Abstract
Purpose
To investigate the prognostic value of BRAF V600E mutation for the recurrence of papillary thyroid cancer (PTC).
Patients and Methods
This was a retrospective multicenter study of the relationship between BRAF V600E mutation and recurrence of PTC in 2,099 patients (1,615 women and 484 men), with a median age of 45 years (interquartile range [IQR], 34 to 58 years) and a median follow-up time of 36 months (IQR, 14 to 75 months).
Results
The overall BRAF V600E mutation prevalence was 48.5% (1,017 of 2,099). PTC recurrence occurred in 20.9% (213 of 1,017) of BRAF V600E mutation–positive and 11.6% (125 of 1,082) of BRAF V600E mutation–negative patients. Recurrence rates were 47.71 (95% CI, 41.72 to 54.57) versus 26.03 (95% CI, 21.85 to 31.02) per 1,000 person-years in BRAF mutation–positive versus –negative patients (P < .001), with a hazard ratio (HR) of 1.82 (95% CI, 1.46 to 2.28), which remained significant in a multivariable model adjusting for patient sex and age at diagnosis, medical center, and various conventional pathologic factors. Significant association between BRAF mutation and PTC recurrence was also found in patients with conventionally low-risk disease stage I or II and micro-PTC and within various subtypes of PTC. For example, in BRAF mutation–positive versus –negative follicular-variant PTC, recurrence occurred in 21.3% (19 of 89) and 7.0% (24 of 342) of patients, respectively, with recurrence rates of 53.84 (95% CI, 34.34 to 84.40) versus 19.47 (95% CI, 13.05 to 29.04) per 1,000 person-years (P < .001) and an HR of 3.20 (95% CI, 1.46 to 7.02) after adjustment for clinicopathologic factors. BRAF mutation was associated with poorer recurrence-free probability in Kaplan-Meier survival analyses in various clinicopathologic categories.
Conclusion
This large multicenter study demonstrates an independent prognostic value of BRAF V600E mutation for PTC recurrence in various clinicopathologic categories.
INTRODUCTION
Papillary thyroid cancer (PTC) is a common endocrine malignancy, which accounts for 80% to 85% of all thyroid cancers, and can be classified into several subtype variants, including the common conventional PTC (CPTC), follicular-variant PTC (FVPTC), and a few uncommon variants.1,2 Although PTC is generally a highly curable disease, disease recurrence is common, and a subgroup of patients die, particularly when disease recurrence occurs.3–5 These patients need to be identified for appropriately more-aggressive treatments to reduce the chance of disease recurrence and progression. Clinical decisions regarding these patients are classically based on clinicopathologic risk criteria, which are often inaccurate, sometimes making the current risk stratification of PTC clinically challenging.
In recent years, prognostic molecular markers have been vigorously sought to improve risk stratification of PTC, among which BRAF V600E mutation has received the widest attention. BRAF V600E is a major oncogenic mutation in PTC, which promotes PTC tumorigenesis by aberrantly activating the MAP kinase pathway.6 Many studies have demonstrated an association of BRAF V600E mutation with aggressive clinicopathologic characteristics of PTC,6–9 showing promise of this mutation as a prognostic molecular marker for PTC. The association of BRAF V600E mutation with PTC recurrence demonstrated in several previous studies has particularly important clinical relevance. However, these studies represented mostly single-institution studies with relatively small series of patients, and the results were sometimes inconsistent. This makes debatable the prognostic value of BRAF V600E mutation in the management of PTC. Also, the important issue of whether the prognostic value of BRAF V600E mutation holds in individual subtype variants of PTC, such as FVPTC, has not been established, because previous studies were mostly performed collectively in all PTC variants, and their sample sizes did not provide sufficient power to stratify by variant. Here, we investigated the role of BRAF V600E mutation in the recurrence of PTC in a large multicenter study with the goal of establishing its prognostic value for PTC recurrence.
PATIENTS AND METHODS
Study Countries and Centers
This study was conducted at 16 medical centers in eight countries, including the Johns Hopkins Medical Institution, University of Pittsburgh Medical Center, Memorial Sloan-Kettering Cancer Center, and Yale University in the United States; medical centers at the University of Pisa, University of Perugia, University of Milan, University of Padua, and University of Bologna in Italy; Kanagawa Cancer Center in Yokohama, Japan; Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology in Poland; medical centers at Griffith University and University of Sydney in Australia; Hospital La Paz Health Research Institute in Spain; the Institute of Endocrinology in Prague, Czech Republic; and the University of Ulsan in South Korea.
Study Patients
The same study patients and institutions from a recent study10 plus additional patients and institutions participated in this study. Briefly, patients were consecutively selected at each center over differing time periods spanning 1978 to 2011. Patients with PTC of all types were selected at all centers, except for Memorial Sloan-Kettering Cancer Center and Kanagawa Cancer Center, where patients with relatively more advanced disease were treated. All patients had been treated for PTC with total thyroidectomy, and therapeutic neck dissection and dissection extents were performed as clinically indicated. Pathologic diagnoses of PTC and variants were made based on WHO criteria and documented in our peer-reviewed publications.11–25 Postoperative treatments included standard thyroid-stimulating hormone suppression at appropriate levels and radioiodine (ie, iodine-131 [131I]) ablation (Appendix Table A1, online only) in patients at all centers, except for Kanagawa Cancer Center, where no 131I treatment was used. PTC recurrence was defined as recurrent or persistent disease per authoritative histologic, cytologic, radiographic, or biochemical criteria.26,27 Local, regional, and distant recurrences were all included. Follow-up time was defined as the time from initial surgical treatment to discovery of PTC recurrence or, in cases of no recurrence, to the most recent clinic visit.
Study Design
This was a retrospective study, as described recently,10 which was approved by the institutional review board of each center, and informed patient consent was obtained where required. Patient consent was waived in some cases after institutional review board review, because the study only involved the use of thyroid tumor tissues and collection of clinicopathologic information. Disease stages of PTC were defined based on the American Joint Committee on Cancer staging system. Genomic DNA isolated from primary PTC tumors was sequenced at exon 15 of the BRAF gene to identify BRAF V600E mutation, as described in our previously published studies.11–25 In all cases, BRAF V600E mutation status was examined after the surgical and radioiodine treatments and had no impact on the selection of treatments for patients. A uniform protocol designed for this study was used at all centers to obtain clinicopathologic information from the medical records. Data from all 16 centers were pooled for the analysis of the relationship between BRAF V600E mutation and recurrence of PTC.
Statistical Analyses
Recurrence rates per person-year were calculated by dividing the number of recurrences by the total follow-up time, and Poisson regression was used to calculate the 95% CIs and compare across BRAF V600E mutation status. Kaplan-Meier survival curves and log-rank tests, censoring patients at the time of last follow-up or 15 years, and Cox proportional hazards regression analysis, censoring patients at the time of last follow-up, were used to compare recurrence by BRAF V600E mutation status. A second proportional hazards regression model adjusted for patient age at diagnosis, sex, and medical center, along with a third model that additionally adjusted for tumor size, extrathyroidal invasion, lymph node metastasis, multifocality, and PTC subtype, was used to examine the independent effect of BRAF V600E mutation. The covariates were tested for the proportional hazards assumption using the assess statement in SAS software (version 9.3; SAS Institute, Cary, NC). The covariate medical center violated the proportional hazards assumption, and consequently, stratified models were used. A sensitivity analysis, excluding patients who did not experience recurrence but were observed for < 3 years, was performed to address concerns of shorter follow-up times at some centers. Synergy indexes (SIs), as described by Hosmer and Lemeshow,28 were calculated to examine the additive interactions of BRAF V600E mutation with classical clinicopathologic risk factors in affecting the recurrence of PTC. All analyses were performed using SAS software (version 9.3). All reported P values were two sided, and significance was set at P < .05.
