with oxygen (human-dissociation-equilibrium-sulfhydryl-absorption-x-ray analysis). Proc Natl Acad Sci U S A. 1972 Jan;69(1):203–207. doi: 10.1073/pnas.69.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Hewitt J. A. The effect of removal of C-terminal residues on cooperative interactions in hemoglobin. Cold Spring Harb Symp Quant Biol. 1972;36:311–314. doi: 10.1101/sqb.1972.036.01.041. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Inhibition of CO2 combination and reduction of the Bohr effect in haemoglobin chemically modified at its alpha-amino groups. Nature. 1969 Jun 28;222(5200):1243–1246. doi: 10.1038/2221243a0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Wootton J. F. Inhibition of Bohr effect after removal of C-terminal histidines from haemoglobin beta-chains. Nature. 1970 Nov 21;228(5273):766–767. doi: 10.1038/228766a0. [DOI] [PubMed] [Google Scholar]
- Meadows D. H., Jardetzky O. Nuclear magnetic resonance studies of the structure and binding sites of enzymes. IV. Cytidine 3'-monophosphate binding to ribonuclease. Proc Natl Acad Sci U S A. 1968 Oct;61(2):406–413. doi: 10.1073/pnas.61.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett F., Raftery M. A. An NMR method for characterizing conformation changes in proteins. Biochem Biophys Res Commun. 1972 May 12;47(3):625–632. doi: 10.1016/0006-291x(72)90924-2. [DOI] [PubMed] [Google Scholar]
- Parsons S. M., Raftery M. A. Ionization behavior of the catalytic carboxyls of lysozyme. Effects of ionic strength. Biochemistry. 1972 Apr 25;11(9):1623–1629. doi: 10.1021/bi00759a013. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Pulsinelli P., Eyck L. T., Kilmartin J. V., Shibata S., Iuchi I., Miyaji T., Hamilton H. B. Haemoglobin Hiroshima and the mechanism of the alkaline Bohr effect. Nat New Biol. 1971 Aug 4;232(31):147–149. doi: 10.1038/newbio232147a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Tyuma I., Shimizu K. Effect of organic phosphates on the difference in oxygen affinity between fetal and adult human hemoglobin. Fed Proc. 1970 May-Jun;29(3):1112–1114. [PubMed] [Google Scholar]
- de Bruin S. H., Janssen L. H., van Os G. A. Effect of 2,3-diphosphoglycerate on the Bohr effect of human adult hemoglobin. Biochem Biophys Res Commun. 1971 Oct 15;45(2):544–550. doi: 10.1016/0006-291x(71)90854-0. [DOI] [PubMed] [Google Scholar]


