Abstract
Purpose
To determine the optimal dose and schedule of anthracycline and taxane administration as adjuvant therapy for early-stage breast cancer.
Patients and Methods
A 2 × 2 factorial design was used to test two hypotheses: (1) that a novel continuous schedule of doxorubicin-cyclophosphamide was superior to six cycles of doxorubicin-cyclophosphamide once every 2 weeks and (2) that paclitaxel once per week was superior to six cycles of paclitaxel once every 2 weeks in patients with node-positive or high-risk node-negative early-stage breast cancer. With 3,250 patients, a disease-free survival (DFS) hazard ratio of 0.82 for each randomization could be detected with 90% power with two-sided α = .05. Overall survival (OS) was a secondary outcome.
Results
Interim analyses crossed the futility boundaries for demonstrating superiority of both once-per-week regimens and once-every-2-weeks regimens. After a median follow-up of 6 years, a significant interaction developed between the two randomization factors (DFS P = .024; OS P = .010) in the 2,716 patients randomly assigned in the original design, which precluded interpretation of the two factors separately. Comparing all four arms showed a significant difference in OS (P = .040) but not in DFS (P = .11), with all treatments given once every 2 weeks associated with the highest OS. This difference in OS seemed confined to patients with hormone receptor–negative/human epidermal growth factor receptor 2 (HER2) –negative tumors (P = .067), with no differences seen with hormone receptor–positive/HER2-negative (P = .90) or HER2-positive tumors (P = .40).
Conclusion
Patients achieved a similar DFS with any of these regimens. Subset analysis suggests the hypothesis that once-every-2-weeks dosing may be best for patients with hormone receptor–negative/HER2-negative tumors.
INTRODUCTION
Since the incorporation of taxanes into adjuvant therapy of operable breast cancer, no new cytotoxic agents have been shown to add any benefit to currently available regimens.1–3 However, dose and schedule alterations have produced improvements in outcome,4,5 indicating that optimizing use of available agents is an effective strategy that should not be abandoned as we search for new approaches. S0221 (Phase III Trial of Continuous Schedule AC + G Versus Q 2 Week Schedule AC, Followed by Paclitaxel Given Either Every 2 Weeks or Weekly for 12 Weeks as Post-Operative Adjuvant Therapy in Node-Positive or High-Risk Node-Negative Breast Cancer) is a clinical trial investigating adjuvant chemotherapy performed by the North American Breast Intergroup. Patients with node-positive or high-risk node-negative breast cancer were randomly assigned to one of two schedules of doxorubicin-cyclophosphamide followed by one of two schedules of paclitaxel.
The schedules of doxorubicin-cyclophosphamide studied were (1) doxorubicin-cyclophosphamide administered once every 2 weeks for six cycles, and (2) a continuous schedule of doxorubicin-cyclophosphamide with filgrastim, with doxorubicin given intravenously once per week and cyclophosphamide given orally once per day for 15 weeks. The duration of therapy with doxorubicin-cyclophosphamide administered once every 2 weeks was chosen to give an equivalent dose of doxorubicin over a duration of treatment similar to that in the continuous dosing schedule. The continuous dosing schedule was based on a series of trials from the University of Washington and SWOG in the adjuvant and preoperative settings that suggested promising activity for continuous doxorubicin-cyclophosphamide with filgrastim as treatment for early-stage breast cancer.6,7 To compare two common schedules for paclitaxel used in this setting,4,5 both of which had been found to be superior to paclitaxel once every 3 weeks, patients were also randomly assigned, after completion of doxorubicin-cyclophosphamide, to receive treatment with paclitaxel either once every 2 weeks or once per week for 12 weeks.
PATIENTS AND METHODS
Patients
S0221 (registered on October 2003) enrolled male and female patients with high-risk pathologic stage I to III breast cancer, defined as node-positive (pN1-3), any primary tumor ≥ 2 cm, or any tumor ≥ 1 cm if it was estrogen receptor (ER) negative/progesterone receptor (PgR) negative or if it was hormone receptor positive with 21-gene recurrence score ≥ 26.8 Other requirements were age ≥ 18 years; SWOG performance status 0 to 2; adequate local therapy, defined as negative resection margins after mastectomy or partial mastectomy with planned radiation; axillary nodal dissection yielding a total of at least six nodes unless sentinel node negative; no history of congestive heart failure or active angina pectoris and a normal left ventricular ejection fraction; adequate major organ function, as specified in the protocol; and a negative pregnancy test if the patient was a woman with reproductive potential. Patients were excluded from participation if it had been more than 84 days since their last surgery to treat breast cancer or if they were known to be infected by HIV; had received prior cytotoxic chemotherapy for the current malignancy; had received prior therapy with an anthracycline, anthracenedione, or taxane; had received prior radiation therapy for the current malignancy (except partial breast radiation); or had a history of prior malignancy other than specified in situ cancers or other cancers from which they were disease-free for ≥ 5 years. The study protocol was approved by the National Cancer Institute Central Institutional Review Board and the institutional review boards of each participating institution. All patients provided written informed consent to participation, and patient safety was monitored by an independent data safety monitoring committee (DSMC).