RESULTS
Patient Demographics
We studied a total of 2,099 patients (1,615 women and 484 men) across the 16 centers, with a median age of 45 years (interquartile range [IQR], 34 to 58 years). Patient age, sex, BRAF V600E mutation status, PTC recurrence, and follow-up time are summarized overall, by medical center, and by country in Table 1. The overall BRAF V600E mutation prevalence was 48.5%, and the overall PTC recurrence was seen in 16.1% of patients, comparable to the literature.6–8 The overall median follow-up time for all patients was 36 months (IQR, 14 to 75 months). The median follow-up time was 35 months (IQR, 15 to 78 months) in the BRAF V600E–positive group and 36 months (IQR, 13 to 72 months) in the BRAF V600E–negative group (P = .37). 131I doses used in the initial treatment of patients were not different between BRAF mutation–positive and –negative groups at most individual centers, but they were higher in BRAF mutation–positive patients at some centers and in the overall analysis of all patients (Appendix Table A1, online only).
Table 1.
Demographic and Clinical Characteristics, BRAF V600E Mutation, Recurrence, and Follow-Up Time by Medical Center and Country
| Location | No. of Patients | Age at Diagnosis (years) |
Male Sex |
BRAF V600E Mutation |
Recurrence, n (%) |
Follow-Up Time (months) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All |
BRAF V600E Positive |
BRAF V600E Negative |
All Patients |
Patients With No Recurrence |
|||||||||||||
| Median | IQR | No. | % | No. | % | No. | % | No. | % | No. | % | Median | IQR | Median | IQR | ||
| Medical Center | |||||||||||||||||
| Johns Hopkins Hospital | 387 | 45 | 35 to 57 | 101 | 26 | 151 | 39 | 53 | 14 | 33 | 22 | 20 | 9 | 12 | 1 to 28 | 11 | 1 to 28 |
| University of Pittsburgh | 169 | 52 | 38 to 63 | 42 | 25 | 101 | 60 | 10 | 6 | 9 | 9 | 1 | 2 | 19 | 11 to 26 | 18 | 10 to 25 |
| Memorial Sloan-Kettering Cancer Center | 135 | 50 | 35 to 63 | 44 | 33 | 64 | 47 | 35 | 26 | 26 | 41 | 9 | 13 | 96 | 1 to 144 | 78 | 1 to 132 |
| Yale University | 18 | 36 | 32 to 49 | 4 | 22 | 8 | 44 | 3 | 17 | 2 | 25 | 1 | 10 | 5 | 1 to 14 | 3 | 1 to 14 |
| University of Pisa | 189 | 38 | 28 to 51 | 47 | 25 | 65 | 34 | 44 | 23 | 22 | 34 | 22 | 18 | 72 | 16 to 180 | 132 | 48 to 192 |
| University of Perugia | 117 | 49 | 37 to 59 | 32 | 27 | 76 | 65 | 23 | 20 | 12 | 16 | 11 | 27 | 21 | 6 to 39 | 18 | 5 to 40 |
| University of Milan | 110 | 42 | 34 to 55 | 24 | 22 | 38 | 35 | 23 | 21 | 7 | 18 | 16 | 22 | 48 | 24 to 64 | 58 | 26 to 70 |
| University of Padua | 135 | 48 | 39 to 57 | 32 | 24 | 87 | 64 | 17 | 13 | 10 | 12 | 7 | 15 | 26 | 22 to 30 | 26 | 22 to 31 |
| University of Bologna | 35 | 40 | 32 to 52 | 8 | 23 | 20 | 57 | 7 | 20 | 5 | 25 | 2 | 13 | 29 | 15 to 40 | 29 | 22 to 40 |
| Kanagawa Cancer Center | 49 | 55 | 41 to 65 | 16 | 33 | 33 | 67 | 19 | 39 | 15 | 45 | 4 | 25 | 68 | 28 to 75 | 73 | 61 to 78 |
| Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology | 99 | 49 | 33 to 59 | 10 | 10 | 42 | 42 | 4 | 4 | 2 | 5 | 2 | 4 | 48 | 42 to 53 | 48 | 43 to 54 |
| Griffith University | 76 | 40 | 34 to 56 | 20 | 26 | 34 | 45 | 4 | 5 | 3 | 9 | 1 | 2 | 42 | 4 to 82 | 40 | 2 to 79 |
| University of Sydney | 95 | 44 | 34 to 59 | 20 | 21 | 55 | 58 | 21 | 22 | 11 | 20 | 10 | 25 | 103 | 63 to 135 | 114 | 74 to 150 |
| Hospital La Paz Health Research Institute | 66 | 42 | 32 to 54 | 11 | 17 | 28 | 42 | 13 | 20 | 9 | 32 | 4 | 10 | 41 | 30 to 57 | 45 | 30 to 57 |
| Institute of Endocrinology, Prague | 222 | 47 | 31 to 60 | 39 | 18 | 71 | 32 | 22 | 10 | 12 | 17 | 10 | 7 | 50 | 29 to 85 | 50 | 30 to 84 |
| University of Ulsan | 197 | 43 | 35 to 52 | 34 | 17 | 144 | 73 | 40 | 20 | 35 | 24 | 5 | 9 | 105 | 58 to 120 | 109 | 69 to 121 |
| Country | |||||||||||||||||
| United States | 709 | 47 | 36 to 58 | 191 | 27 | 324 | 46 | 101 | 14 | 70 | 22 | 31 | 8 | 16 | 2 to 35 | 15 | 1 to 30 |
| Italy | 586 | 44 | 34 to 55 | 143 | 24 | 286 | 49 | 114 | 19 | 56 | 20 | 58 | 19 | 32 | 18 to 63 | 36 | 23 to 75 |
| Japan | 49 | 55 | 41 to 65 | 16 | 33 | 33 | 67 | 19 | 39 | 15 | 45 | 4 | 25 | 62 | 28 to 75 | 73 | 61 to 78 |
| Poland | 99 | 49 | 33 to 59 | 10 | 10 | 42 | 42 | 4 | 4 | 2 | 5 | 2 | 4 | 48 | 42 to 53 | 48 | 43 to 54 |
| Australia | 171 | 43 | 34 to 57 | 40 | 23 | 89 | 52 | 25 | 15 | 14 | 16 | 11 | 13 | 74 | 32 to 118 | 78 | 35 to 120 |
| Spain | 66 | 42 | 32 to 54 | 11 | 17 | 28 | 42 | 13 | 20 | 9 | 32 | 4 | 10 | 41 | 30 to 57 | 45 | 30 to 57 |
| Czech Republic | 222 | 47 | 31 to 60 | 39 | 18 | 71 | 32 | 22 | 10 | 12 | 17 | 10 | 7 | 50 | 29 to 85 | 50 | 30 to 84 |
| South Korea | 197 | 43 | 35 to 52 | 34 | 17 | 144 | 73 | 40 | 20 | 35 | 24 | 5 | 9 | 105 | 58 to 120 | 109 | 69 to 121 |
| Overall | 2,099 | 45 | 34 to 58 | 484 | 23 | 1,017 | 48 | 338 | 16 | 213 | 21 | 125 | 12 | 36 | 14 to 75 | 37 | 15 to 79 |
Abbreviation: IQR, interquartile range.
