Abstract
Purpose
Long-standing diabetes is a risk factor for pancreatic cancer, and recent-onset diabetes in the several years before diagnosis is a consequence of subclinical pancreatic malignancy. However, the impact of diabetes on survival is largely unknown.
Patients and Methods
We analyzed survival by diabetes status among 1,006 patients diagnosed from 1986 to 2010 from two prospective cohort studies: the Nurses' Health Study (NHS) and Health Professionals Follow-Up Study (HPFS). We validated our results among 386 patients diagnosed from 2004 to 2013 from a clinic-based case series at Dana-Farber Cancer Institute (DFCI). We estimated hazard ratios (HRs) for death using Cox proportional hazards models, with adjustment for age, sex, race/ethnicity, smoking, diagnosis year, and cancer stage.
Results
In NHS and HPFS, HR for death was 1.40 (95% CI, 1.15 to 1.69) for patients with long-term diabetes (> 4 years) compared with those without diabetes (P < .001), with median survival times of 3 months for long-term diabetics and 5 months for nondiabetics. Adjustment for a propensity score to reduce confounding by comorbidities did not change the results. Among DFCI patient cases, HR for death was 1.53 (95% CI, 1.07 to 2.20) for those with long-term diabetes compared with those without diabetes (P = .02), with median survival times of 9 months for long-term diabetics and 13 months for nondiabetics. Compared with nondiabetics, survival times were shorter for long-term diabetics who used oral hypoglycemics or insulin. We observed no statistically significant association of recent-onset diabetes (< 4 years) with survival.
Conclusion
Long-standing diabetes was associated with statistically significantly decreased survival among patients with pancreatic cancer enrolled onto three longitudinal studies.
INTRODUCTION
Pancreatic cancer is the fourth-leading cause of cancer-related death in the United States, and most patients survive < 12 months after diagnosis.1 Aside from disease stage, few prognostic factors have been well characterized.2 Individuals with diabetes are known to be at increased risk of developing of pancreatic cancer,3 but few studies have assessed diabetes and survival among patients with pancreatic cancer.
The relationship between diabetes and pancreatic cancer is complex. Numerous studies have demonstrated an increased risk of pancreatic cancer among individuals with long-standing diabetes, generally classified as diabetes for > 3 to 5 years.3–7 On the basis of laboratory and epidemiologic studies, the chronic metabolic and inflammatory consequences of diabetes are thought to promote cancer initiation and early progression.8,9 However, studies have also indicated that 20% to 30% of patients with pancreatic cancer develop diabetes in the 2 to 4 years before cancer diagnosis.10–12 Hyperglycemia in this setting is the result of intact, but not yet clinically diagnosed, pancreatic cancer and has been classified as secondary or type 3c diabetes.12,13 In these patients, pancreatic tumors are thought to secrete factors that cause paraneoplastic hyperglycemia, and recent-onset diabetes will resolve in some patients after resection of the pancreatic tumor.14–16 Given the different pathophysiology of long-standing and recent-onset diabetes in patients with pancreatic cancer, we sought to evaluate their association with survival in two large cohort studies, in which dates of diagnosis for diabetes and pancreatic cancer were collected prospectively. To validate our findings in a third independent data set, we examined survival in patients from a prospective clinic-based study of pancreatic cancer.
PATIENTS AND METHODS
Study Populations
We evaluated patients with pancreatic cancer enrolled onto two prospective cohort studies: the Nurses' Health Study (NHS) and Health Professionals Follow-Up Study (HPFS). NHS was initiated in 1976, when 121,700 female registered nurses age 30 to 55 years returned a mailed questionnaire describing demographics, lifestyle choices, and medical history.17 HPFS began in 1986, when 51,529 men age 40 to 75 years working as dentists, veterinarians, pharmacists, optometrists, osteopathic physicians, or podiatrists returned a mailed questionnaire on health-related behaviors and medical history.18 Participants have updated exposures and medical history through biennial follow-up questionnaires. These studies were approved by the Brigham and Women's Hospital Human Research Committee (Boston, MA), and participants provided informed consent.
We then examined patients from an ongoing clinic-based study at Dana-Farber Cancer Institute (DFCI; Boston, MA). Patients with pancreatic cancer were prospectively recruited by reviewing provider schedules in the GI Cancer Center between November 5, 2004, and July 15, 2013. Eligible patients were age ≥ 21 years with pathologically confirmed pancreatic adenocarcinoma. The study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (Boston, MA), and participants provided informed consent.
Patient Cases of Pancreatic Cancer
In NHS and HPFS, 1,006 patient cases of pancreatic cancer were diagnosed after return of the 1986 questionnaire through May 31, 2010, in NHS or January 31, 2010, in HPFS. Patient cases were identified by participant self-report or death follow-up. Deaths were ascertained from next of kin or the postal service and by searching the National Death Index.19 Diagnoses were confirmed by review of medical records, death certificates, and/or tumor registry data by study physicians blinded to exposure data. Patients with nonadenocarcinoma histology were excluded.
At DFCI, 1,038 patients were approached for consent, and 743 (72%) agreed to participate. Of consenting patients, 485 completed the structured questionnaire. Characteristics were similar between patients who did and did not complete the structured questionnaire, including age at diagnosis (63.6 v 64.1 years), sex (47.4% v 48.8% female), race/ethnicity (93.6% v 92.2% white), and stage at diagnosis (60.0% v 55.4% with metastatic disease). Of 485 patients who completed the structured questionnaire, we excluded 88 patients who completed the questionnaire > 6 months after cancer diagnosis, to minimize misclassification of diabetes duration, and 11 patients with unknown diabetes status or duration.
