Abstract
Purpose
Interleukin-15 (IL-15) has significant potential in cancer immunotherapy as an activator of antitumor CD8 T and natural killer (NK) cells. The primary objectives of this trial were to determine safety, adverse event profile, dose-limiting toxicity, and maximum-tolerated dose of recombinant human IL-15 (rhIL-15) administered as a daily intravenous bolus infusion for 12 consecutive days in patients with metastatic malignancy.
Patients and Methods
We performed a first in-human trial of Escherichia coli–produced rhIL-15. Bolus infusions of 3.0, 1.0, and 0.3 μg/kg per day of IL-15 were administered for 12 consecutive days to patients with metastatic malignant melanoma or metastatic renal cell cancer.
Results
Flow cytometry of peripheral blood lymphocytes revealed dramatic efflux of NK and memory CD8 T cells from the circulating blood within minutes of IL-15 administration, followed by influx and hyperproliferation yielding 10-fold expansions of NK cells that ultimately returned to baseline. Up to 50-fold increases of serum levels of multiple inflammatory cytokines were observed. Dose-limiting toxicities observed in patients receiving 3.0 and 1.0 μg/kg per day were grade 3 hypotension, thrombocytopenia, and elevations of ALT and AST, resulting in 0.3 μg/kg per day being determined the maximum-tolerated dose. Indications of activity included clearance of lung lesions in two patients.
Conclusion
IL-15 could be safely administered to patients with metastatic malignancy. IL-15 administration markedly altered homeostasis of lymphocyte subsets in blood, with NK cells and γδ cells most dramatically affected, followed by CD8 memory T cells. To reduce toxicity and increase efficacy, alternative dosing strategies have been initiated, including continuous intravenous infusions and subcutaneous IL-15 administration.
INTRODUCTION
The goal of cancer immunotherapy is to stimulate the immune system to attack cancer cells. Recombinant interleukin-2 (IL-2) is a prototypic immunotherapeutic treatment for patients with metastatic malignancy.1 Despite its accepted role, IL-2 has negative effects. IL-2 has a dual role as an immunomodulator, stimulating proliferation of effector cells that kill cancer cells but also suppressing immune responses by maintenance of inhibitory CD25+ Foxp3+ T regulatory cells (Tregs) and activation-induced cell death (AICD).2,3 These issues prompted a search for other immunotherapeutics with benefits of IL-2 but fewer negative adverse effects (AEs).4
IL-15 was described nearly simultaneously by the Waldmann laboratory and by Grabstein et al.5–7 IL-2 and IL-15 use unique cytokine-specific receptor α chains: IL-2 receptor α (IL-2Rα; CD25) for IL-2 and IL-15Rα (CD215) for IL-15. They share a common IL-2/IL-15Rβ chain (CD122)6,7 and, with other cytokines, the common γc chain (CD132).8–11 Both cytokines stimulate proliferation of T cells, induce generation of cytotoxic lymphocytes (CTLs), and stimulate prolonged expansion of natural killer (NK) cells.8,12–14 However, in many adaptive immune responses, IL-2 and IL-15 have distinct roles.8,12–16 In contrast to IL-2, IL-15 inhibited IL-2–mediated AICD, less consistently activated Tregs, and did not cause a significant capillary leak syndrome in mice or nonhuman primates (NHPs).4,17 In preclinical toxicology studies, IL-15 induced prolonged expansion and activation of NK cells and CD8 memory T cells.8,12–13
After distinct roles of IL-2 and IL-15 were defined, the question emerged of how these two cytokines manifest different functions. One feature that might account for their distinct modes of action is that IL-2 is a secreted molecule. In contrast, IL-15 is only secreted in small amounts but rather induces signaling in the context of cell-cell contact at an immunologic synapse.8,18–21 Dendritic cells (DCs) stimulated by interferons or activated through CD40 coordinately express IL-15 and IL-15Rα.18–21 Trans-presentation of IL-15 to NK and CD8 memory phenotype T cells expressing IL-2/IL-15Rβ and γc occurs via heterodimeric IL-15 and IL-15Rα associated at cell surfaces.18–21 The ability of IL-15 to activate crucial effector T and NK cells, its inhibition of AICD, and its capacity to maintain function of CD8 memory T cells provided the scientific basis for evaluation of IL-15 as a cancer immunotherapeutic. IL-15 demonstrated activity in syngeneic murine TRAMP (Transgenic Adenocarcinoma Mouse Prostate) C2 prostatic cancer, Pmel-1 and B16 melanoma, and MC38 and CT26 colon carcinoma models.8,21–29
On the basis of these data, an Escherichia coli (E coli) recombinant DNA–based process was developed to provide clinical-grade recombinant human IL-15 (rhIL-15) that ultimately produced a nonglycosylated single-chain peptide of 150 amino acids with a calculated molecular weight of 12,901 Da. This material underwent preclinical toxicology testing with 12 daily intravenous bolus infusions of rhIL-15 in rhesus macaques.4,30,31 Laboratory analyses were unremarkable, with the exception of transient grade 3 to 4 neutropenia in three of six animals because of redistribution from circulation to tissues.4,31 Bolus rhIL-15 treatment produced a four- to eight-fold increase in numbers of circulating NK and CD8 T cells.4,31 We initiated a first-in-human phase I clinical trial of bolus intravenous infusions of rhIL-15 in adults with metastatic malignant melanoma and metastatic renal cell cancer.