Relationship Between BRAF V600E Mutation and Recurrence of PTC
The number of patients and proportion with recurrence, recurrence rates per 1,000 person-years, and hazard ratios (HRs) for all patients with PTC and by subtype are listed in Table 2. For all patients, 20.9% (213 of 1,017) of BRAF mutation–positive patients and 11.6% (125 of 1,082) of BRAF mutation–negative patients experienced recurrence. Recurrence rates were significantly higher for BRAF mutation–positive compared with –negative patients (47.71 v 26.03 per 1,000 person-years), with an unadjusted HR of 1.82 (95% CI, 1.46 to 2.28), which remained significant after adjustment for patient age and sex and stratification by medical center (HR, 1.63; 95% CI, 1.29 to 2.06) and after additional adjustment for tumor size, extrathyroidal invasion, lymph node metastasis, multifocality, and PTC subtype (HR, 1.38; 95% CI, 1.07 to 1.80).
Table 2.
Relationship Between BRAF V600E Mutation and Tumor Recurrence in PTC of Various Subtype Variants
| Type of PTC |
BRAF Mutation |
Tumor Recurrence |
Person-Years of Follow-Up | Recurrence Rates |
Model One* |
Model Two† |
Model Three |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
BRAF V600E Positive |
BRAF V600E Negative |
BRAF V600E Positive |
BRAF V600E Negative |
P§ | |||||||||||||||
| No. | % | No. | % | No. | % | No. | % | Per 1,000 Person-Years | 95% CI | Per 1,000 Person-Years | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| All types | 1,017 of 2,099 | 48.5 | 338 of 2,099 | 16.1 | 213 of 1,017 | 20.9 | 125 of 1,082 | 11.6 | 9,266.1 | 47.71 | 41.72 to 54.57 | 26.03 | 21.85 to 31.02 | < .001 | 1.82 | 1.46 to 2.28 | 1.63 | 1.29 to 2.06 | 1.38 | 1.07 to 1.80 |
| CPTC | 813 of 1,448 | 56.1 | 247 of 1,448 | 17.1 | 168 of 813 | 20.7 | 79 of 635 | 12.4 | 6,822.2 | 44.92 | 38.62 to 52.26 | 25.63 | 20.56 to 31.95 | < .001 | 1.75 | 1.34 to 2.29 | 1.48 | 1.11 to 1.96 | 1.46 | 1.08 to 1.99 |
| FVPTC | 89 of 431 | 20.6 | 43 of 431 | 10.0 | 19 of 89 | 21.3 | 24 of 342 | 7.0 | 1,585.7 | 53.84 | 34.34 to 84.40 | 19.47 | 13.05 to 29.04 | < .001 | 2.76 | 1.51 to 5.06 | 4.02 | 1.95 to 8.28 | 3.20 | 1.46 to 7.02 |
Abbreviations: CPTC, conventional papillary thyroid cancer; FVPTC, follicular-variant papillary thyroid cancer; HR, hazard ratio; PTC, papillary thyroid cancer.
Model one was unadjusted.
Model two was adjusted for patient age and sex and stratified by medical center.
Model three was additionally adjusted for tumor size, extrathyroidal invasion, lymph node metastasis, and multifocality (and PTC subtypes for all-types group).
P values from Poisson regression comparing BRAF mutation–positive and –negative groups.
Restricting the analysis to patients with CPTC (Table 2), BRAF V600E mutation prevalence was 56.1% (813 of 1,448). In CPTC, 20.7% (168 of 813) of BRAF mutation–positive patients and 12.4% (79 of 635) of BRAF mutation–negative patients experienced recurrence. Recurrence rates were significantly higher for BRAF mutation–positive compared with –negative patients (44.92 v 25.63 recurrences per 1,000 person-years), with an unadjusted HR of 1.75 (95% CI, 1.34 to 2.29), which remained significant after adjustment for patient age and sex and stratification by center (HR, 1.48; 95% CI, 1.11 to 1.96) and after additional adjustment for pathologic characteristics (HR, 1.46; 95% CI, 1.08 to 1.99).
Restricting the analysis to patients with FVPTC (Table 2), the BRAF V600E mutation prevalence was 20.6% (89 of 431). In FVPTC, 21.3% (19 of 89) of BRAF mutation–positive patients and 7.0% (24 of 342) of BRAF mutation–negative patients experienced recurrence. Recurrence rates were significantly higher for BRAF mutation–positive compared with –negative patients (53.84 v 19.47 per 1,000 person-years), with an HR of 2.76 (95% CI, 1.51 to 5.06), which increased after adjustment for patient age and sex and stratification by center (HR, 4.02; 95% CI, 1.95 to 8.28) and remained significant after additional adjustment for pathologic characteristics (HR, 3.20; 95% CI, 1.46 to 7.02).
A sensitivity analysis excluding patients who did not experience recurrence but were observed for < 3 years was performed. The resulting person-year rates were slightly higher for both BRAF V600E mutation–positive and –negative patients, but the risk ratios were similar to those reported for the full sample (data not shown).
Kaplan-Meier Analyses of PTC Recurrence-Free Probability
A significant association of BRAF V600E mutation with decreased recurrence-free probability is shown in Kaplan-Meier survival curves for all PTC (Fig 1A), CPTC only (Fig 1B), and FVPTC only (Fig 1C). We also compared the effects of BRAF V600E mutation and several classical clinicopathologic factors (Fig 2). In comparison with patients negative for both BRAF V600E mutation and lymph node metastasis, those with either BRAF mutation or lymph node metastasis had a lower recurrence-free probability, and the probability was further reduced with coexisting mutation and lymph node metastasis (Fig 2A). Similarly, in comparison with patients negative for both BRAF mutation and extrathyroidal invasion, presence of either BRAF mutation or extrathyroidal invasion was significantly associated with a more rapid decline in the recurrence-free probability curve, and the curve declined further with coexisting mutation and extrathyroidal invasion (Fig 2B). Regarding patient age, in comparison with age < 60 years and BRAF mutation negativity, age < 60 years with BRAF mutation or age ≥ 60 years without BRAF mutation was significantly associated with a more rapid decline in the recurrence-free probability curve, and the curve declined further in patients age ≥ 60 years who were BRAF V600E mutation positive (Fig 2C).
Fig 1.