Study Procedures
The study design was an open-label 2 × 2 factorial design with equal probability of receiving each treatment combination. The first factor compared doxorubicin 60 mg/m2 intravenously (IV) day 1, cyclophosphamide 600 mg/m2 IV day 1, and pegfilgrastim 6 mg subcutaneously day 2, administered every 2 weeks for six cycles versus a continuous schedule of doxorubicin 24 mg/m2 IV once per week, cyclophosphamide 60 mg/m2 orally once per day, and filgrastim 5 μg/kg rounded to the nearer of 300 or 480 μg subcutaneously once per day, except for the days of intravenous drug administration, for 15 weeks. The second factor was subsequent paclitaxel 175 mg/m2 IV day 1 once every 2 weeks and pegfilgrastim 6 mg subcutaneously day 2 repeated every 2 weeks for six cycles versus paclitaxel 80 mg/m2 IV once per week for 12 weeks.
Radiation therapy was given after completion of chemotherapy to patients undergoing partial mastectomy and was administered according to institutional practice for patients undergoing mastectomy. Patients with ER-positive or PgR-positive tumors were prescribed endocrine therapy for at least 5 years after completion of chemotherapy. After October 2005, patients with human epidermal growth factor receptor 2 (HER2) –positive tumors were allowed to receive trastuzumab concurrently with or after treatment with paclitaxel for a total of 1 year. Toxicity was graded according to Common Terminology Criteria for Adverse Events version 3.0, and chemotherapy doses were modified on the basis of toxicity.
At the first interim analysis in September 2010, the SWOG DSMC recommended that random assignment to the two schedules of doxorubicin-cyclophosphamide be halted on the basis of futility. At the time of closure of the doxorubicin-cyclophosphamide randomization, 2,716 patients had been randomly assigned in the 2 × 2 design. The trial was amended to assign all future patients to treatment with doxorubicin-cyclophosphamide once every 2 weeks for four cycles, based on evidence that patients treated with four cycles of this regimen had outcomes similar to those treated with six cycles,9 followed by either six cycles of paclitaxel once every 2 weeks (arm 5) or paclitaxel once per week for 12 weeks (arm 6). An additional 578 patients were enrolled under the modified design, resulting in a total of 3,294 patients being randomly assigned between the two paclitaxel schedules.
Statistical Methods
The primary outcome was disease-free survival (DFS) defined as time from registration (random assignment) to first instance of disease recurrence (local, regional, or distant), new breast primary tumor, or death as a result of any cause. A secondary outcome was overall survival (OS) defined as time from registration to death as a result of any cause. For the purpose of this article, follow-up for survival was locked on October 23, 2013.
Stratification factors were not used because of the large size of the trial. The original sample size goal of 4,500 was reduced to 3,250 in November 2006 on the basis of revised accrual and follow-up projections. Each treatment factor was to be tested separately in a joint model with both factors (at a two-sided α = .05) if there was no significant interaction of the two factors (α = .10). Power was 90% to test whether continuous administration was superior to dose-dense administration with a hazard ratio (HR) of 0.82 or less. Annual interim analyses were planned after 30% of the expected number of failures had occurred. The significance levels for the interim analyses were chosen by using the Lan-DeMets function.10 In addition to efficacy tests, a test of futility was to be conducted at each interim analysis for each factor. If the 99.5% confidence limit for the HR excluded the alternate hypothesis (HR, 0.82), then the trial would not be able to be positive and a recommendation would be made to the DSMC to discontinue random assignment to that factor.
Survival analysis methods include Kaplan-Meier plots, log-rank tests, and Cox regression analyses. The Cox regression analysis included both factors in a joint model.
RESULTS
Patient Characteristics and Trial Progress
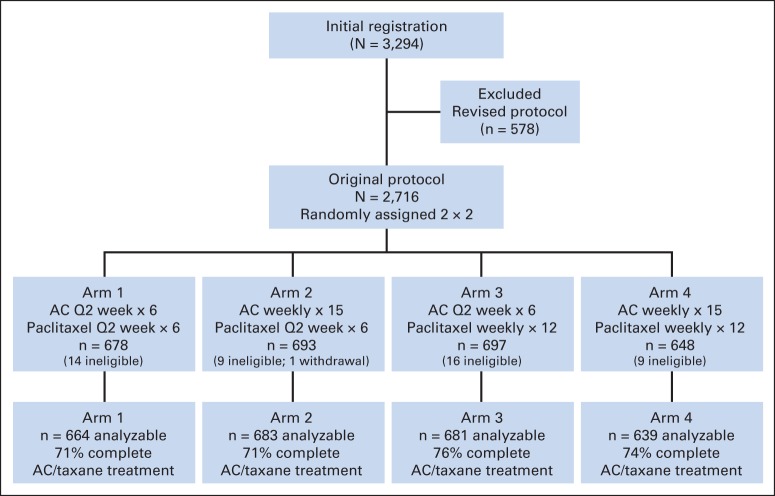
A total of 2,716 patients were randomly assigned from December 2003 to November 2010 to the original 2 × 2 design (Fig 1 and Table 1). At the time of the first interim analysis in September 2010, the observed HR for DFS for doxorubicin-cyclophosphamide with filgrastim versus doxorubicin-cyclophosphamide once every 2 weeks was 1.21 (adjusting for the paclitaxel randomization). The 99.5% CI was 0.90 to 1.64, suggesting that it would be futile to continue randomization to this factor. On the basis of the recommendation of the DSMC, accrual to the trial was suspended in November 2010. The trial reopened in December 2010 with all patients assigned to four cycles of doxorubicin-cyclophosphamide administered once every 2 weeks and randomly assigned only to the paclitaxel factor. At the third interim analysis in September 2012, the futility boundary for the comparison of the two paclitaxel schedules was crossed, with a Cox model adjusting for the doxorubicin-cyclophosphamide arms producing an HR of 1.08 (99.5% CI, 0.83 to 1.39), thus excluding 0.82. On the basis of this analysis, the DSMC recommended releasing the results.