PATIENTS AND METHODS
The primary objectives of this trial were to determine the safety, AE profile, dose-limiting toxicity (DLT), and maximum-tolerated dose (MTD) of intravenous bolus rhIL-15 administered as a daily intravenous bolus infusion for 12 consecutive days in patients with metastatic malignant melanoma or metastatic renal cell cancer. rhIL-15 was produced under current good manufacturing practice conditions in an E coli expression system as previously described.4 This was a single-institution, open-label, nonrandomized 3 + 3 design, phase I dose-escalation study of IL-15 in patients with metastatic malignancy. Groups of three to six patients were scheduled to receive IL-15 daily by bolus infusion for 12 days. The initial study plan included intergroup escalating doses; however, reduced levels of 1.0 and 0.3 μg/kg per day were added after two of the first patients treated with 3.0 μg/kg per day experienced DLTs (Appendix, online only).
Flow-Cytometry Analysis
Polychromatic flow-cytometry analysis was performed, as described previously, on heparinized blood preinfusion and at various times after the first infusion, with analysis of multiple leukocyte populations including naïve, central memory, and effector memory CD4 and CD8 T cells.32 Proliferating cells were detected by analysis of intracellular Ki-67 on T-cell subsets (Appendix, online only).
Pharmacokinetic Analysis
The rhIL-15 concentrations in serum were assayed using human IL-15–specific enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN). Noncompartmental pharmacokinetic analysis of data was undertaken using the software package WINNonlin (version 5.0; Scientific Consultant, Apex, NC; Pharsight, St Louis, MO; Appendix, online only).
RESULTS
Redistribution, Hyperproliferation, and Activation of Multiple Lymphocyte Subsets After IL-15
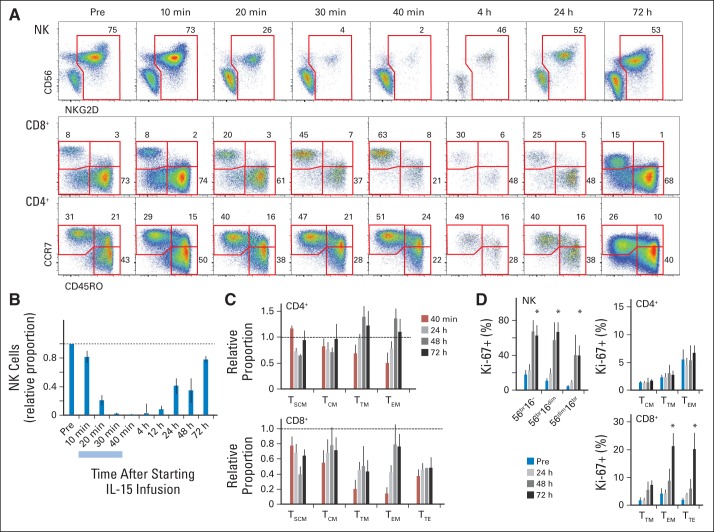
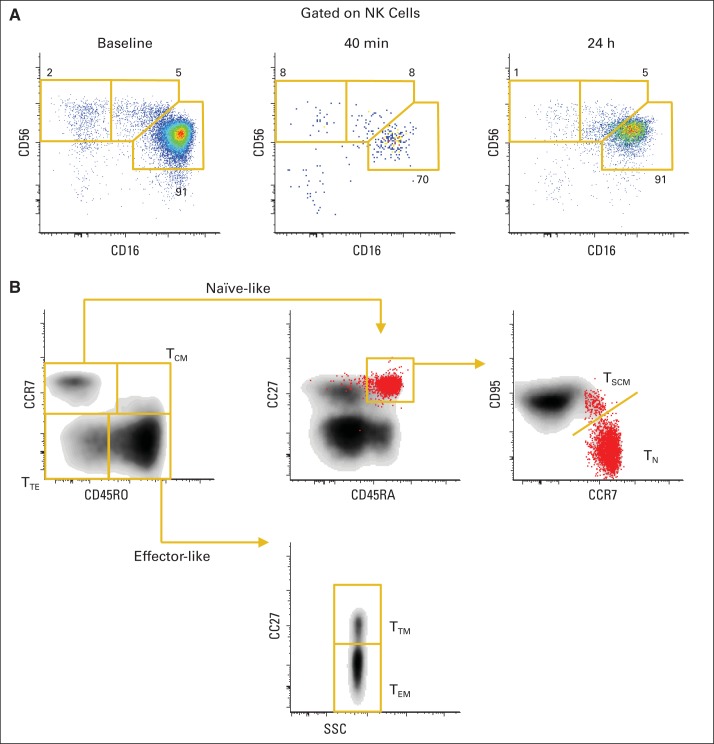