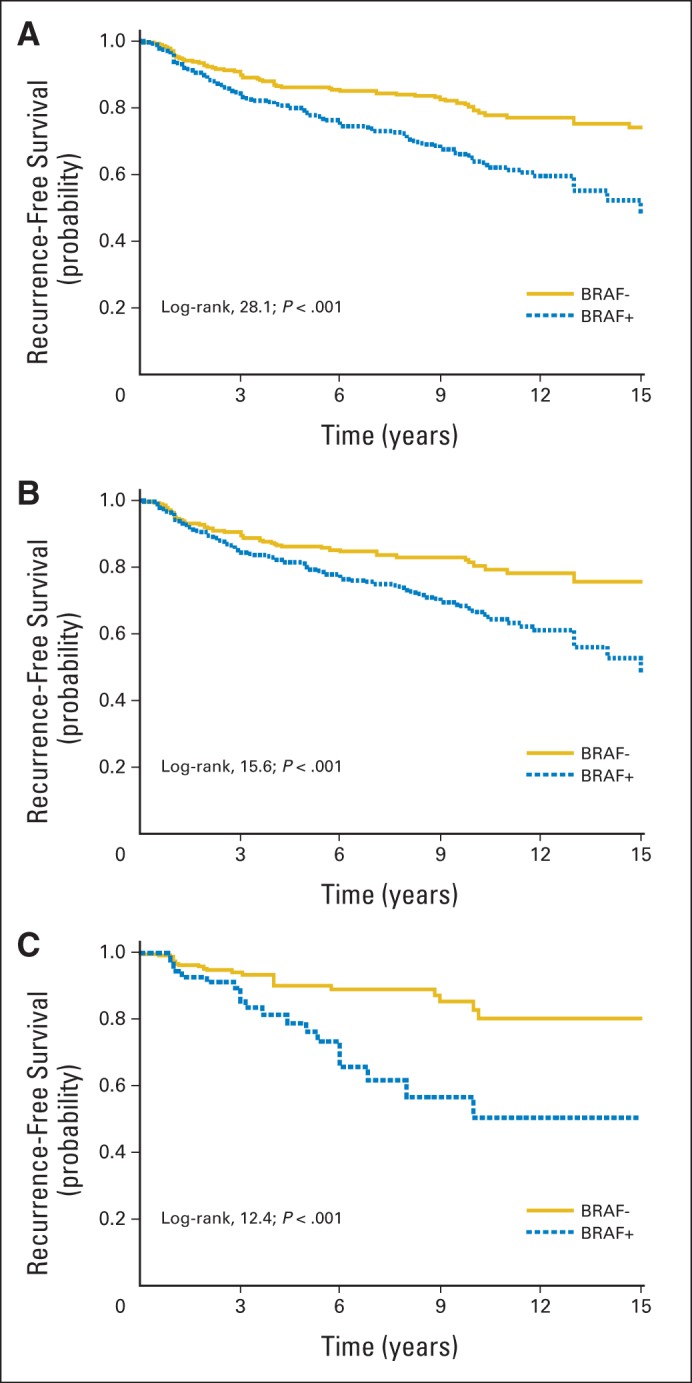
Kaplan-Meier survival curves of effect of BRAF V600E mutation status on disease recurrence–free probability in patients with various types of papillary thyroid cancer (PTC). Comparison of recurrence-free survival of patients, represented by indicated log-rank and P values in each panel, was performed between BRAF V600E–negative and –positive groups for (A) all patients, (B) those with conventional PTC, and (C) those with follicular-variant PTC. Follow-up time truncated at 15 years.
Fig 2.
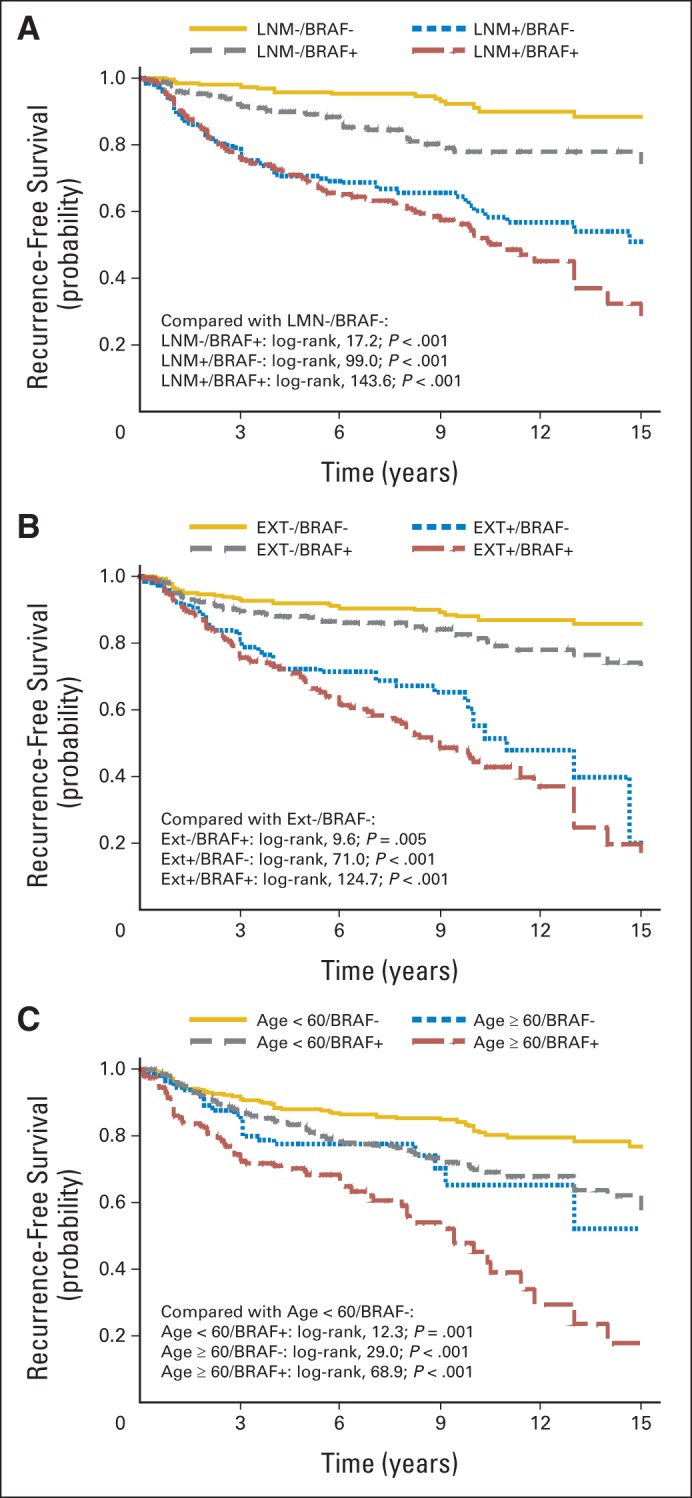
Kaplan-Meier survival curves of interaction of BRAF V600E mutation with clinicopathologic risk factors in affecting disease-free probability in patients with papillary thyroid cancer (all types). (A) Lymph node metastasis (LNM) and BRAF V600E mutation, (B) tumor extrathyroidal extension (EXT) and BRAF V600E mutation, and (C) patients age ≥ 60 years and BRAF V600E mutation. In each panel, P values were from log-rank tests, adjusted for multiple comparisons, comparing each stratum with patients negative for both BRAF V600E mutation and indicated clinicopathologic factor. Follow-up time truncated at 15 years.
To further examine the interactions of BRAF V600E mutation with clinicopathologic risk factors, we calculated the SI,28 which tests for an additive interaction, representing synergism if the SI is > 1 and antagonism between the two factors if the value is < 1. We found a significant synergistic interaction between BRAF V600E mutation and patient age ≥ 60 years, with an SI of 2.15 (95% CI, 1.11 to 4.19; Table 3).
Table 3.
Interactions of BRAF V600E With Conventional Risk Factors in Recurrence of PTC (all types): Synergy Test
| Risk Factor for Interaction With BRAF V600E | Synergy Index* | 95% CI |
|---|---|---|
| Patient age ≥ 45 years | 3.22 | 0.69 to 15.01 |
| Patient age ≥ 60 years | 2.15 | 1.11 to 4.19 |
| Lymph node metastasis | 1.10 | 0.80 to 1.49 |
| Extrathyroidal invasion | 1.12 | 0.76 to 1.66 |
NOTE. Test method from Hosmer and Lemeshow.28
Abbreviation: PTC, papillary thyroid cancer.
Synergy index different than 1 represents significant additive interaction; > 1 represents synergism; < 1 represents antagonism. There was significant synergistic interaction between BRAF V600E mutation and patient age ≥ 60 years in affecting recurrence of PTC. There were no significant interactions between BRAF V600E mutation and patient age ≥ 45 years, lymph node metastasis, or extrathyroidal invasion.