Assessment of Diabetes Exposure
In HPFS and NHS, diabetes status was determined from patient report on biennial questionnaires. A supplementary questionnaire was used to verify the diagnosis, as described previously.20,21 Of 181 patients with type 2 diabetes included in the current analysis, 132 (73%) returned the supplementary questionnaire allowing confirmation. On the basis of prior studies of pancreatic cancer,10–12 long-term diabetes was defined as diabetes for > 4 years before pancreatic cancer diagnosis, whereas short-term diabetes was defined as diabetes ≤ 4 years before cancer diagnosis. In sensitivity analyses, results were largely unchanged using 3 or 5 years duration to define long- and short-term diabetes.
In the DFCI study, a structured questionnaire was used to collect demographic information and risk factors. For patients who indicated a diabetes history, diabetes duration was assessed by the question: “How many years ago were you diagnosed with diabetes or high blood sugar?” Answer options were 0 to 1, 2 to 4, 5 to 9, 10 to 14, or ≥ 15 years, and for type of diabetes treatment, options were medications by mouth or insulin injections. As in prior studies10–12 and the HPFS and NHS cohorts, patient cases were grouped as long term for those with diabetes for > 4 years and short term for those with diabetes ≤ 4 years.
Assessment of Covariates
In NHS and HPFS, covariate data were obtained from questionnaires. Cancer diagnosis date and stage were obtained from medical record review. Cancer stage was classified as: local disease amenable to surgical resection, locally advanced disease that was unresectable but without metastases, or distant metastatic disease. In the DFCI study, the structured questionnaire provided covariate data, including age, race/ethnicity, smoking status, and body-mass index (BMI) 6 months before clinic visit. Cancer diagnosis date and stage were obtained from medical records. Cancer stage was classified similarly to the prospective cohorts.
Statistical Analysis
We examined the association of long- and short-term diabetes with survival using Cox proportional hazards regression to calculate hazard ratios (HRs) and 95% CIs for mortality. Survival was calculated from cancer diagnosis to death or last follow-up. Last follow-up was May 31, 2010, in NHS; January 31, 2010, in HPFS; and July 23, 2013, in the DFCI study. Proportionality of hazards assumption was satisfied by evaluating a time-dependent variable, which was the cross product of diabetes and time (all P ≥ .15).
In multivariable models, we adjusted for potential confounders, including age at diagnosis, sex, race/ethnicity, smoking status, year of diagnosis, and cancer stage. We noted similar results in a sensitivity analysis in which we removed NHS and HPFS patient cases with unknown stage; no DFCI patient cases had unknown stage. Subsequently, we adjusted for BMI, which may lie along the causal pathway of diabetes and survival. To consider overall comorbidity, we adjusted for a continuous propensity score in NHS and HPFS derived by regressing diabetes on comorbidities and lifestyle factors with the potential to limit survival,22 including physical activity, alcohol intake, calorie intake, and history of high cholesterol, stroke, hypertension, or heart disease (eg, angina pectoris, coronary bypass/angioplasty/stent, myocardial infarction). To combine results, we conducted a pooled analysis by calculating an HR for each study and computing a summary HR using the DerSimonian and Laird random effects model.23 Heterogeneity was tested using Cochran's Q statistic.24
We performed stratified analyses among cohort patients and assessed statistical interaction by entering main effect terms and a cross-product term of diabetes and stratification variable into the model, evaluating likelihood ratio tests. Survival curves were investigated using the Kaplan-Meier method and log-rank test. Statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC), and all P values are two sided.
RESULTS
Characteristics of patients with pancreatic cancer are listed in Table 1. Among NHS and HPFS patients with known stage, 20.0% had localized disease, 15.6% had locally advanced disease, and 64.4% had metastatic disease. Among DFCI patients, 15.3% had localized disease, 17.9% had locally advanced disease, and 66.8% had metastatic disease. In NHS and HPFS, median survival times were 16 months for patients with localized, 8 months for those with locally advanced, and 3 months for those with metastatic disease, closely mirroring survival times in the general US population for patients with pancreatic cancer.25 As commonly noted at large tertiary cancer centers, DFCI patients had longer median survival times, consistent with the self-selecting populations seeking care at these centers. Median survival times were 29 months for patients with localized, 18 months for those with locally advanced, and 10 months for those with metastatic disease. By end of follow-up, 971 (96.5%) and 268 patients (69.4%) had died in NHS/HPFS and the DFCI study, respectively. Characteristics of patients with pancreatic cancer by diabetes status are listed in Appendix Table A1 (online only).
Table 1.