Five patients with metastatic malignant melanoma or renal cell cancer received bolus intravenous infusions of 3.0 μg/kg per day, four received 1.0 μg/kg per day, and nine received 0.3 μg/kg per day for 12 days (Appendix Table A1, online only). Accompanying IL-15 administration was dynamic in the blood lymphocyte compartment, beginning immediately during the first infusion. Reduction in the number of current lymphocyte subsets could be seen within 20 minutes of the infusion of IL-15 (Figs 1A to 1D) for all four patients sampled. The most rapid and marked decline in circulation level was observed with NK and CD8 memory cells (Fig 1A). Even 10 minutes after infusion, margination or efflux of NK populations could be seen (Fig 1B), and the cells were nearly absent by 30 minutes. All subsets of NK cells were equally affected (Appendix Figs A1A and A1B, online only). CD8 cells also showed an acute efflux from the circulating blood, most pronounced for transitional and effector (TEM) memory subsets (Fig 1C). For CD4 cells, in contrast, only CD4 TEM showed a mild response. A mild decrease in all circulating lymphocyte subsets was observed during this period.
Fig 1.
Acute lymphocyte dynamics after interleukin-15 (IL-15) administration. (A) Representation of natural killer (NK) cells among CD3− lymphocytes (top) and T-cell differentiation stages among CD8 (middle) or CD4 (bottom) cells are shown for one individual administered 3 μg/kg of IL-15 during 30-minute infusion; 10-, 20-, and 30-minute time points were taken during administration. Numbers outside red lines indicate percentage of cells inside each gate. (B) Change from baseline in proportion of NK cells is shown as mean ± SEM for four individuals. Light blue bar indicates IL-15 infusion time. (C) Changes from baseline in representation of relative proportions of CD4 and CD8 subsets are shown as mean ± SEM. Subsets were gated. (D) Proliferation (percentage of Ki-67) among NK, CD4, and CD8 subsets is shown for four individuals. TCM, T central memory; TEM, T effector memory; TSCM, T stem-cell memory; TTE, T terminal effector; TTM, T transitional memory.
After infusion, influx of NK cells back to circulating blood was detected by 4 hours, followed by a slow normalization of cell numbers over 2 to 3 days. Notably, influx of cells during the first 2 days was the result of redistribution, because no evidence of proliferation (Ki-67) was seen until at least 48 hours (Fig 1D).