Fig 1.
CONSORT diagram for the original protocol of Southwest Oncology Group S0221 trial. AC, doxorubicin-cyclophosphamide; Q2 week, once every 2 weeks.
Table 1.
Baseline Characteristics for Patients in All Arms in the Original Protocol
| Characteristic | Arm 1 |
Arm 2 |
Arm 3 |
Arm 4 |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. randomly assigned | 678 | 693 | 697 | 648 | 2,716 | |||||
| No. ineligible/withdrew consent | 14 | 10 | 16 | 9 | 49 | |||||
| No. analyzed | 664 | 683 | 681 | 639 | 2,667 | |||||
| Age, years | ||||||||||
| Median | 50.5 | 50.9 | 51.8 | 50.7 | 51.0 | |||||
| Range | 25-77 | 23-79 | 23-86 | 21-76 | 21-86 | |||||
| Black race | 73 | 11 | 77 | 11 | 74 | 11 | 78 | 12 | 302 | 11 |
| Male | 6 | 0.9 | 4 | 0.6 | 5 | 0.7 | 3 | 0.5 | 18 | 0.7 |
| Menopausal status (females) | ||||||||||
| Premenopausal | 326 | 50 | 325 | 48 | 308 | 46 | 299 | 47 | 1,258 | 47 |
| Postmenopausal | 332 | 50 | 354 | 52 | 368 | 54 | 337 | 53 | 1,391 | 53 |
| Nodal status (n = 9 unknown) | ||||||||||
| Negative | 161 | 24 | 153 | 22 | 159 | 23 | 146 | 23 | 619 | 23 |
| 1-3 positive nodes | 260 | 39 | 266 | 39 | 276 | 41 | 245 | 39 | 1,047 | 39 |
| ≥ 4 positive nodes | 241 | 36 | 264 | 39 | 243 | 36 | 244 | 38 | 992 | 37 |
| ER/PgR (n = 9 unknown) | ||||||||||
| Negative (both negative) | 212 | 32 | 226 | 33 | 232 | 34 | 206 | 32 | 876 | 33 |
| Positive (either or both positive) | 450 | 68 | 456 | 67 | 446 | 66 | 430 | 68 | 1,782 | 67 |
| HER2 (n = 29 unknown) | ||||||||||
| Negative | 528 | 81 | 556 | 82 | 554 | 82 | 525 | 83 | 2,163 | 82 |
| Positive | 125 | 19 | 123 | 18 | 118 | 18 | 109 | 17 | 475 | 18 |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PgR, progesterone receptor.
This report updates outcomes through October 2013. Median follow-up was 6 years in those without an event. A total of 550 DFS events were observed, giving an overall event incident rate per year of 0.039 versus rates of 0.04 to 0.06 hypothesized in the study design. Although no significant interaction of the doxorubicin-cyclophosphamide and paclitaxel factors was found in the early interim analyses, the interaction was now significant (P = .024), and the two factors could not be analyzed separately. This analysis was therefore limited to the 2,716 patients who participated in the original protocol and did not include the 578 patients entered onto the study under the revised protocol. One patient withdrew consent, and 48 other patients were determined to be ineligible at baseline allowing analysis of 2,667 (98.2%) of the 2,716 patients randomly assigned (Fig 1 [CONSORT diagram]).