Demographic and Clinical Characteristics of Patients With Pancreatic Cancer by Study
| Characteristic | NHS (n = 635) |
HPFS (n = 371) |
DFCI (n = 386) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age at diagnosis, years | ||||||
| Mean | 70.8 | 72.3 | 64.2 | |||
| SD | 7.9 | 9.0 | 10.8 | |||
| Female sex | 635 | 100.0 | 0 | 0.0 | 184 | 47.7 |
| Race/ethnicity | ||||||
| White | 620 | 97.6 | 334 | 90.0 | 359 | 93.0 |
| Black | 7 | 1.1 | 7 | 1.9 | 4 | 1.0 |
| Other | 8 | 1.3 | 12 | 3.2 | 6 | 1.6 |
| Unknown | 0 | 0.0 | 18 | 4.9 | 17 | 4.4 |
| BMI, kg/m2 | ||||||
| Mean | 26.6 | 25.4 | 29.3 | |||
| SD | 5.6 | 3.2 | 7.5 | |||
| Physical activity, MET-hr/wk | ||||||
| Mean | 16.8 | 30.3 | 13.8 | |||
| SD | 21.1 | 34.5 | 24.6 | |||
| Smoking status | ||||||
| Never | 258 | 40.6 | 144 | 38.8 | 131 | 33.9 |
| Past | 267 | 42.0 | 180 | 48.5 | 177 | 45.9 |
| Current | 100 | 15.7 | 37 | 10.0 | 37 | 9.6 |
| Missing | 10 | 1.6 | 10 | 2.7 | 41 | 10.6 |
| Cancer stage | ||||||
| Localized | 83 | 13.1 | 66 | 17.8 | 59 | 15.3 |
| Locally advanced | 59 | 9.3 | 57 | 15.4 | 69 | 17.9 |
| Metastatic | 300 | 47.2 | 180 | 48.5 | 258 | 66.8 |
| Unknown | 193 | 30.4 | 68 | 18.3 | 0 | 0.0 |
| Diagnosis period | ||||||
| 1986-2000 | 281 | 44.3 | 227 | 61.2 | 0 | 0.0 |
| 2001-2013 | 354 | 55.7 | 144 | 38.8 | 386 | 100.0 |
| Median survival time by stage, months | ||||||
| Overall | 5 | 5 | 12 | |||
| Localized | 16 | 16 | 29 | |||
| Locally advanced | 9 | 7 | 18 | |||
| Metastatic | 3 | 3 | 10 | |||
Abbreviations: BMI, body-mass index; DFCI, Dana-Farber Cancer Institute; HPFS, Health Professionals Follow-Up Study; MET, metabolic equivalent; NHS, Nurses' Health Study; SD, standard deviation.
Patients with pancreatic cancer with long-term diabetes had reduced survival compared with those without diabetes (Table 2). In NHS and HPFS, median survival was 5 months for nondiabetics and 3 months for long-term diabetics. In multivariable-adjusted Cox regression models, the HR for death was 1.40 (95% CI, 1.15 to 1.69; P < .001), comparing patients with long-term diabetes with those without diabetes. The HR was not materially changed after further adjustment by BMI (HR, 1.38; 95% CI, 1.14 to 1.68; P = .001). We considered whether patients with diabetes had a greater degree of comorbid illness, which might have led to worse survival. After adjustment for a propensity score to account for differences in comorbidity, our results remained unchanged (HR, 1.46; 95% CI, 1.17 to 1.80; P < .001). In a sensitivity analysis involving only patients with confirmed diabetes status, our results also remained unchanged. Although data are conflicting, it has been suggested that diabetes may increase perioperative morbidity and mortality among patients undergoing pancreatectomy.26,27 Only patients with localized disease undergo pancreatectomy, and after exclusion of patients with localized disease, our results were not materially altered (HR, 1.47; 95% CI, 1.15 to 1.87; P = .002). In contrast to the results for long-term diabetes, short-term diabetes was not associated with survival among patients from NHS and HPFS (Table 2).
Table 2.
HRs for Death by Diabetes Status at Diagnosis Among Patients With Pancreatic Cancer
| Cohort | No Diabetes HR | Short Term (≤ 4 years) |
Long Term (> 4 years) |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| NHS/HPFS | |||||||
| Person-months | 8,544 | 441 | 873 | ||||
| No. of patient cases | 825 | 47 | 134 | ||||
| No. of deaths | 793 | 47 | 131 | ||||
| Median OS, months | 5 | 6 | 3 | ||||
| Age adjusted | 1.0 | 1.00 | 0.74 to 1.35 | 1.00 | 1.41 | 1.14 to 1.75 | .002 |
| Multivariable adjusted* | 1.0 | 1.01 | 0.75 to 1.36 | .96 | 1.43 | 1.18 to 1.74 | < .001 |
| Multivariable adjusted† | 1.0 | 1.03 | 0.76 to 1.39 | .84 | 1.40 | 1.15 to 1.69 | < .001 |
| DFCI | |||||||
| Person-months | 4,185 | 995 | 508 | ||||
| No. of patient cases | 265 | 72 | 49 | ||||
| No. of deaths | 179 | 51 | 38 | ||||
| Median OS, months | 13 | 11 | 9 | ||||
| Age adjusted | 1.0 | 1.19 | 0.87 to 1.63 | .27 | 1.44 | 1.01 to 2.07 | .04 |
| Multivariable adjusted* | 1.0 | 1.22 | 0.89 to 1.67 | .22 | 1.56 | 1.08 to 2.23 | .02 |
| Multivariable adjusted† | 1.0 | 1.22 | 0.88 to 1.68 | .23 | 1.53 | 1.07 to 2.20 | .02 |
Abbreviations: DFCI, Dana-Farber Cancer Institute; HPFS, Health Professionals Follow-Up Study; HR, hazard ratio; NHS, Nurses' Health Study; OS, overall survival.
Adjusted for age at diagnosis (continuous), sex (male or female), race/ethnicity (white, black, other, or unknown), smoking status (never, past, current, or missing), and year of diagnosis (continuous).
Further adjusted for stage at diagnosis (localized, locally advanced, metastatic, or unknown).
We next sought to confirm these results in an independent patient population. Among patients in the DFCI case series, long-term diabetes was similarly associated with reduced survival (Table 2). Median survival was 13 months for nondiabetics and 9 months for long-term diabetics. In the multivariable-adjusted model, the HR for death was 1.53 (95% CI, 1.07 to 2.20; P = .02), comparing patients with long-term diabetes with those without diabetes. These results were unchanged after further adjustment for BMI and after excluding patients with localized disease. Short-term diabetes was not associated with survival (Table 2).