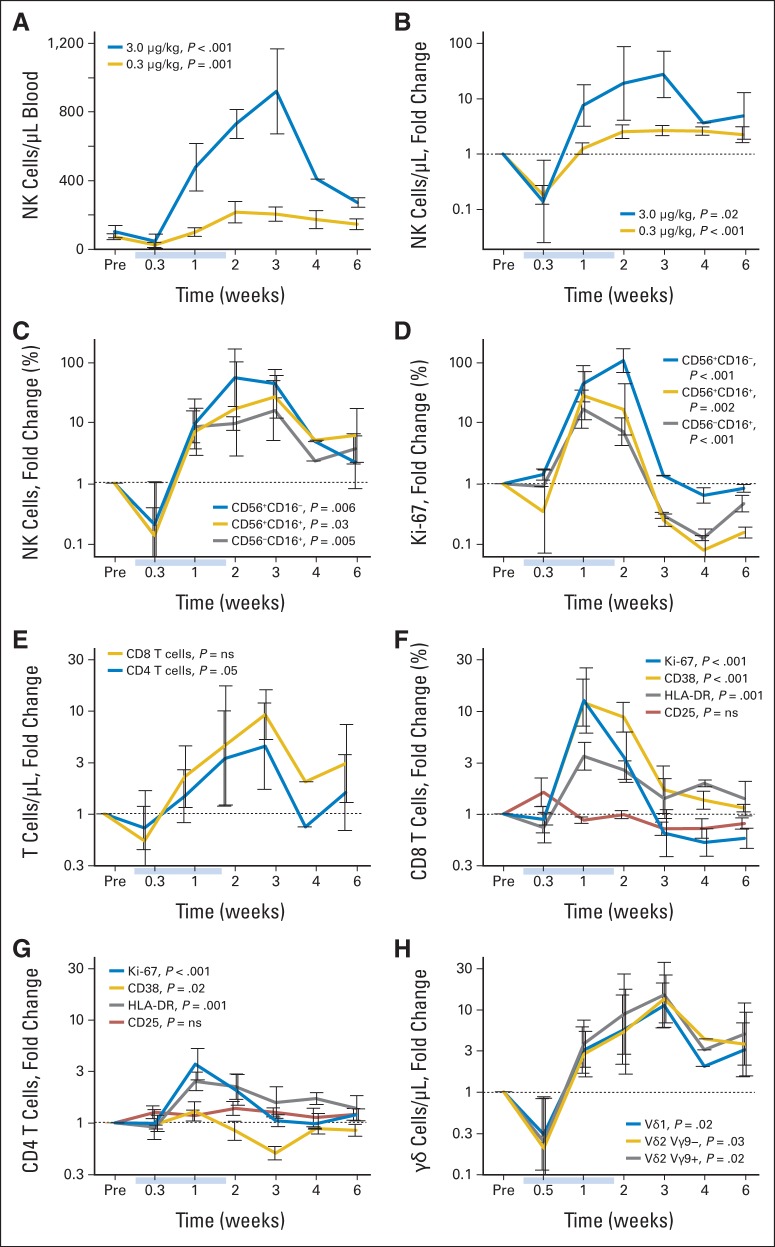
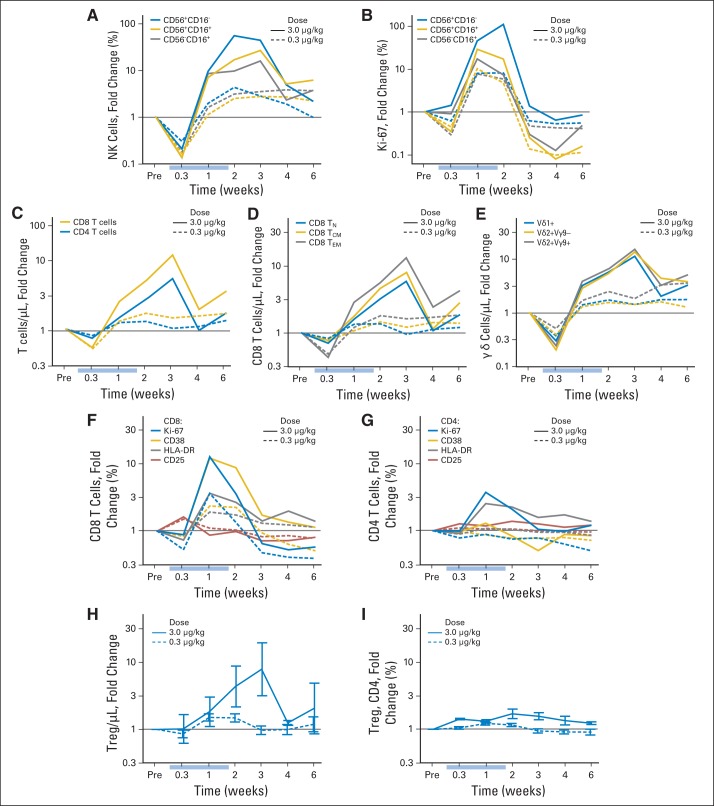
Peripheral-blood mononuclear cells from nearly all patients were analyzed beginning 2 days after the first daily infusion and continuing through 6 weeks after the last infusion (Figs 2A to 2H). Changes in subset proportions (and absolute counts) were far more evident for patients receiving 3 μg/kg than for those receiving the lower doses (Appendix Figs A2A to A2I, online only). In the chronic phase (> 3 days), hyperproliferation (Ki-67) and increases in circulating NK-cell numbers occurred. For example, absolute NK-cell counts increased > 10-fold to > 800/μL with the high 3-μg/kg per day dose but only two- to three-fold to < 200/μL with the low 0.3-μg/kg per day dose (Fig 2A). The increase in NK-cell counts (Fig 2C) was accompanied by increases in the proportions of proliferating cells as demonstrated by Ki-67 staining (Fig 2D). After IL-15 administrations, cell counts slowly returned to normal over more than 6 weeks, accompanied by hypoproliferation. As cell counts returned to baseline, proliferation likewise returned to normal homeostatic levels.