Toxicity
Toxicities are classified as occurring during either doxorubicin-cyclophosphamide treatment or paclitaxel treatment. Table 2 shows the number of patients who had a grade 3 or higher toxicity that was possibly, probably, or definitely related to doxorubicin-cyclophosphamide treatment. Results in Table 2 are limited to those who started doxorubicin-cyclophosphamide treatment, who did not have a major deviation in the treatment protocol, and whose toxicity profile has been completed. Toxicity was greater for doxorubicin-cyclophosphamide once every 2 weeks with regard to hemoglobin and leukocytes. Toxicity was higher for doxorubicin-cyclophosphamide with filgrastim for mucositis and dermatologic toxicity. Five patients in the group given doxorubicin-cyclophosphamide once every 2 weeks had fatal toxicity: apnea and asystole (1); renal failure, neutropenia, and wound infection (1); hepatitis B reactivation (1); multiorgan failure (1); and left ventricular diastolic dysfunction (1). Three patients given doxorubicin-cyclophosphamide with filgrastim had fatal toxicity: two patients had neutropenia and pulmonary infection and one patient was coded as having grade 5 pleural effusion. Four patients randomly assigned to doxorubicin-cyclophosphamide once every 2 weeks received no assigned treatment versus 18 patients assigned to doxorubicin-cyclophosphamide with filgrastim. The percentage of patients who completed all doxorubicin-cyclophosphamide treatment after random assignment to the arms receiving doxorubicin-cyclophosphamide once every 2 weeks was 88% versus 83% in the arms receiving doxorubicin-cyclophosphamide with filgrastim (P < .001). More patients in the arms receiving doxorubicin-cyclophosphamide with filgrastim (11.0%) stopped doxorubicin-cyclophosphamide treatment early because of toxicity compared with those randomly assigned to the arms receiving doxorubicin-cyclophosphamide once every 2 weeks (7.9%; P = .006).
Table 2.
Number of Patients with Type and Grade of Toxicity for Doxorubicin-Cyclophosphamide Every 2 Weeks Versus Doxorubicin-Cyclophosphamide Plus Figrastim
| Adverse Event | Grade for Doxorubicin-Cyclophosphamide Every 2 Weeks (n = 1,323) |
Grade for Doxorubicin-Cyclophosphamide Plus Filgrastim (n = 1,280) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 2 |
3 |
4 |
5 |
≤ 2 |
3 |
4 |
5 |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Hemoglobin | 1,193 | 90.2 | 122 | 9.2 | 8 | 0.6 | 0 | 0.0 | 1,211 | 94.6 | 66 | 5.2 | 3 | 0.2 | 0 | 0.0 |
| Leukocytes | 1,064 | 80.4 | 108 | 8.2 | 151 | 11.4 | 0 | 0.0 | 1,090 | 85.2 | 145 | 11.3 | 45 | 3.5 | 0 | 0.0 |
| Neutrophils | 979 | 74.0 | 111 | 8.4 | 233 | 17.6 | 0 | 0.0 | 980 | 76.6 | 196 | 15.3 | 104 | 8.1 | 0 | 0.0 |
| Platelets | 1,288 | 97.4 | 24 | 1.8 | 11 | 0.8 | 0 | 0.0 | 1,241 | 97.0 | 33 | 2.6 | 6 | 0.5 | 0 | 0.0 |
| General cardiac | 1,312 | 99.2 | 8 | 0.6 | 3 | 0.2 | 1 | 0.1 | 1,275 | 99.6 | 5 | 0.4 | 0 | 0.0 | 0 | 0.0 |
| Clinical mucositis | 1,296 | 98.0 | 27 | 2.0 | 0 | 0.0 | 0 | 0.0 | 1,173 | 91.6 | 105 | 8.2 | 2 | 0.2 | 0 | 0.0 |
| Dermatologic/skin | 1,295 | 97.9 | 28 | 2.1 | 0 | 0.0 | 0 | 0.0 | 1,092 | 85.3 | 188 | 14.7 | 0 | 0.0 | 0 | 0.0 |
| Infection | 1,210 | 91.5 | 96 | 7.3 | 16 | 1.2 | 1 | 0.1 | 1,222 | 95.5 | 50 | 3.9 | 6 | 0.5 | 2 | 0.2 |
| Febrile neutropenia | 1,242 | 93.9 | 65 | 4.9 | 16 | 1.2 | 1 | 0.1 | 1,255 | 98.0 | 22 | 1.7 | 3 | 0.2 | 0 | 0.0 |
| Flu-like symptoms | 1,209 | 91.4 | 107 | 8.1 | 7 | 0.5 | 0 | 0.0 | 1,188 | 92.8 | 90 | 7.0 | 2 | 0.2 | 0 | 0.0 |
| Other or undetermined | 2 | 0.2 | 1 | 0.1 | ||||||||||||
| All adverse events | 702 | 53.1 | 329 | 24.9 | 288 | 21.8 | 5 | 0.4 | 556 | 43.4 | 587 | 45.9 | 134 | 10.5 | 3 | 0.2 |
Table 3 summarizes the toxicity of the two paclitaxel regimens. Grade 3 to 4 leukopenia and neutropenia were observed more commonly in patients treated with once-per-week paclitaxel, although the rate of neutropenic fever did not differ between the two schedules. Grade 3 to 4 allergic reactions, musculoskeletal pain, and neurologic toxicity were more commonly observed in patients treated with paclitaxel once every 2 weeks. Fatal toxicities during therapy with paclitaxel once every 2 weeks were heart failure after doxorubicin-cyclophosphamide once every 2 weeks (1), pneumonitis (1), and unclear/multifactorial (2). Fatal toxicities during treatment with paclitaxel once per week were heart failure after doxorubicin-cyclophosphamide once every 2 weeks (1) and pneumonitis (2).