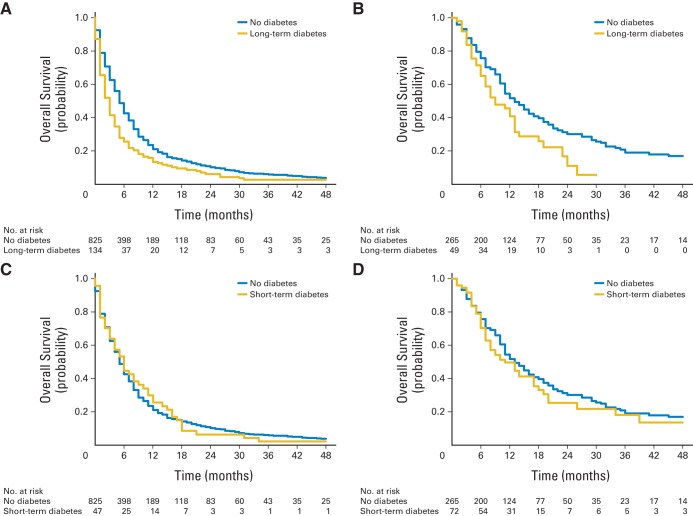
Kaplan-Meier curves comparing survival by diabetes status are shown in Figure 1. In NHS and HPFS, the 6-, 12-, and 24-month survival rates were 25.6%, 13.4%, and 6.0% among long-term diabetics and 42.7%, 20.9%, and 10.1% among nondiabetics, respectively (log-rank P < .001). Among DFCI patients, the 6-, 12-, and 24-month survival rates were 65.1%, 40.8%, and 11.1% among long-term diabetics and 75.7%, 51.7%, and 30.0% among nondiabetics, respectively (log-rank P = .01). The HRs for death were similar across the three patient populations (Fig 2). To examine extremes of survival, we divided patient cases into quintiles by survival time and noted a higher proportion of long-term diabetics in the bottom 20% of survival time in both NHS/HPFS and the DFCI study (Appendix Table A2, online only).
Fig 1.
Kaplan-Meier curves of overall survival by (A, B) long- and (C, D) short-term diabetes status at diagnosis among patients with pancreatic cancer from (A, C) Nurses' Health Study and Health Professionals Follow-up Study and (B, D) Dana-Farber Cancer Institute.
Fig 2.
Forest plot and meta-analysis of hazard ratios (HRs) for death among patients with pancreatic cancer by (A) long- and (B) short-term diabetes status at diagnosis from Nurses' Health Study, Health Professionals Follow-up Study, and Dana-Farber Cancer Institute. Solid squares and horizontal lines correspond to study-specific multivariable-adjusted HRs and 95% CIs; area of solid square reflects cohort-specific weight (inverse of variance). Diamonds represent meta-analysis multivariable-adjusted HRs and 95% CIs. Vertical line indicates odds ratio of 1.0. HRs adjusted for age at diagnosis (continuous), sex (male or female), race/ethnicity (white, black, other, or unknown), smoking status (never, past, current, or missing), year of diagnosis (continuous), and stage at diagnosis (localized, locally advanced, metastatic, or unknown).
Although somewhat limited by sample size, we examined survival among long-term diabetics by diabetes treatment (Table 3). Compared with nondiabetics, long-term diabetics receiving oral hypoglycemics or insulin seemed to have reduced survival. Among patients with long-term diabetes, similar HRs for death were observed among patients with > 10 and ≤ 10 years exposure to diabetes (Appendix Table A3, online only). Within NHS and HPFS, no statistically significant interactions were seen by sex, smoking status, BMI, or disease stage (all interaction P > .50; Appendix Table A4, online only); sample size was limited for stratified analyses in the DFCI study.
Table 3.
HRs for Death by Diabetes Treatment Among Patients With Pancreatic Cancer With Long-Term Diabetes
| Cohort | No Diabetes HR | Oral Medication |
Insulin Injection |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| NHS/HPFS | |||||||
| Person-months | 8,544 | 235 | 42 | ||||
| No. of patient cases | 825 | 43 | 13 | ||||
| No. of deaths | 793 | 41 | 13 | ||||
| Median OS, months | 5 | 3 | 1 | ||||
| Age adjusted | 1.0 | 1.45 | 1.06 to 1.99 | .02 | 2.10 | 1.21 to 3.66 | .009 |
| Multivariable adjusted* | 1.0 | 1.51 | 1.10 to 2.08 | .01 | 2.10 | 1.20 to 3.68 | .009 |
| Multivariable adjusted† | 1.0 | 1.71 | 1.24 to 2.36 | .001 | 1.60 | 0.91 to 2.79 | .10 |
| DFCI | |||||||
| Person-months | 4,185 | 225 | 241 | ||||
| No. of patient cases | 265 | 19 | 26 | ||||
| No. of deaths | 179 | 16 | 19 | ||||
| Median OS, months | 13 | 12 | 8 | ||||
| Age adjusted | 1.0 | 1.37 | 0.81 to 2.31 | .24 | 1.53 | 0.94 to 2.48 | .08 |
| Multivariable adjusted* | 1.0 | 1.48 | 0.87 to 2.51 | .15 | 1.72 | 1.05 to 2.80 | .03 |
| Multivariable adjusted† | 1.0 | 1.38 | 0.81 to 2.34 | .24 | 1.79 | 1.10 to 2.94 | .02 |
Abbreviations: DFCI, Dana-Farber Cancer Institute; HPFS, Health Professionals Follow-Up Study; HR, hazard ratio; NHS, Nurses' Health Study; OS, overall survival.
Adjusted for age at diagnosis (continuous), sex (male or female), race/ethnicity (white, black, other, or unknown), smoking status (never, past, current, or missing), and year of diagnosis (continuous).