Fig 2.
Lymphocyte dynamics during and after daily interleukin-15 (IL-15) infusions. All data are shown as mean ± SEM for administration of IL-15 3 μg/kg (n = 4; all panels) or (A, B) 0.3 μg (n = 9). All fold-change values were computed by individual relative to baseline (Pre). Light blue bars indicate IL-15 infusion times. (A) Absolute count and (B) change in absolute count for total natural killer (NK) cells. (C) Representation within NK cells and (D) proliferation state of differentiation stages of NK cells. (E) Absolute CD4 and CD8 T-cell counts. Activation and proliferation states of (F) CD8 and (G) CD4 cells based on any of four markers. (H) Absolute counts of γδ T-cell lineages. P values are from two-tailed Student's t test for peak time point.
T-cell subsets showed a similar but more nuanced dynamic. CD8 T cells (eight-fold expansion) were more strongly affected than CD4 T cells (three-fold expansion; Fig 2E) and showed evidence of activation, including proliferation (Ki-67) and increased expression of CD38 and HLA-DR. Interestingly, there was no change in expression of CD25 (Fig 2F). CD4 T cells showed mild proliferation and a slight increase only in HLA-DR (Fig 2G). Tregs (defined as CD4+CD127−CD45RO+CD25+ T cells) increased with CD4 T cells (Appendix Fig A2H, online only) at the highest dose of IL-15, but this increase was not greater than that observed with other CD4 subsets (Appendix Fig A2I, online only). Finally, γδ T cells responded nearly as strongly and in the same fashion as NK cells (Fig 2H).
Pharmacokinetics
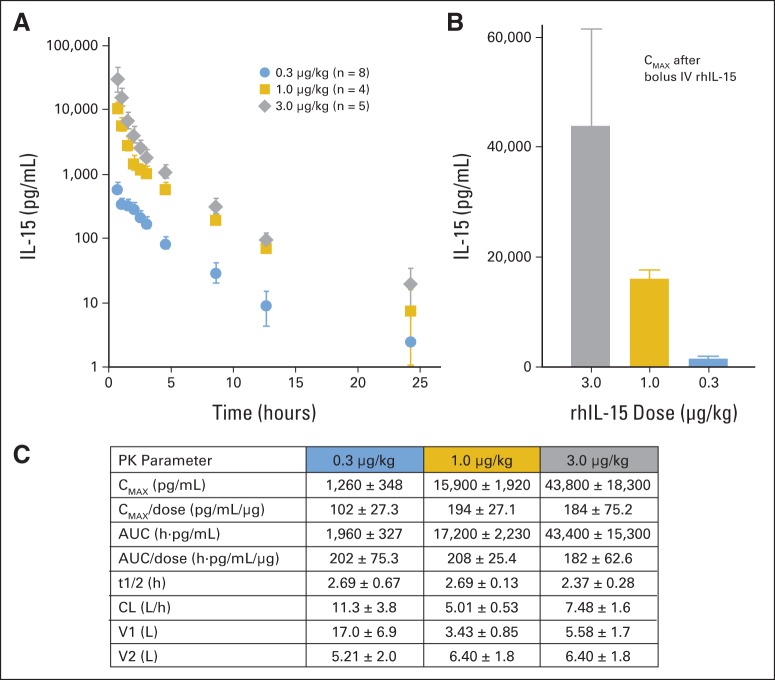
Pharmacokinetic results for rhIL-15 for patients are summarized in Figures 3A to 3C. Mean values (± SEM) for various parameters were as follows: 3.0-, 1.0-, and 0.3-μg/kg per day doses resulted in a maximum serum concentration (Cmax) of 43,800 ± 18,300, 15,900 ± 1,900, and 1,260 ± 350 pg/mL, respectively; areas under the serum concentration versus time curve were 43,400 ± 5,300, 17,200 ± 2,200, and 1,960 ± 330 pg*h/mL. Half-lives were similar for the three dose levels: 2.4 ± 0.25, 2.7 ± 0.13, and 2.7 ± 0.67 hours. The nonlinearity of the Cmax and shorter survival half-life observed with the lowest IL-15 dose suggested with this dose, there may be clearance by binding to the high-affinity IL-15 receptors as well as by a non–IL-15R–mediated mechanism shared with the higher doses.