Table 3.
Number of Patients with Type and Grade of Toxicity for Paclitaxel Once Every 2 Weeks Versus Once Per Week
| Adverse Event | Grade for Paclitaxel Every 2 Weeks (n = 1,162) |
Grade for Paclitaxel Once Per Week (n = 1,139) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 2 |
3 |
4 |
5 |
≤ 2 |
3 |
4 |
5 |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Hemoglobin | 1,135 | 97.7 | 25 | 2.2 | 2 | 0.2 | 0 | 0.0 | 1,123 | 98.6 | 16 | 1.4 | 0 | 0.0 | 0 | 0.0 |
| Leukocytes | 1,153 | 99.2 | 7 | 0.6 | 2 | 0.2 | 0 | 0.0 | 1,057 | 92.8 | 72 | 6.3 | 10 | 0.9 | 0 | 0.0 |
| Neutrophils | 1,140 | 98.1 | 17 | 1.5 | 5 | 0.4 | 0 | 0.0 | 1,000 | 87.8 | 105 | 9.2 | 34 | 3.0 | 0 | 0.0 |
| Neurologic | 961 | 82.7 | 193 | 16.6 | 8 | 0.7 | 0 | 0.0 | 1,020 | 89.6 | 115 | 10.1 | 4 | 0.4 | 0 | 0.0 |
| Dermatologic/skin | 1,132 | 97.4 | 30 | 2.6 | 0 | 0.0 | 0 | 0.0 | 1,117 | 98.1 | 22 | 1.9 | 0 | 0.0 | 0 | 0.0 |
| Allergy | 1,146 | 98.6 | 16 | 1.4 | 0 | 0.0 | 0 | 0.0 | 1,133 | 99.5 | 6 | 0.5 | 0 | 0.0 | 0 | 0.0 |
| Febrile neutropenia | 1,160 | 99.8 | 2 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1,138 | 99.9 | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 |
| Musculoskeletal pain | 1,035 | 89.1 | 124 | 10.7 | 3 | 0.3 | 0 | 0.0 | 1,106 | 97.1 | 33 | 2.9 | 0 | 0.0 | 0 | 0.0 |
| Fatal event | 1,161 | 99.9 | 0 | 0.0 | 0 | 0.0 | 4 | 0.3 | 1,139 | 100 | 0 | 0.0 | 0 | 0.0 | 3 | 0.3 |
| All adverse events | 750 | 64.5 | 384 | 33.0 | 24 | 2.1 | 4 | 0.3 | 727 | 63.8 | 363 | 31.9 | 48 | 4.2 | 3 | 0.3 |
Outcome
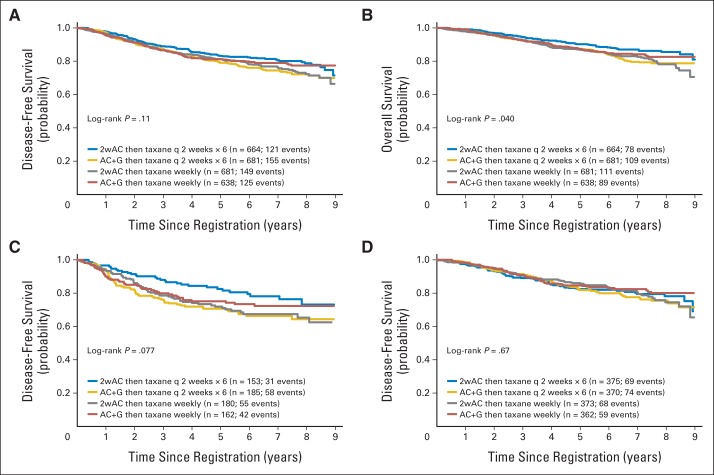
A log-rank test comparing all four arms simultaneously was not significant for the primary outcome DFS (P = .11). A Cox model yielded the following HRs relative to arm 1 (doxorubicin-cyclophosphamide once every 2 weeks; paclitaxel once every 2 weeks): for arm 2 (doxorubicin-cyclophosphamide with filgrastim; paclitaxel once every 2 weeks), the HR was 1.32 (95% CI, 1.04 to 1.68; P = .022); for arm 3 (doxorubicin-cyclophosphamide once every 2 weeks; paclitaxel once per week), the HR was 1.24 (95% CI, 0.98 to 1.59; P = .072); and for arm 4 (doxorubicin-cyclophosphamide with filgrastim; paclitaxel once per week), the HR was 1.12 (95% CI, 0.87 to 1.44; P = .38). Figures 2A and 2B show the Kaplan-Meier plots, and the Data Supplement shows the estimated 5-year DFS and OS for the four arms.
Fig 2.