Effects of BRAF V600E Mutation on Recurrence of Conventionally Low-Risk PTC
BRAF V600E mutation was also significantly associated with PTC recurrence in conventionally low-risk patients (Table 4). In patients with stage I PTC, 12.1% (66 of 547) of BRAF mutation–positive patients and 7.3% (53 of 726) of BRAF mutation–negative patients experienced recurrence. Recurrence rates were significantly higher for BRAF mutation–positive versus –negative patients (25.61 v 15.75 per 1,000 person-years; P = .008), with an HR of 1.61 (95% CI, 1.12 to 2.31), which remained significant at 1.56 (95% CI, 1.04 to 2.34) after adjustment for patient age, sex, medical center, tumor size, extrathyroidal invasion, lymph node metastasis, and multifocality. In patients with stage II PTC, 20.7% (19 of 92) of BRAF mutation–positive patients and 9.2% (13 of 142) of BRAF mutation–negative patients experienced recurrence. Although these numbers were relatively small, BRAF mutation was still significantly associated with higher recurrence rates (54.99 v 22.65 per 1,000 person-years; P = .01) and risk (fully adjusted HR, 4.45; 95% CI, 1.70 to 11.67). In patients with micro-PTC, 17.8% (39 of 219) of BRAF mutation–positive patients and 5.7% (18 of 315) of BRAF mutation–negative patients experienced recurrence. Again, BRAF mutation was significantly associated with higher recurrence rates (43.85 v 13.04 per 1,000 person-years; P < .001) and risk (fully adjusted HR, 2.40; 95% CI, 1.00 to 5.75).
Table 4.
Relationship Between BRAF V600E Mutation and Tumor Recurrence in Low-Risk Clinicopathologic Categories of PTC
| Clinicopathologic Category | Tumor Recurrence |
Person- Years of Follow-Up | Recurrence Rates |
Model One* |
Model Two† |
Model Three‡ |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BRAF V600E Positive |
BRAF V600E Negative |
BRAF V600E Positive |
BRAF V600E Negative |
P§ | ||||||||||||
| No. | % | No. | % | Per 1,000 Person-Years | 95% CI | Per 1,000 Person-Years | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Stage I | 66 of 547 | 12.1 | 53 of 726 | 7.3 | 5,941.8 | 25.61 | 20.12 to 32.60 | 15.75 | 12.03 to 20.62 | .008 | 1.61 | 1.12 to 2.31 | 1.58 | 1.07 to 2.34 | 1.56 | 1.04 to 2.34 |
| Stage II | 19 of 92 | 20.6 | 13 of 142 | 9.2 | 919.5 | 54.99 | 35.08 to 86.22 | 22.65 | 13.15 to 39.00 | .01 | 2.44 | 1.20 to 4.97 | 3.22 | 1.41 to 7.34 | 4.45 | 1.70 to 11.67 |
| Tumor ≤ 1.0 cm | 39 of 219 | 17.8 | 18 of 315 | 5.7 | 2,270.2 | 43.85 | 32.04 to 60.02 | 13.04 | 8.21 to 20.69 | < .001 | 3.33 | 1.90 to 5.84 | 2.74 | 1.50 to 5.02 | 2.40 | 1.00 to 5.75 |
Abbreviations: HR, hazard ratio; PTC, papillary thyroid cancer.
Model one was unadjusted.
Model two was adjusted for patient age and sex and stratified by medical center.
Model three was additionally adjusted for tumor size, extrathyroidal invasion, lymph node metastasis, and multifocality (and PTC subtypes for all-types group).
P values from Poisson regressions comparing BRAF mutation–positive and –negative groups.
Significant effects of BRAF V600E mutation on PTC recurrence were also found with various tumor sizes (Appendix Tables A2 and A3, online only). When examined in various patient sex and age categories (Appendix Table A4, online only), significant effects of BRAF mutation on PTC recurrence were observed in both male and female patients and patients age ≥ 60 or ≥ 45 years. These effect patterns of BRAF mutation were reproduced in CPTC and FVPTC variants. Among most of these categories, the impact of BRAF V600E mutation on PTC recurrence was greatest in men age ≥ 60 years (Appendix Table A4, online only).
DISCUSSION
It is often a challenging task to risk stratify patients with PTC for optimal treatments. In recent years, promise for better prognostication of PTC has come from molecular markers.9 The BRAF V600E mutation has emerged as one such promising molecular marker that has attracted considerable attention.6–9 However, previous studies, which were relatively small and mostly single institution oriented, yielded inconsistent results, making BRAF V600E mutation debatable as a prognostic marker for PTC.29–31
In this study, we demonstrated a significant association of BRAF V600E mutation with recurrence of PTC, which was independent of conventional clinicopathologic risk factors, representing an incremental prognostic value of BRAF V600E mutation beyond the power of conventional clinicopathologic risk factors. We also observed a synergistic interaction between BRAF V600E mutation and older patient age in affecting PTC recurrence, which was similar to their synergistic effect on PTC-associated patient mortality.10 It is worth noting that even in conventionally low-risk stage I or II disease and micro-PTC, BRAF V600E mutation was strongly associated with recurrence, confirming the findings in a recent smaller study.32 Management of these patients is highly controversial.33 The prognostic value of BRAF V600E mutation may help improve the risk stratification and treatment of these patients.
The prognostic value of BRAF V600E mutation in specific individual subtype variants of PTC has been rarely investigated in previous studies.6–9 With the large size of this study, we were able to examine CPTC and FVPTC individually and similarly demonstrated a strong prognostic value of BRAF V600E mutation. It was particularly interesting to see, for the first time to our knowledge, a strong association of BRAF V600E mutation with recurrence of FVPTC. In fact, BRAF V600E mutation showed the most significant association and highest HRs for recurrence of FVPTC compared with CPTC and all PTCs. BRAF V600E mutation was previously reported to be most common in infiltrative FVPTC with lymph node metastases and extrathyroidal invasion,34 consistent with the association of BRAF V600E mutation with FVPTC recurrence found in this study. FVPTC has been increasingly documented, and some studies have suggested an overall better prognosis than other PTC variants,35 whereas other studies have suggested a prognosis for FVPTC similar to that for CPTC,36 which tends to promote under-treatment in some practices, whereas unnecessary over-treatments may occur in other practices. The prognostic value of BRAF V600E mutation in FVPTC may now help better define the vigorous levels of treatment for this cancer.
The aggressive role and prognostic value of BRAF V600E mutation in PTC can be explained by several molecular mechanisms, including its aberrant regulation of various signaling pathways, such as the MAP kinase pathway, NFκB pathway, and RASSF1A pathway; upregulation of various pro-oncogenic molecules; and downregulation of various tumor suppressor genes in thyroid cancer.37 BRAF V600E mutation also uniquely downregulates thyroid iodide–metabolizing genes, such as sodium-iodide symporter (NIS),37 thus explaining the initial finding of the association of BRAF V600E mutation with the loss of radioiodine avidity and hence radioiodine treatment failure in PTC.13 The molecular mechanism for the silencing of NIS by BRAF V600E mutation was recently demonstrated to involve histone deacetylation at the NIS promoter.38
One weakness in this study was the potential patient inhomogeneity, as is often seen in multicenter studies. Some centers treated patients with more-advanced diseases, but the number of such patients was relatively small. Center stratification performed in this study helped minimize the effect of variations among centers. Also, the large multicenter study with worldwide geographic reach makes the findings highly generalizable. The median follow-up time of 36 months was relatively short, but this should have captured most recurrence events, because PTC recurs mostly within the first several years after the initial treatments. Treatment doses of radioiodine varied at different centers. However, within most centers, there was no significant difference in dose between BRAF mutation–positive and –negative patients. A higher overall dose of radioiodine was received by BRAF mutation–positive patients, presumably because these patients had more aggressive disease, which prompted more-aggressive treatments. This may have caused an underestimation of the effect of BRAF V600E mutation on PTC recurrence, because radioiodine treatment has been shown to reduce recurrence of PTC, particularly in patients with high-stage disease.27
In summary, this was a large multicenter study that provided sufficient power to address the prognostic value of BRAF V600E mutation for the recurrence of PTC in various clinicopathologic categories. These results, together with the recent demonstration of the strong association of BRAF V600E mutation with PTC-associated patient mortality, help establish a prognostic value of BRAF V600E mutation in PTC.