Fig 3.
Pharmacokinetic (PK) analysis after infusions of recombinant human interleukin-15 (rhIL-15) in patients with metastatic malignancy. Mean ± SEM shown for patients in each dose group. (A) Plasma concentrations of rHIL-15 after infusion. (B) Maximum serum concentration (Cmax) after bolus intravenous infusions of rhIL-15 3.0, 1.0, and 0.3 μg/kg per day. (C) PK parameters for all patients. AUC, area under plasma concentration versus time curve; CL, clearance; t1/2, half-life; V1, volume of distribution into central compartment; V2, volume of distribution into peripheral compartment.
Toxicity
Primary objectives of this phase I first-in-human study of bolus intravenous rhIL-15 in adults with metastatic malignant melanoma or metastatic renal cell cancer were to determine toxicity profile, safety, DLT, and MTD of intravenous rhIL-15 administered as daily intravenous bolus infusions for 12 consecutive days. Appendix Tables A1 and A2 (online only) summarize the enrolled patients' diagnoses, doses of rhIL-15, number of doses of rhIL-15 received, reasons treatment was halted, and patients' best responses.
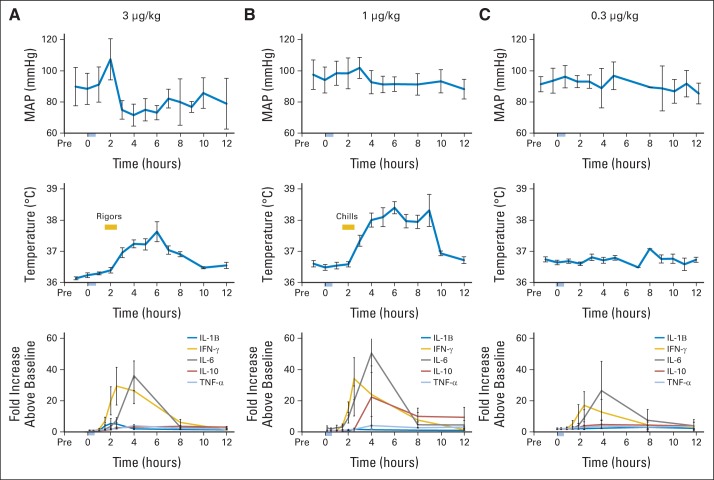
Clinical and laboratory toxicities for patients treated in this trial are summarized in Figures 4A to 4C and Appendix Table A3 (online only). Grade 3 toxicities were fever and DLTs of thrombocytopenia and hypotension. The only grade 4 toxicity was transient non-DLT lymphocytopenia (Appendix Table A3, online only). After an 83-year-old woman with metastatic melanoma developed symptomatic grade 3 hypotension, and another patient developed grade 3 thrombocytopenia (platelet count nadir, 35,000/μL) after six doses, in part because of preprotocol radiotherapy to his right hemipelvis just before starting rhIL-15 in the 3.0-μg/kg dosing cohort, the protocol was amended to add two lower dose levels (1.0 and 0.3 μg/kg per day). Two of four patients at the 1.0-μg/kg per day dose had persistent grade 3 ALT and AST elevations that were DLTs. One of these patients was known to have a history of steatohepatitis, and the other developed micrometastases to the liver. All nine patients with IL-15 administered at 0.3 μg/kg per day received all 12 doses without DLTs, and four patients completed a second course of treatment. One of these patients developed grade 3 pneumonitis/pneumonia. The MTD of bolus rhIL-15 was determined to be 0.3 μg/kg per day. There was a consistent temporal pattern of post-treatment AEs noted in patients receiving 3.0 μg/kg per day, with fever beginning 2.5 to 4 hours after start of rhIL-15 infusions, reliably peaking at 3 to 4 hours (Fig 4A). Rigors occurred at approximately the 1.5- to 2-hour time point, and blood pressure dropped to a nadir approximately 20 mm/Hg below pretreatment level, usually beginning not long after rigors. These changes overlapped with maximum IL-6 and interferon gamma (IFN-γ) levels (Figs 4A to 4C). In contrast to the severe