(A) Kaplan-Meier disease-free survival plot for all randomly assigned patients by randomization arm. Number at risk at the beginning of each 12-month period is shown in the table. Three patients with no follow-up were excluded. (B) Kaplan-Meier overall survival plot of all randomly assigned patients by randomization arm. Three patients with no follow-up were excluded. (C) Kaplan-Meier disease-free survival plot by randomization arm for patients with triple-negative tumors (estrogen receptor negative, progesterone receptor negative, and human epidermal growth factor receptor 2 [HER2] negative). (D) Kaplan-Meier disease-free survival plot by randomization arm for patients with hormone receptor–positive (estrogen receptor–positive and progesterone receptor–positive) and HER2–negative tumors. 2wAC, doxorubicin-cyclophosphamide once every 2 weeks; AC + G, doxorubicin-cyclophosphamide with filgrastim; q 2 weeks, once every 2 weeks.
For the secondary OS outcome, the interaction remained statistically significant (P = .010). The HRs showed statistically significant differences among the four arms (P = .040), with the highest OS in the arm using once-every-2-weeks treatment for both doxorubicin-cyclophosphamide and paclitaxel. A Cox model yielded the following HRs relative to arm 1 (doxorubicin-cyclophosphamide once every 2 weeks; paclitaxel once every 2 weeks): for arm 2 (doxorubicin-cyclophosphamide with filgrastim; paclitaxel once every 2 weeks), the HR was 1.44 (95% CI, 1.08 to 1.93; P = .013); for arm 3 (doxorubicin-cyclophosphamide once every 2 weeks; paclitaxel once per week), the HR was 1.46 (95% CI, 1.09 to 1.95; P = .011); and for arm 4 (doxorubicin-cyclophosphamide with filgrastim; paclitaxel once per week), the HR was 1.24 (95% CI, 0.91 to 1.68; P = .17).
To better understand the possible interaction between the doxorubicin-cyclophosphamide schedule and the paclitaxel schedule, we performed separate unplanned subset analyses by biologic type of breast cancer based on local testing. Breast cancer was categorized as hormone receptor positive (ER positive or PgR positive) and HER2 negative (n = 1,481; 56%); ER negative, PgR negative, and HER2 negative (triple negative; n = 681; 26%); or HER2 positive (n = 475; 18%).
Outcomes for each biologic type are shown in Figure 2C, 2D, online-only Appendix, and the Data Supplement. For hormone receptor–positive tumors and HER2-positive tumors, there were no significant differences by treatment and no interaction between doxorubicin-cyclophosphamide and paclitaxel schedules. For triple negative tumors, there were nonsignificant trends for DFS (P = .077) and OS (P = .067), with the interactions between doxorubicin-cyclophosphamide and paclitaxel being significant (DFS P = .018; OS P = .010). OS was best when all treatment was administered every 2 weeks.
DISCUSSION
Continuous doxorubicin-cyclophosphamide with filgrastim and doxorubicin-cyclophosphamide once every 2 weeks differ in their toxicity profiles, with continuous doxorubicin-cyclophosphamide with filgrastim producing more stomatitis and dermatologic toxicity and doxorubicin-cyclophosphamide once every 2 weeks producing more myelosuppression and neutropenic fever. A nonsignificant trend for reduced cardiac toxicity was observed for once-per-week modest doses of doxorubicin compared with a higher dose given less frequently, consistent with previous reports.11,12 Quality-of-life assessments were not performed as a part of this trial, but continuous doxorubicin-cyclophosphamide with filgrastim would likely be associated with the greater degree of disruption of daily living, because it involves daily parenteral drug administration and is associated with an increased incidence of stomatitis. Furthermore, more patients receiving continuous doxorubicin-cyclophosphamide with filgrastim discontinued treatment compared with those receiving doxorubicin-cyclophosphamide once every 2 weeks; we do not recommend routine use of the continuous doxorubicin-cyclophosphamide with filgrastim regimen. Fatal toxicities were observed, underscoring the need for careful weighing of risks and benefits as our results and those from other trials are applied to clinical practice.
The two paclitaxel regimens studied represent the two most commonly used schedules in the adjuvant setting in the United States. Although paclitaxel once per week was associated with a higher incidence of leukopenia and neutropenia, this difference could be due in part to ascertainment bias, because patients treated once per week had blood counts performed once per week whereas those treated once every 2 weeks had mandated blood counts only once every 2 weeks. Moreover, the incidence of neutropenic fever did not differ between the two schedules. The once-every-2-weeks schedule produced more allergic-type reactions, musculoskeletal pain, and neurologic toxicity than the once-per-week schedule. It must be noted that six cycles of paclitaxel once every 2 weeks were given, rather than the four cycles commonly used. Four cycles of chemotherapy was found to be equivalent to six cycles in Cancer and Leukemia Group B 40101 (CALGB-40101; Cyclophosphamide and Doxorubicin [CA; 4 v 6 Cycles] Versus Paclitaxel [12 Weeks v 18 Weeks] As Adjuvant Therapy for Women With Node-Negative Breast Cancer: A 2 × 2 Factorial Phase III Randomized Study),9 and although that trial was performed in patients with lower risk than those entered onto our study, four cycles will likely continue to be the standard. In CALGB-40101, four cycles of paclitaxel produced less neurotoxicity than did six cycles, and it is not possible to directly compare 12 weeks of once-per-week paclitaxel with four cycles of once-every-2-weeks treatment. Musculoskeletal pain was clearly less with the once-per-week schedule, however, and it is unlikely that limiting treatment to four cycles would eliminate this difference.