Appendix
Table A1.
Initial Radioiodine Treatment Doses by BRAF V600E Mutation Status in PTC (all types)
| Location | No. of Patients |
BRAF Mutation Positive |
BRAF Mutation Negative |
P* | ||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | |||
| Medical Center | ||||||
| Johns Hopkins Hospital | 387 | 76 | 0 to 100 | 30 | 0 to 100 | .03 |
| University of Pittsburgh | 162 | 135 | 106 to 161 | 105 | 0 to 134 | < .001 |
| Memorial Sloan-Kettering Cancer Center | 90 | 104 | 30 to 197 | 75 | 0 to 150 | .05 |
| Yale University | 17 | 158 | 51 to 243 | 100 | 0 to 209 | .38 |
| University of Pisa | 189 | 30 | 30 to 30 | 30 | 30 to 30 | .60 |
| University of Perugia | 117 | 100 | 50 to 100 | 100 | 50 to 100 | .37 |
| University of Milan | 110 | 80 | 50 to 80 | 50 | 0 to 80 | .07 |
| University of Padua | 135 | 100 | 100 to 150 | 100 | 100 to 150 | .57 |
| University of Bologna | 32 | 100 | 50 to 100 | 100 | 98 to 100 | .86 |
| Kanagawa Cancer Center | 49 | 0 | 0 to 0 | 0 | 0 to 0 | 1.0 |
| Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology | 98 | 100 | 100 to 100 | 100 | 100 to 100 | .84 |
| Griffith Medical School | 0 | — | — | — | ||
| University of Sydney | 84 | 143 | 108 to 162 | 162 | 135 to 270 | .26 |
| Hospital La Paz Health Research Institute | 66 | 120 | 100 to 150 | 100 | 100 to 150 | .13 |
| Institute of Endocrinology, Prague | 221 | 100 | 0 to 102 | 100 | 0 to 119 | .93 |
| University of Ulsan | 197 | 150 | 150 to 150 | 150 | 150 to 150 | .008 |
| Country | ||||||
| United States | 656 | 100 | 0 to 140 | 53 | 0 to 103 | < .001 |
| Italy | 583 | 100 | 30 to 100 | 50 | 30 to 100 | < .001 |
| Japan | 49 | 0 | 0 to 0 | 0 | 0 to 0 | 1.0 |
| Poland | 98 | 100 | 100 to 100 | 100 | 100 to 100 | .84 |
| Australia | 84 | 143 | 108 to 162 | 162 | 135 to 270 | .26 |
| Spain | 66 | 120 | 100 to 150 | 100 | 100 to 150 | .13 |
| Czech Republic | 221 | 100 | 0 to 102 | 100 | 0 to 119 | .93 |
| South Korea | 197 | 150 | 150 to 150 | 150 | 150 to 150 | .008 |
| Overall | 1954 | 100 | 50 to 150 | 100 | 27 to 103 | < .001 |
Abbreviations: IQR, interquartile range; PTC, papillary thyroid cancer.
P value from Wilcoxon signed-rank test.
Table A2.
Recurrence and HRs for BRAF V600E Mutation–Positive Versus -Negative Patients in Various Tumor Size Groups of PTC (all types)
| Tumor Size Category (cm) | Recurrence |
HR | 95% CI | P | |||
|---|---|---|---|---|---|---|---|
|
BRAF Mutation Positive |
BRAF Mutation Negative |
||||||
| No. | % | No. | % | ||||
| 1.0 to 2.0 | 68 of 472 | 14.4 | 40 of 451 | 8.9 | 1.69 | 1.14 to 2.50 | .009 |
| 2.0 to 3.0 | 60 of 292 | 20.6 | 37 of 263 | 14.1 | 1.66 | 1.09 to 2.50 | .02 |
| 3.0 to 4.0 | 49 of 164 | 29.9 | 34 of 171 | 19.9 | 1.41 | 0.90 to 2.19 | .13 |
| ≥ 4.0 | 55 of 129 | 42.6 | 29 of 146 | 19.9 | 1.88 | 1.20 to 2.95 | .006 |
Abbreviations: HR, hazard ratio; PTC, papillary thyroid cancer.
Table A3.
Recurrence per 1,000 Person-Years and Relative Risk in BRAF V600E Mutation–Positive Versus –Negative Patients in Various Tumor Size Groups of PTC (all types)
| Tumor Size Category (cm) | Recurrence |
Relative Risk | 95% CI | |||
|---|---|---|---|---|---|---|
|
BRAF Mutation Positive |
BRAF Mutation Negative |
|||||
| Per 1,000 Person-Years | 95% CI | Per 1,000 Person-Years | 95% CI | |||
| 1.0 to 2.0 | 31.71 | 25.00 to 40.22 | 18.36 | 13.47 to 25.03 | 1.73 | 1.17 to 2.55 |
| 2.0 to 3.0 | 45.33 | 35.20 to 58.39 | 27.36 | 19.82 to 37.76 | 1.66 | 1.10 to 2.50 |
| 3.0 to 4.0 | 64.02 | 48.39 to 84.71 | 46.48 | 33.21 to 65.05 | 1.38 | 0.89 to 2.13 |
| ≥ 4.0 | 91.92 | 70.57 to 119.73 | 49.28 | 34.25 to 70.92 | 1.87 | 1.19 to 2.92 |
Abbreviation: PTC, papillary thyroid cancer.
Table A4.