Further adjusted for stage at diagnosis (localized, locally advanced, metastatic, or unknown).
DISCUSSION
In this study of three patient populations drawn from two prospective cohorts and a clinic-based case series, we identified a consistent 40% to 50% relative reduction in survival time comparing long-term diabetics with nondiabetics, translating to a 2- to 4-month shorter median survival. This reduction in survival remained statistically significant after several sensitivity analyses and adjustment for potential confounding factors, including a propensity score that provided an aggregate measure of comorbid illness. Reduced survival was noted among diabetics receiving oral hypoglycemic medications and insulin, compared with nondiabetics. In contrast, we did not observe differences in survival for patients with recent-onset diabetes compared with those without diabetes. Taken together, these data implicate long-standing diabetes as a prognostic factor in pancreatic cancer.
Studies have demonstrated an increased risk of dying as a result of pancreatic cancer among diabetics or those with increased serum glucose, compared with those without diabetes or with lower serum glucose.5,6,28,29 However, the long-term survival rate for patients with pancreatic cancer is < 5%,2 such that nearly all patients with incident pancreatic cancer also die as a result of the disease. Therefore, identifying an increased risk of dying as a result of pancreatic cancer provides similar information to analyses of incident disease and does not inform length of survival after diagnosis. Few studies have evaluated survival time after diagnosis by diabetes status, and much of the available data pertain only to patients who underwent pancreatectomy, a small subset that comprises 15% to 20% of patients diagnosed with pancreatic cancer. With these limitations, studies evaluating duration of diabetes and survival are conflicting. In a retrospective cohort study of 3,147 patients in the Health Improvement Network,30 a primary care medical record database in the United Kingdom, modest increases in mortality were noted for patients with pancreatic cancer and diabetes for 3 to 5 years (HR, 1.23; 95% CI, 0.98 to 1.54) and > 5 years (HR, 1.16; 95% CI, 1.00 to 1.33), compared with patients without diabetes. No increase in mortality was seen for patients with pancreatic cancer and diabetes < 3 years in duration. Among 209 patients who underwent pancreatectomy at Emory University (Atlanta, GA),31 survival seemed reduced among patients with new-onset diabetes (< 2 years duration; HR, 1.75; 95% CI, 1.10 to 2.78) and longer-standing diabetes (≥ 2 years; HR, 1.30; 95% CI, 0.75 to 2.25). In a study of 475 patients from Memorial Sloan-Kettering Cancer Center (New York, NY),32 long-standing diabetes (≥ 3 years) was not associated with survival among patients with pancreatic cancer.
We observed a reduction in survival among patients with long-standing diabetes that was consistent across three separate patient populations drawn from studies with different designs: two prospective cohort studies and a clinic-based case series. An important strength of the prospective cohort design is its ability to fully capture the spectrum of patients with pancreatic cancer in terms of disease aggressiveness and stage of disease, because individuals are enrolled before their diagnosis and are not identified at selected tertiary care centers. Notably, survival times and stage distribution for patients in NHS and HPFS were highly similar to 121,713 patients included in the National Cancer Data Base, which is thought to capture 76% of pancreatic cancer patient cases diagnosed in the United States each year.25 Cohort studies also prospectively collect data on exposures, including diabetes and other factors that may affect survival. Thus, these studies limit issues with misclassification and recall bias and allow for rigorous adjustment for comorbidities.
After noting a reduction in survival for patients with long-standing diabetes in two prospective cohorts, we confirmed our results in a separate clinic-based study. As expected from prior studies performed at tertiary care centers,32 median survival was longer stage for stage among patients from DFCI compared with the prospective cohorts or general US population.25 Tertiary centers tend to attract patients who are younger and healthier and present with earlier-stage disease, leading to longer overall survival times. Nevertheless, among the DFCI patient population, the relative reduction in survival for patients with long-standing diabetes was highly similar to that seen in the prospective cohorts, translating into a 4-month reduction in median survival. Of note, two multiagent chemotherapy regimens were recently incorporated into treatment for patients with metastatic pancreatic cancer.33,34 These two chemotherapy regimens improved median overall survival by 1.8 (gemcitabine plus nab-paclitaxel34) and 4.3 months (FOLFIRINOX [folinic acid, fluorouracil, irinotecan, and oxaliplatin]33) in comparison with single-agent gemcitabine.
Multiple metabolic and inflammatory changes related to glucose intolerance have enhanced pancreatic cancer development in preclinical models.9,35–38 Furthermore, experimental data indicate that pancreatic tumors develop and metastasize over years, providing opportunities for chronic alterations in systemic metabolism to influence malignant characteristics and growth.39,40 Our results suggest that the chronic consequences of diabetes affect survival after diagnosis of pancreatic cancer. A potential explanation for this finding is that the milieu of chronic glucose intolerance has an impact on the genetic architecture of the developing tumor, rendering it more aggressive and leading to shorter patient survival. In this regard, recent studies have suggested differing patient survival in molecularly defined subgroups of pancreatic cancer.41–43 However, long-term diabetes also leads to a number of non–malignancy-associated complications, such as heart and kidney disease, which might influence the intensity of administered therapies and patient survival. To improve survival among patients with long-term diabetes, future studies should examine tumor-specific and host factors that may be amenable to therapeutic intervention. Our results also suggest that clinical trials including patients with pancreatic cancer should account for diabetes status and duration across treatment arms.