capillary leak observed in patients receiving high-dose IL-2, patients had evidence of only modest capillary leak, seen as an imbalance between fluid intake and urinary output, decreases in serum albumin concentrations, and weight gain or pulmonary toxicity during treatment. Some patients received infusions of 25% albumin to maintain serum albumin > 3 g/dL, with diuretics as needed. Chills rather than rigors and increased temperature, but no significant blood pressure changes, were seen in patients receiving 1.0 μg/kg per day (Fig 4B). All common cytokine-related AEs including fever, rigors, and hypotension occurred much less frequently in patients treated with the 0.3-μg/kg dose level (Fig 4C). Importantly, when evaluated 21, 28, and 42 days after initiation of the 12 infusions of rhIL-15, none of the patients produced an antibody to administered rhIL-15, as assessed using a two-arm capture ELISA assay.
Fig 4.
Clinical and immunologic responses to recombinant human interleukin-15 (rhIL-15) infusions. For patients administered rhIL-15 (A) 3, (B) 1, or (C) 0.3 μg/kg, mean arterial pressure (MAP; upper panels) and temperature (middle panels) are shown as mean ± SEM after 12 daily infusions of rhIL-15 for one patient in each group; plasma cytokine concentrations are shown as mean ± SEM for all patients in each group after first infusion (lower panel). Fever, rigors (occurring within 2.5 to 4 hours), and hypertension followed by hypotension occurred contemporaneously with elevations in IL-6 and interferon gamma (IFN-γ). TNF-α, tumor necrosis factor alpha.
Cytokine Production After rhIL-15 Treatment
Toxicities were associated with a high Cmax for IL-15 and consequent inflammatory cytokine secretion. Serial assays of inflammatory cytokine levels are shown in Figures 4A to 4C. After bolus rhIL-15 treatment with 3.0 μg/kg per day, there was cytokine release, with marked increases in serum inflammatory cytokine concentrations that manifested a relationship consistent in time with AEs including fever, rigors, and hypotension (Figs 4A to 4C). Decreases in mean arterial blood pressure were primarily the result of lower diastolic pressures, strongly suggesting a transient loss of vascular tone. There were marked elevations of IFN-α and IL-6, with maximum-fold increases (± standard deviation) for the latter of 30- ± 35-, 47- ± 50-, and 21- ± 20-fold elevations with the 3.0-, 1.0-, and 0.3-μg/kg per day dose groups, respectively, with a peak at 2 to 4 hours after the infusion and 4 hours before the onset of hypotension. There were consistently high serum concentrations of IL-6Rα and IL-8 that peaked at the 8-hour time point—a period after fever, rigors, and initiation of hypotension. As another indicator of immune activation, elevations of IL-2Rα were observed on days 8 and 14 after initiation of infusions.
Preliminary Information on Antitumor Effects of rhIL-15 in Patients With Metastatic Malignancy.