Recurrence and HRs for BRAF V600E Mutation–Positive Versus –Negative Patients With PTC (all types) in Various Age and Sex Groups
| Patient Age (years) | Recurrence |
HR | 95% CI | |||
|---|---|---|---|---|---|---|
|
BRAF Mutation Positive |
BRAF Mutation Negative |
|||||
| No. | % | No. | % | |||
| All PTCs | ||||||
| All patients | ||||||
| All ages | 213 of 1,017 | 20.9 | 125 of 1,082 | 11.6 | 1.82 | 1.46 to 2.28 |
| < 45 | 75 of 443 | 16.9 | 69 of 576 | 12.0 | 1.37 | 0.99 to 1.91 |
| ≥ 45 | 138 of 574 | 24.0 | 56 of 506 | 11.1 | 2.20 | 1.61 to 3.00 |
| ≥ 60 | 80 of 251 | 31.9 | 31 of 195 | 15.9 | 1.84 | 1.22 to 2.79 |
| Women | ||||||
| All ages | 133 of 767 | 17.3 | 86 of 848 | 10.1 | 1.72 | 1.31 to 2.26 |
| < 45 | 50 of 351 | 14.2 | 50 of 468 | 10.7 | 1.33 | 0.90 to 1.98 |
| ≥ 45 | 83 of 416 | 20.0 | 36 of 380 | 9.5 | 2.08 | 1.40 to 3.07 |
| ≥ 60 | 50 of 187 | 26.7 | 22 of 140 | 15.7 | 1.47 | 0.89 to 2.42 |
| Men | ||||||
| All ages | 80 of 250 | 32.0 | 39 of 234 | 16.7 | 1.90 | 1.30 to 2.79 |
| < 45 | 25 of 92 | 27.2 | 19 of 108 | 17.6 | 1.30 | 0.72 to 2.37 |
| ≥ 45 | 55 of 158 | 34.8 | 20 of 126 | 15.9 | 2.35 | 1.41 to 3.93 |
| ≥ 60 | 30 of 64 | 46.9 | 9 of 55 | 16.4 | 3.08 | 1.46 to 6.51 |
| CPTC | ||||||
| All patients | ||||||
| All ages | 168 of 813 | 20.7 | 79 of 635 | 12.4 | 1.75 | 1.34 to 2.29 |
| < 45 | 64 of 368 | 17.4 | 46 of 345 | 13.3 | 1.26 | 0.86 to 1.85 |
| ≥ 45 | 104 of 445 | 23.4 | 33 of 290 | 11.4 | 2.33 | 1.57 to 3.46 |
| ≥ 60 | 56 of 193 | 29.0 | 18 of 111 | 16.2 | 1.90 | 1.11 to 3.24 |
| Women | ||||||
| All ages | 104 of 612 | 17.0 | 50 of 501 | 10.0 | 1.74 | 1.24 to 2.44 |
| < 45 | 43 of 296 | 14.5 | 30 of 281 | 10.7 | 1.35 | 0.84 to 2.16 |
| ≥ 45 | 61 of 316 | 19.3 | 20 of 220 | 9.1 | 2.23 | 1.34 to 3.70 |
| ≥ 60 | 34 of 143 | 23.8 | 13 of 81 | 16.0 | 1.41 | 0.74 to 2.70 |
| Men | ||||||
| All ages | 64 of 201 | 31.8 | 29 of 134 | 21.6 | 1.70 | 1.09 to 2.65 |
| < 45 | 21 of 72 | 29.2 | 16 of 64 | 25.0 | 1.01 | 0.53 to 1.95 |
| ≥ 45 | 43 of 129 | 33.3 | 13 of 70 | 18.6 | 2.47 | 1.32 to 4.63 |
| ≥ 60 | 22 of 50 | 44.0 | 5 of 30 | 16.7 | 3.90 | 1.47 to 10.32 |
| FVPTC | ||||||
| All patients | ||||||
| All ages | 19 of 89 | 21.4 | 24 of 342 | 7.0 | 2.76 | 1.51 to 5.06 |
| < 45 | 6 of 35 | 17.1 | 15 of 175 | 8.6 | 2.06 | 0.79 to 5.38 |
| ≥ 45 | 13 of 54 | 24.1 | 9 of 167 | 5.4 | 3.50 | 1.49 to 8.23 |
| ≥ 60 | 8 of 16 | 50.0 | 4 of 60 | 6.7 | 3.43 | 0.97 to 12.13 |
| Women | ||||||
| All ages | 12 of 70 | 17.1 | 21 of 266 | 7.9 | 2.17 | 1.06 to 4.45 |
| < 45 | 4 of 28 | 14.3 | 15 of 143 | 10.5 | 1.43 | 0.47 to 4.36 |
| ≥ 45 | 8 of 42 | 19.0 | 6 of 123 | 4.9 | 3.50 | 1.21 to 10.12 |
| ≥ 60 | 5 of 12 | 41.7 | 3 of 41 | 7.3 | 4.86 | 0.90 to 26.37 |
| Men | ||||||
| All ages | 7 of 19 | 36.8 | 3 of 76 | 4.0 | 5.60 | 1.44 to 21.76 |
| < 45 | 2 of 7 | 28.6 | 0 of 32 | 0.0 | * | |
| ≥ 45 | 5 of 12 | 41.7 | 3 of 44 | 6.8 | 2.71 | 0.64 to 11.45 |
| ≥ 60 | 3 of 4 | 75.0 | 1 of 19 | 5.3 | 2.18 | 0.17 to 28.05 |
Abbreviations: CPTC, conventional papillary thyroid cancer; FVPTC, follicular-variant papillary thyroid cancer; HR, hazard ratio; PTC, papillary thyroid cancer.
Could not be estimated.
Footnotes
See accompanying editorial on page 7; listen to the podcast by Dr Haddad at www.jco.org/podcasts
Support information appears at the end of this article.
The funding organizations had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health or the funding entities of the individual centers participating in this study.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Mingzhao Xing
Financial support: Mingzhao Xing
Administrative support: Mingzhao Xing
Provision of study materials or patients: Mingzhao Xing, Ali S. Alzahrani, Young Kee Shong, Tae Yong Kim, David Viola, Rossella Elisei, Bela Bendlová, Linwah Yip, Caterina Mian, Federica Vianello, R. Michael Tuttle, Eyal Robenshtok, James A. Fagin, Efisio Puxeddu, Laura Fugazzola, Agnieszka Czarniecka, Barbara Jarzab, Christine J. O'Neill, Mark S. Sywak, Alfred K. Lam, Garcilaso Riesco-Eizaguirre, Pilar Santisteban, Hirotaka Nakayama, Roderick Clifton-Bligh, Giovanni Tallini, Elizabeth H. Holt, Vlasta Sýkorová
Collection and assembly of data: All authors
Data analysis and interpretation: Mingzhao Xing, Kathryn A. Carson
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported by National Institutes of Health (NIH) Grants No. R01CA134225 and RO1CA113507 (M.X.); by Grant No. UL1 RR 025005 from the National Center for Advancing Translational Sciences of NIH and NIH Roadmap for Medical Research (K.A.C.); and by the following funding to individual study centers: National Science Centre Poland Grants No. N N403 194340 (A.C.) and N N401 612440 (B.J.); grants from Griffith Health Institute (Australia; A.K.L.); Grants No. BFU2010-16025, RD06/0020/0060-RD12/0036/0030 FIS, ISCIII, and S2011/BMD-2328 TIRONET (Spain; P.S.); NIH Grant No. RO1-CA50706 and the Byrne Foundation (J.A.F.); Grant No. IG 9338 from the Fondazione Cassa di Risparmio di Perugia and Associazione Italiana per la Ricerca sul Cancro (Italy) and the Beadle Family Foundation (San Antonio, TX; E.P.); Grant No. IGA MH CR NT 13901-4 (Czech Republic; B.B., V.S.); grants from the New South Wales Cancer Institute (C.J.O.) and Cancer Council of New South Wales (Australia; R.C.-B.); Grant No. MIUR 20074zw8la from the Ministero della Istruzione Universitaria e Ricerca Scientifica and the Associazione Italiana per la Ricerca sul Cancro (Italy; G.T.); NIH/National Institute on Aging Grant No. 5R03AG042334-02 (L.Y.); grants from the Ministero della Istruzione Universitaria e Ricerca Scientifica, the Associazione Italiana per la Ricerca sul Cancro, the Istituto Toscano Tumori, and the Ministero della Salute (Italy; D.V., R.E.); and Grant No. CB-2011-03-02 from the Korean Foundation for Cancer Research (South Korea; Y.K.S., T.Y.K.).