A formal cost-effectiveness study was not performed. However, the costs of the protocol-specified treatment and related tests are less for 12 weeks of once-per-week paclitaxel compared with either six or four cycles of paclitaxel with pegfilgrastim once every 2 weeks.
S0221 was designed to compare chemotherapeutic regimens in patients in whom chemotherapy was felt to be the standard of care. Recent studies in node-negative and node-positive breast cancer indicate that tumor biology may be more important than traditional prognostic factors in determining the efficacy of chemotherapy. It is possible that patients with disease that was at high risk of recurrence but who had a poor likelihood of response to cytotoxic chemotherapy were entered onto this trial.13,14 It is also possible that biologic subtypes differ by global sensitivity to chemotherapy and also to the particular agents used and the doses and schedules of those agents. Many of the patients enrolled onto S0221 also consented to submitting tissue and blood samples. Evaluations of genetic factors associated with neurologic, hematologic, and GI toxicities have been performed, and additional studies are planned.15,16 Future studies of tissue biomarkers will allow assessment of their association with prognosis and possibly prediction of treatment benefit. Patients with highly endocrine-responsive disease may not have benefitted from chemotherapy, and inclusion of these patients may have obscured some differences between treatments in the sensitive subsets.
At a median follow-up of 6 years, an interaction between the two randomized factors had emerged, so that the two randomizations could not be analyzed independently. A trend toward a significant difference in DFS between the treatment arms has been observed, and a significant difference in OS between the arms is now present, favoring patients who received all treatment once every 2 weeks (Figures 2A and 2B). Examination of relevant biologic subsets reveals that this advantage for treatment once every 2 weeks is observed only in patients with hormone receptor–negative/HER2-negative disease. This observation is the result of a subset analysis that was not protocol specified and should be regarded as hypothesis generating.
It is unusual to see a significant difference in OS in the absence of a significant difference in DFS. OS is a summation of both breast cancer and treatment-related deaths and unrelated causes of mortality. Differences in OS then, are the result of either treatment-related differences in the former or random differences in the latter. Assessment of cause of death is difficult in a multicenter trial, and random differences in non–breast cancer–related deaths may have been a factor in the observed survival difference. However, we believe that this result may be, in part, a consequence of the higher relapse rate and relatively short postrelapse survival of patients with hormone receptor–negative/HER2-negative disease (median, 1.17 years), the subset that is driving the observed difference between the treatment arms compared with the lower relapse rate and longer postrelapse survival of patients with recurrent hormone receptor–positive (median, 1.82 years) or HER2-positive disease (median, 2.13 years).
Patients treated in this trial achieved equivalent DFS with any of our regimens, and treatment selection for this end point can be based on toxicity, cost, or patient preference. Patients with HER2-positive disease should be treated according to protocols that specifically studied this subset. Our study suggests the hypothesis that patients with hormone receptor–negative/HER2-negative high-risk breast cancers may have improved outcome when treated with the once-every-2-weeks regimen, and we recommend that this hypothesis be investigated in other studies.
Supplementary Material
Appendix
Authors and Study Groups
The following authors helped write this article for their respective study groups: George T. Budd, SWOG; Timothy J. Hobday, North Central Cancer Treatment Group (NCCTG); James A. Stewart, Eastern Cooperative Oncology Group (ECOG); Claudine Isaacs, Cancer and Leukemia Group B (CALGB); and Muhammad Salim, National Cancer Institute of Canada (NCIC).
Footnotes
Supported in part by Public Health Service Cooperative Agreement Grants No. CA32102, CA38926, CA21115, CA21076, CA77597, CA25224, CA77202, and CCSRI15469 awarded by the National Cancer Institute, Department of Health and Human Services; by Grants No. 021039, 015469, CA63844, CA46282, CA20319, CA63848, CA46441, CA35261, CA14028, CA76447, CA58658, CA67575, CA128567, CA04919, CA37981, CA22433, CA35281, CA58882, CA45560, CA45808, CA58861, CA95860, CA45807, CA13612, CA46368, CA27057, CA42777, CA86780, CA35176, CA35178, CA74647, CA68183, CA12644, CA58416, CA67663, CA63845, CA35431, CA11083, CA45377, CA35128, CA35262, CA35090, CA52654, CA45461, CA45450, CA76132, and CA35119 from the Canadian Cancer Society Research Institute; and in part by Amgen.
Presented at the 47th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, June 3-7, 2011, and at the 49th Annual Meeting of ASCO, Chicago, IL, May 31-June 4, 2013.