Limitations of our study also require consideration. Among study participants, treatment programs may have varied by diabetes status, and we could not control for differences in treatment, because we did not systematically collect this information across the studies. Nevertheless, chemotherapy and radiotherapy options are limited and have had only a modest impact on patient survival.2 Furthermore, our results remained unchanged after exclusion of patients with localized disease, thus limiting the potential of increased perioperative morbidity and mortality among diabetics to explain our results. We used overall mortality in our analyses, as opposed to pancreatic cancer–specific mortality. However, < 5% of patients with pancreatic cancer are cured of their disease, such that almost all patients die as a result of their cancer rather than other causes. We cannot rule out that our findings may have been influenced in part by residual confounding. Nonetheless, we considered multiple possible confounding covariates and adjusted for a propensity score of comorbid illness without change in our results. Finally, our study participants were predominantly of European descent, and additional studies in other populations are warranted.
In conclusion, long-term diabetes was associated with statistically significantly decreased survival among patients with pancreatic cancer in two large prospective cohort studies and one clinic-based study. In contrast, short-term diabetes was not associated with survival in our three patient populations. These data demonstrate a link between chronic glucose intolerance and pancreatic cancer survival, while suggesting that recent-onset diabetes does not portend a worse prognosis among patients with pancreatic adenocarcinoma.
Acknowledgment
We thank the participants and staff of the Health Professionals Follow-up Study, Nurses' Health Study, and Dana-Farber Cancer Institute pancreatic cancer study for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Lousiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Appendix
Table A1.
Demographic and Clinical Characteristics of Patients With Pancreatic Cancer by Diabetes Status at Diagnosis
| Characteristic | NHS and HPFS |
DFCI |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Diabetes (n = 825) |
Short Term (≤ 4 years; n = 47) |
Long Term (> 4 years; n = 134) |
No Diabetes (n = 265) |
Short Term (≤ 4 years; n = 72) |
Long Term (> 4 years; n = 49) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age at diagnosis, years | ||||||||||||
| Mean | 70.8 | 71.9 | 74.6 | 63.2 | 65.0 | 68.0 | ||||||
| SD | 8.4 | 7.9 | 7.0 | 11.2 | 10.1 | 9.1 | ||||||
| Female sex | 520 | 63.0 | 33 | 70.2 | 82 | 61.2 | 138 | 52.1 | 29 | 40.3 | 17 | 34.7 |
| Race/ethnicity | ||||||||||||
| White | 781 | 94.7 | 46 | 97.9 | 127 | 94.8 | 248 | 93.6 | 65 | 90.3 | 46 | 93.9 |
| Black | 13 | 1.6 | 0 | 0.0 | 1 | 0.7 | 2 | 0.8 | 2 | 2.8 | 0 | 0.0 |
| Other | 16 | 1.9 | 0 | 0.0 | 4 | 3.0 | 4 | 1.5 | 1 | 1.4 | 1 | 2.0 |
| Unknown | 15 | 1.8 | 1 | 2.1 | 2 | 1.5 | 11 | 4.2 | 4 | 5.6 | 2 | 4.1 |
| BMI, kg/m2 | ||||||||||||
| Mean | 25.7 | 27.3 | 28.1 | 28.4 | 30.8 | 31.8 | ||||||
| SD | 4.6 | 5.3 | 5.5 | 7.8 | 5.7 | 7.5 | ||||||
| Physical activity, MET-hr/wk | ||||||||||||
| Mean | 22.8 | 23.3 | 16.6 | 15.7 | 8.4 | 11.5 | ||||||
| SD | 27.9 | 33.3 | 25.1 | 27.1 | 13.3 | 22.4 | ||||||
| Smoking status | ||||||||||||
| Never | 330 | 40.0 | 20 | 42.6 | 52 | 38.8 | 93 | 35.1 | 22 | 30.6 | 16 | 32.7 |
| Past | 367 | 44.5 | 22 | 46.8 | 58 | 43.3 | 117 | 44.2 | 35 | 48.6 | 25 | 51.0 |
| Current | 108 | 13.1 | 5 | 10.6 | 24 | 17.9 | 24 | 9.1 | 9 | 12.5 | 4 | 8.2 |
| Missing | 20 | 2.4 | 0 | 0.0 | 0 | 0.0 | 31 | 11.7 | 6 | 8.3 | 4 | 8.2 |
| Cancer stage | ||||||||||||
| Localized | 127 | 15.4 | 7 | 14.9 | 15 | 11.2 | 46 | 17.4 | 6 | 8.3 | 7 | 14.3 |
| Locally advanced | 100 | 12.1 | 7 | 14.9 | 9 | 6.7 | 43 | 16.2 | 20 | 27.8 | 6 | 12.2 |
| Metastatic | 380 | 46.1 | 21 | 44.7 | 79 | 59.0 | 176 | 66.4 | 46 | 63.9 | 36 | 73.5 |
| Unknown | 218 | 26.4 | 12 | 25.5 | 31 | 23.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Diagnosis period | ||||||||||||
| 1986-2000 | 437 | 53.0 | 23 | 48.9 | 48 | 35.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 2001-2013 | 388 | 47.0 | 24 | 51.1 | 86 | 64.2 | 265 | 100.0 | 72 | 100.0 | 49 | 100.0 |
Abbreviations: BMI, body-mass index; DFCI, Dana-Farber Cancer Institute; HPFS, Health Professionals Follow-Up Study; MET, metabolic equivalent; NHS, Nurses' Health Study; SD, standard deviation.
Table A2.