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association Between BRAF V600E Mutation and Recurrence of Papillary Thyroid Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Mingzhao Xing
Honoraria: Bayer/Onyx
Consulting or Advisory Role: Bayer/Onyx
Patents, Royalties, Other Intellectual Property: Receiving royalties as coholder of licensed US patent related to BRAF V600E mutation in thyroid cancer
Travel, Accommodations, Expenses: Bayer/Onyx
Ali S. Alzahrani
No relationship to disclose
Kathryn A. Carson
No relationship to disclose
Young Kee Shong
Honoraria: Bayer, Genzyme
Consulting or Advisory Role: Bayer, Genzyme
Travel, Accommodations, Expenses: Bayer, Genzyme
Tae Yong Kim
No relationship to disclose
David Viola
Consulting or Advisory Role: SOBI
Speakers' Bureau: Genzyme
Rossella Elisei
Consulting or Advisory Role: Bayer, Genzyme, AstraZeneca, Exelixis
Speakers' Bureau: Bayer, Genzyme, Exelixis
Travel, Accommodations, Expenses: Bayer, Genzyme, AstraZeneca
Bela Bendlová
No relationship to disclose
Linwah Yip
No relationship to disclose
Caterina Mian
No relationship to disclose
Federica Vianello
No relationship to disclose
R. Michael Tuttle
Honoraria: Genzyme, Bayer/Onyx, sanofi-aventis
Consulting or Advisory Role: Genzyme, Bayer/Onyx, sanofi-aventis
Eyal Robenshtok
Consulting or Advisory Role: Genzyme
James A. Fagin
Honoraria: Quest Diagnostics
Consulting or Advisory Role: Novartis
Research Funding: Biomed Valley, AstraZeneca
Expert Testimony: Novo Nordisk
Efisio Puxeddu
Travel, Accommodations, Expenses: IBSA
Laura Fugazzola
Travel, Accommodations, Expenses: Genzyme
Agnieszka Czarniecka
No relationship to disclose
Barbara Jarzab
Honoraria: AstraZeneca, Novartis, Oxigene, Ipsen, SOBI, BiPar/sanofi-aventis, Bayer, Roche, Eisai
Consulting or Advisory Role: SOBI, AstraZeneca
Speakers' Bureau: Eisai
Expert Testimony: AstraZeneca, SOBI
Travel, Accommodations, Expenses: Ipsen, BiPar/sanofi-aventis, Novartis
Christine J. O'Neill
No relationship to disclose
Mark S. Sywak
No relationship to disclose
Alfred K. Lam
No relationship to disclose
Garcilaso Riesco-Eizaguirre
No relationship to disclose
Pilar Santisteban
No relationship to disclose
Hirotaka Nakayama
No relationship to disclose
Roderick Clifton-Bligh
Consulting or Advisory Role: Amgen, Bayer
Speakers' Bureau: Amgen, Bayer, Novartis, Novo Nordisk
Research Funding: Amgen (Inst), Eisai (Inst)
Giovanni Tallini
No relationship to disclose
Elizabeth H. Holt
No relationship to disclose
Vlasta Sýkorová
No relationship to disclose
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. http://seer.cancer.gov/csr/1975_2010/
- 3.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle RM, Ball DW, Byrd D, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–1274. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 5.Brown RL, de Souza JA, Cohen EE. Thyroid cancer: Burden of illness and management of disease. J Cancer. 2011;2:193–199. doi: 10.7150/jca.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 7.Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 8.Kim TH, Park YJ, Lim JA, et al. The association of the BRAF (V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: A meta-analysis. Cancer. 2012;118:1764–1773. doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 9.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puxeddu E, Moretti S, Elisei R, et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–2420. doi: 10.1210/jc.2003-031425. [DOI] [PubMed] [Google Scholar]
- 12.Fugazzola L, Mannavola D, Cirello V, et al. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf) 2004;61:239–243. doi: 10.1111/j.1365-2265.2004.02089.x. [DOI] [PubMed] [Google Scholar]
- 13.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 14.Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, et al. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama H, Yoshida A, Nakamura Y, et al. Clinical significance of BRAF (V600E) mutation and Ki-67 labeling index in papillary thyroid carcinomas. Anticancer Res. 2007;27:3645–3649. [PubMed] [Google Scholar]
- 16.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 17.Xing M, Clark D, Guan H, et al. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol. 2009;27:2977–2982. doi: 10.1200/JCO.2008.20.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yip L, Nikiforova MN, Carty SE, et al. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery. 2009;146:1215–1223. doi: 10.1016/j.surg.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sykorova V, Dvorakova S, Ryska A, et al. BRAFV600E mutation in the pathogenesis of a large series of papillary thyroid carcinoma in Czech Republic. J Endocrinol Invest. 2010;33:318–324. doi: 10.1007/BF03346593. [DOI] [PubMed] [Google Scholar]
- 21.Czarniecka A, Rusinek D, Stobiecka E, et al. Occurrence of BRAF mutations in a Polish cohort of PTC patients: Preliminary results. Endokrynol Pol. 2010;61:462–466. [PubMed] [Google Scholar]
- 22.O'Neill CJ, Bullock M, Chou A, et al. BRAF(V600E) mutation is associated with an increased risk of nodal recurrence requiring reoperative surgery in patients with papillary thyroid cancer. Surgery. 2010;148:1139–1145. doi: 10.1016/j.surg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Pelizzo MR, Boschin IM, Barollo S, et al. BRAF analysis by fine needle aspiration biopsy of thyroid nodules improves preoperative identification of papillary thyroid carcinoma and represents a prognostic factor: A mono-institutional experience. Clin Chem Lab Med. 2011;49:325–329. doi: 10.1515/CCLM.2011.031. [DOI] [PubMed] [Google Scholar]
- 24.Smith RA, Salajegheh A, Weinstein S, et al. Correlation between BRAF mutation and the clinicopathological parameters in papillary thyroid carcinoma with particular reference to follicular variant. Hum Pathol. 2011;42:500–506. doi: 10.1016/j.humpath.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–368. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 26.Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 27.Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association (ATA) Guidelines Taskforce on thyroid nodules and differentiated thyroid cancer: Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Sarne DH. A piece of the puzzle: What does BRAF status mean in the management of patients with papillary thyroid carcinoma? J Clin Endocrinol Metab. 2012;97:3094–3096. doi: 10.1210/jc.2012-2760. [DOI] [PubMed] [Google Scholar]
- 30.Xing M. BRAFV600E mutation and papillary thyroid cancer: Chicken or egg? J Clin Endocrinol Metab. 2012;97:2295–2298. doi: 10.1210/jc.2012-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puxeddu E, Filetti S. BRAF mutation assessment in papillary thyroid cancer: Are we ready to use it in clinical practice? Endocrine. 2014;45:341–343. doi: 10.1007/s12020-013-0139-0. [DOI] [PubMed] [Google Scholar]
- 32.Elisei R, Viola D, Torregrossa L, et al. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: Single-institution results from a large cohort study. J Clin Endocrinol Metab. 2012;97:4390–4398. doi: 10.1210/jc.2012-1775. [DOI] [PubMed] [Google Scholar]
- 33.McLeod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet. 2013;381:1046–1057. doi: 10.1016/S0140-6736(12)62205-3. [DOI] [PubMed] [Google Scholar]
- 34.Rivera M, Ricarte-Filho J, Knauf J, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23:1191–1200. doi: 10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam AK, Lo CY, Lam KS. Papillary carcinoma of thyroid: A 30-yr clinicopathological review of the histological variants. Endocr Pathol. 2005;16:323–330. doi: 10.1385/ep:16:4:323. [DOI] [PubMed] [Google Scholar]
- 36.Lin HW, Bhattacharyya N. Clinical behavior of follicular variant of papillary thyroid carcinoma: Presentation and survival. Laryngoscope. 2010;120:712–716. doi: 10.1002/lary.20828. [DOI] [PubMed] [Google Scholar]
- 37.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Liu D, Murugan AK, et al. Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocr Relat Cancer. 2014;21:161–173. doi: 10.1530/ERC-13-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]