Clinical trial information: NCT00070564.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: George T. Budd, William E. Barlow, Kathy S. Albain, Robert B. Livingston, Julie R. Gralow
Administrative support: Gabriel N. Hortobagyi
Provision of study materials or patients: George T. Budd, Halle C.F. Moore, James A. Stewart, Kristine J. Rinn, Kathy S. Albain, Helen K. Chew, Timothy D. Moore, Gordan Srkalovic, Lawrence E. Flaherty, Julie R. Gralow
Collection and assembly of data: George T. Budd, William E. Barlow, Halle C.F. Moore, Timothy J. Hobday, Claudine Isaacs, Jonathan K. Cho, Gary V. Burton, Timothy D. Moore, Gordan Srkalovic, Bradley A. McGregor, Lawrence E. Flaherty, Gabriel N. Hortobagyi
Data analysis and interpretation: George T. Budd, William E. Barlow, Halle C.F. Moore, James A. Stewart, Claudine Isaacs, Muhammad Salim, Kristine J. Rinn, Helen K. Chew, Danika L. Lew, Julie R. Gralow, Gabriel N. Hortobagyi
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
SWOG S0221: A Phase III Trial Comparing Chemotherapy Schedules in High-Risk Early-Stage Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
George T. Budd
Research Funding: Genentech (Inst), Threshold Pharmaceuticals (Inst), MabVax Therapeutics (Inst), ZIOPHARM Oncology (Inst)
William E. Barlow
No relationship to disclose
Halle C.F. Moore
Research Funding: Clarient (Inst)
Timothy J. Hobday
No relationship to disclose
James A. Stewart
No relationship to disclose
Claudine Isaacs
Honoraria: Genentech/Roche, Celgene
Speakers' Bureau: Genentech, Celgene
Research Funding: Novartis (Inst), Pfizer (Inst), Nektar Therapeutics (Inst)
Muhammad Salim
No relationship to disclose
Jonathan K. Cho
No relationship to disclose
Kristine J. Rinn
No relationship to disclose
Kathy S. Albain
No relationship to disclose
Helen K. Chew
No relationship to disclose
Gary V. Burton
No relationship to disclose
Timothy D. Moore
No relationship to disclose
Gordan Srkalovic
No relationship to disclose
Bradley A. McGregor
Stock or Other Ownership: Gilead, Celgene
Lawrence E. Flaherty
No relationship to disclose
Robert B. Livingston
No relationship to disclose
Danika L. Lew
No relationship to disclose
Julie R. Gralow
Consulting or Advisory Role: Pfizer
Research Funding: Roche/Genetech (Inst), Novartis (Inst), Amgen (Inst)
Gabriel N. Hortobagyi
Consulting or Advisory Role: Pfizer, Antigen Express, Novartis, Galena Biopharma, Genentech, Amgen
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: Novartis
REFERENCES
- 1.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Adjuvant capecitabine, docetaxel, cyclophosphamide, and epirubicin for early breast cancer: Final analysis of the randomized FinXX trial. J Clin Oncol. 2011;30:11–18. doi: 10.1200/JCO.2011.35.4639. [DOI] [PubMed] [Google Scholar]
- 2.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swain SM, Tang G, Geyer CE, Jr, et al. Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: The NSABP B-38 trial. J Clin Oncol. 2013;31:3197–3204. doi: 10.1200/JCO.2012.48.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 5.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis GK, Livingston RB, Gralow JR, et al. Dose-dense anthracycline-based chemotherapy for node-positive breast cancer. J Clin Oncol. 2002;20:3637–3643. doi: 10.1200/JCO.2002.12.113. [DOI] [PubMed] [Google Scholar]
- 7.Ellis GK, Barlow WE, Gralow JR, et al. Phase III comparison of standard doxorubicin and cyclophosphamide versus weekly doxorubicin and daily oral cyclophosphamide plus granulocyte colony-stimulating factor as neoadjuvant therapy for inflammatory and locally advanced breast cancer: SWOG 0012. J Clin Oncol. 2011;29:1014–1021. doi: 10.1200/JCO.2009.27.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 9.Shulman LN, Cirrincione CT, Berry DA, et al. Six cycles of doxorubicin and cyclophosphamide or paclitaxel are not superior to four cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. J Clin Oncol. 2012;30:4071–4076. doi: 10.1200/JCO.2011.40.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demets DL. Group sequential procedures: Calendar versus information time. Stat Med. 1989;8:1191–1198. doi: 10.1002/sim.4780081003. [DOI] [PubMed] [Google Scholar]
- 11.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 12.Chlebowski RT, Paroly WS, Pugh RP, et al. Adriamycin given as a weekly schedule without a loading course: Clinically effective with reduced incidence of cardiotoxicity. Cancer Treat Rep. 1980;64:47–51. [PubMed] [Google Scholar]
- 13.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 14.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sucheston LE, Zhao H, Yao S, et al. Genetic predictors of taxane-induced neurotoxicity in a SWOG phase III intergroup adjuvant breast cancer treatment trial (S0221) Breast Cancer Res Treat. 2011;130:993–1002. doi: 10.1007/s10549-011-1671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao S, Sucheston LE, Zhao H, et al. Germline genetic variants in ABCB1, ABCC1 and ALDH1A1, and risk of hematological and gastrointestinal toxicities in a SWOG Phase III trial S0221 for breast cancer. Pharmacogenomics J. 2014;14:241–247. doi: 10.1038/tpj.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.