No. and Proportion of Patients With Long-Term Diabetes by Quintile of Survival Time
| Quintile of Survival Time | Range of Survival Time (months) | No. of Patients | Patients With Long-Term Diabetes |
|
|---|---|---|---|---|
| No. | % | |||
| NHS/HPFS | ||||
| 1 | < 2 | 231 | 46 | 19.9 |
| 2 | 2-3 | 172 | 32 | 18.6 |
| 3 | 4-6 | 193 | 21 | 10.9 |
| 4 | 7-12 | 201 | 16 | 8.0 |
| 5 | > 12 | 197 | 17 | 8.6 |
| DFCI | ||||
| 1 | < 5 | 67 | 12 | 17.9 |
| 2 | 5-7 | 55 | 8 | 14.5 |
| 3 | 8-12 | 60 | 8 | 13.3 |
| 4 | 13-23 | 60 | 8 | 13.3 |
| 5 | > 23 | 60 | 3 | 5.0 |
Abbreviations: DFCI, Dana-Farber Cancer Institute; HPFS, Health Professionals Follow-Up Study; NHS, Nurses' Health Study.
Table A3.
HRs for Death by Time Interval of Diabetes Among Patients With Pancreatic Cancer With Long-Term Diabetes
| Cohort | No DM | > 4 to 10 Years |
> 10 Years |
||
|---|---|---|---|---|---|
| HR | HR | 95% CI | HR | 95% CI | |
| NHS/HPFS | |||||
| Person-months | 8,544 | 516 | 357 | ||
| No. of patient cases | 825 | 62 | 72 | ||
| No. of deaths | 793 | 59 | 72 | ||
| Multivariable adjusted* | 1.0 | 1.38 | 1.05 to 1.81 | 1.42 | 1.10 to 1.83 |
| DFCI | |||||
| Person-months | 4,185 | 181 | 327 | ||
| No. of patient cases | 265 | 19 | 30 | ||
| No. of deaths | 179 | 13 | 25 | ||
| Multivariable adjusted* | 1.0 | 1.52 | 0.85 to 2.73 | 1.53 | 0.99 to 2.36 |
Abbreviations: DFCI, Dana-Farber Cancer Institute; HPFS, Health Professionals Follow-Up Study; HR, hazard ratio; NHS, Nurses' Health Study.
Adjusted for age at diagnosis (continuous), sex (male or female), race/ethnicity (white, black, other, or unknown), smoking status (never, past, current, or missing), year of diagnosis (continuous), and stage at diagnosis (localized, locally advanced, metastatic, or unknown).
Table A4.
HRs for Death by Diabetes Status Stratified by Covariates Among Patients With Pancreatic Cancer From NHS and HPFS
| Covariate | No. of Patients | Diabetes Status at Diagnosis* |
P (interaction)† | ||||
|---|---|---|---|---|---|---|---|
| No Diabetes | Short Term (≤ 4 years) |
Long Term (> 4 years) |
|||||
| HR | HR | 95% CI | HR | 95% CI | |||
| Sex | .80 | ||||||
| Female (NHS) | 635 | 1.0 | 0.97 | 0.68 to 1.39 | 1.43 | 1.12 to 1.82 | |
| Male (HPFS) | 371 | 1.0 | 1.19 | 0.69 to 2.07 | 1.35 | 0.99 to 1.85 | |
| Smoking status | .66 | ||||||
| Never | 402 | 1.0 | 1.12 | 0.70 to 1.78 | 1.56 | 1.14 to 2.14 | |
| Ever | 584 | 1.0 | 0.94 | 0.63 to 1.39 | 1.32 | 1.04 to 1.68 | |
| BMI, kg/m2 | .98 | ||||||
| < 25 | 419 | 1.0 | 0.97 | 0.60 to 1.57 | 1.51 | 1.04 to 2.20 | |
| ≥ 25 | 492 | 1.0 | 1.16 | 0.78 to 1.74 | 1.39 | 1.09 to 1.77 | |
| Cancer stage | .54 | ||||||
| Localized | 149 | 1.0 | 1.43 | 0.64 to 3.18 | 1.18 | 0.65 to 2.12 | |
| Locally advanced | 116 | 1.0 | 1.28 | 0.57 to 2.84 | 1.19 | 0.58 to 2.42 | |
| Metastatic | 480 | 1.0 | 1.15 | 0.74 to 1.80 | 1.45 | 1.11 to 1.88 | |
Abbreviations: BMI, body-mass index; HPFS, Health Professionals Follow-Up Study; HR, hazard ratio; NHS, Nurses' Health Study.
Adjusted for age at diagnosis (continuous), sex (male or female), race/ethnicity (white, black, other, or unknown), smoking status (never, past, current, or missing), year of diagnosis (continuous), and stage at diagnosis (localized, locally advanced, metastatic, or unknown), excluding stratification covariate.
P interaction for long-term diabetics compared with nondiabetics.
Footnotes
Support information appears at the end of this article.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Chen Yuan, Brian M. Wolpin
Financial support: Matthew H. Kulke, Charles S. Fuchs, Brian M. Wolpin
Administrative support: Lauren K. Brais
Collection and assembly of data: Chen Yuan, Douglas A. Rubinson, Zhi Rong Qian, Chen Wu, Peter Kraft, Shuji Ogino, Kimmie Ng, Thomas E. Clancy, Richard S. Swanson, Megan J. Gorman, Lauren K. Brais, Tingting Li, Meir J. Stampfer, Frank B. Hu, Edward L. Giovannucci, Matthew H. Kulke, Charles S. Fuchs, Brian M. Wolpin
Data analysis and interpretation: Chen Yuan, Douglas A. Rubinson, Zhi Rong Qian, Chen Wu, Peter Kraft, Ying Bao, Shuji Ogino, Kimmie Ng, Thomas E. Clancy, Richard S. Swanson, Megan J. Gorman, Tingting Li, Meir J. Stampfer, Frank B. Hu, Edward L. Giovannucci, Matthew H. Kulke, Charles S. Fuchs, Brian M. Wolpin
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported by National Cancer Institute (NCI; National Institutes of Health [NIH]) Grants No. P01 CA87969, P01 CA55075, U54 CA155626, P50 CA127003, R01 CA124908, R01 CA49449, and 1UM1 CA167552 for the Nurses' Health Study and Health Professionals Follow-up Study; in part by NCI NIH Grant No. P50 CA127003, the Robert T. and Judith B. Hale Fund for Pancreatic Cancer Research, and the Lustgarten Foundation for the Dana-Farber Cancer Institute; in part by NCI NIH Grant No. K07 CA140790, the American Society of Clinical Oncology Conquer Cancer Foundation, the Howard Hughes Medical Institute, the Lustgarten Foundation, and Promises for Purple (B.M.W.); and in part by NIH/NCI Grant No. K07 CA148894 and the American Society of Clinical Oncology Conquer Cancer Foundation (K.N.).
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Survival Among Patients With Pancreatic Cancer and Long-Standing or Recent-Onset Diabetes Mellitus
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Chen Yuan
No relationship to disclose
Douglas A. Rubinson
No relationship to disclose
Zhi Rong Qian
No relationship to disclose
Chen Wu
No relationship to disclose
Peter Kraft
No relationship to disclose
Ying Bao
No relationship to disclose
Shuji Ogino
No relationship to disclose
Kimmie Ng
Consulting or Advisory Role: Genentech/Roche, McCann Regan Campbell Ward, Havas Life Metro, CBPartners
Research Funding: Genentech/Roche (Inst), Pharmavite (Inst)
Travel, Accommodations, Expenses: Gilead Sciences
Thomas E. Clancy
No relationship to disclose
Richard S. Swanson
No relationship to disclose
Megan J. Gorman
No relationship to disclose
Lauren K. Brais
No relationship to disclose
Tingting Li
No relationship to disclose
Meir J. Stampfer
No relationship to disclose
Frank B. Hu
No relationship to disclose
Edward L. Giovannucci
No relationship to disclose
Matthew H. Kulke
Consulting or Advisory Role: Ipsen Pharmaceuticals, Novartis Pharmaceuticals
Research Funding: Ipsen Pharmeceuticals
Charles S. Fuchs
Consulting or Advisory Role: Amgen, Eli Lilly, sanofi-aventis, Pfizer, Takeda, Acceleron, Momenta Pharm, Bayer, Genentech, Roche, Medimmune, Pharmacyclics, Vertex Pharm, Pozen, Celgene, Karyopharm Thera
Brian M. Wolpin
No relationship to disclose
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Jee SH, Ohrr H, Sull JW, et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 6.Gapstur SM, Gann PH, Lowe W, et al. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 7.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: A consensus report. CA Cancer J Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 9.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 10.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: Prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pannala R, Leibson CL, Rabe KG, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol. 2009;104:2318–2325. doi: 10.1038/ajg.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen DK, Andren-Sandberg A, Duell EJ, et al. Pancreatitis-diabetes-pancreatic cancer: Summary of an NIDDK-NCI workshop. Pancreas. 2013;42:1227–1237. doi: 10.1097/MPA.0b013e3182a9ad9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pannala R, Basu A, Petersen GM, et al. New-onset diabetes: A potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: Chicken or egg? Pancreas. 2011;40:339–351. doi: 10.1097/MPA.0b013e318209e05d. [DOI] [PubMed] [Google Scholar]
- 16.Sah RP, Nagpal SJ, Mukhopadhyay D, et al. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10:423–433. doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colditz GA, Hankinson SE. The Nurses' Health Study: Lifestyle and health among women. Nat Rev Cancer. 2005;5:388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 20.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Leitzmann MF, Stampfer MJ, et al. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 25.Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: Report from the National Cancer Database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 26.Chu CK, Mazo AE, Sarmiento JM, et al. Impact of diabetes mellitus on perioperative outcomes after resection for pancreatic adenocarcinoma. J Am Coll Surg. 2010;210:463–473. doi: 10.1016/j.jamcollsurg.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Nakata B, Ishikawa T, Amano R, et al. Impact of preoperative diabetes mellitus on clinical outcome after pancreatectomy. Int J Surg. 2013;11:757–761. doi: 10.1016/j.ijsu.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Coughlin SS, Calle EE, Teras LR, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 29.Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang A, Narayan V, Yang YX. Type 2 diabetes mellitus and survival in pancreatic adenocarcinoma: A retrospective cohort study. Cancer. 2013;119:404–410. doi: 10.1002/cncr.27731. [DOI] [PubMed] [Google Scholar]
- 31.Chu CK, Mazo AE, Goodman M, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17:502–513. doi: 10.1245/s10434-009-0789-6. [DOI] [PubMed] [Google Scholar]
- 32.Olson SH, Chou JF, Ludwig E, et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int J Cancer. 2010;127:2412–2419. doi: 10.1002/ijc.25240. [DOI] [PubMed] [Google Scholar]
- 33.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 34.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zyromski NJ, Mathur A, Pitt HA, et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146:258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Vansaun MN. Molecular pathways: Adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19:1926–1932. doi: 10.1158/1078-0432.CCR-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gukovsky I, Li N, Todoric J, et al. Inflammation, autophagy, and obesity: Common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199.e4–1209.e4. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: Evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res. 1996;63:310–313. doi: 10.1006/jsre.1996.0266. [DOI] [PubMed] [Google Scholar]
- 39.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haeno H, Gonen M, Davis MB, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–375. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yachida S, White CM, Naito Y, et al. Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long-term survivors. Clin Cancer Res. 2012;18:6339–6347. doi: 10.1158/1078-0432.CCR-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]