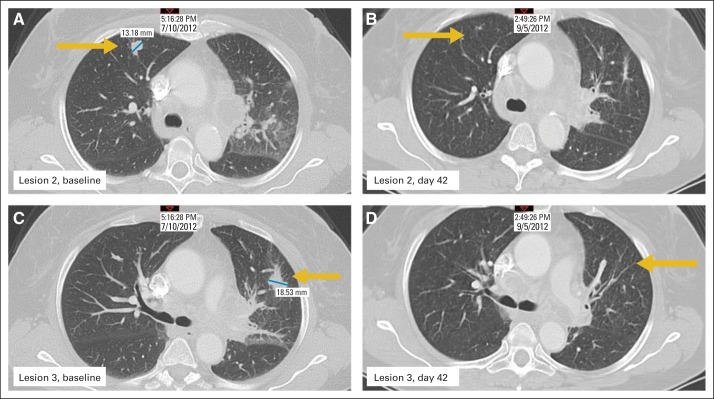
In this first in-human phase I trial, evidence of antitumor activity was assessed by restaging computed tomography scans and determination of clinical responses by RECIST criteria. Overall, there were no responses, with stable disease as a best response. However, five patients manifested a decrease between 10% and 30% in their marker lesions, with two of these patients experiencing clearing of lung lesions, as shown in Figures 5A to 5D.
Fig 5.
Clinical activity of recombinant human interleukin-15 (rhIL-15) in patient with metastatic malignant melanoma. There were no objective remissions in 18 patients, with best response being stable disease. However, five patients had decreases of 10% to 30% in marker lesions. Two patients, including the one shown (patient No. 16), experienced clearance of lung lesions; lesion 2 at (A) baseline and (B) day 42, and lesion 3 at (C) baseline and (D) day 42. Yellow arrows indicate lung lesions; blue lines indicate area measured.
DISCUSSION
IL-15 administration altered homeostasis of lymphocyte subsets in blood. NK cells and γδ T cells were most substantially affected, followed by CD8 memory cells. Response of CD8 naïve T cells and CD4 T cells were less marked. Coordinate measurement of redistribution, proliferation, and activation markers allowed us to develop a model of homeostasis accompanying IL-15 therapy (Fig 6). Immediately after infusion of IL-15, cells marginated or effluxed from circulating blood so that they were nearly absent by 30 minutes. Cell counts then returned over the next 48 hours in absence of proliferation, suggesting that the early reduction in circulating cell numbers was the result of redistribution, not cytolysis. After 48 hours, however, substantial hyperproliferation occurred, leading to continued increases in cell numbers to as high as 10-fold above baseline. After cessation of IL-15 administration, proliferation fell quickly to below-baseline levels. This hypoproliferation state was maintained until counts returned to baseline, after which normal homeostasis was restored. Thus, IL-15 affected homeostasis primarily through two mechanisms: first, redistribution immediately after administration, and second, regulation of proliferation initially to augment circulating numbers and then hypoproliferation to achieve and maintain normal cell numbers. There was only a modest and nonselective increase in the number of Tregs with 0.3-μg/kg per day IL-15 infusions in contrast to the three- to > five-fold increases observed with high-dose IL-2 administration.33–36
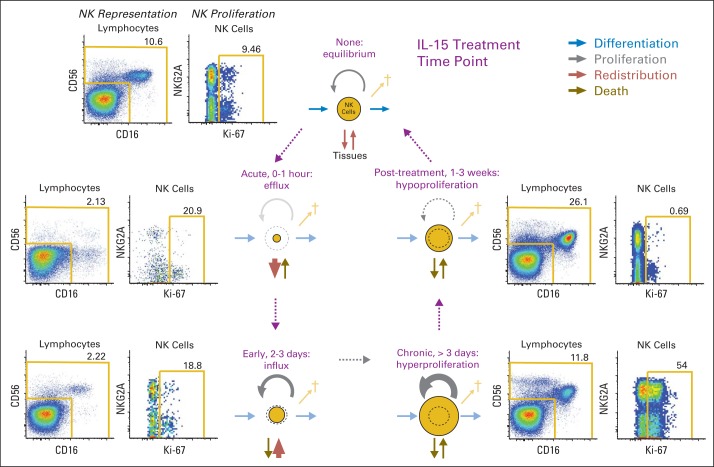
Fig 6.
Model of interleukin-15 (IL-15) –driven homeostasis. Accompanying IL-15 administration, absolute numbers of population of cells in circulating blood (eg, natural killer [NK] cells) were affected by several pathways. During and immediately after infusions, there was margination or efflux of NK populations from circulating blood so that they were nearly absent in circulation by 30 minutes. By 4 hours after infusion, influx of NK cells was detected, followed by slow normalization of cells over 2 to 3 days. After 3 days from initiation of infusions, there was hyperproliferation yielding absolute NK-cell counts > 10-fold over baseline. In the 1- to 3-week period after treatment, there was hypoproliferation until cell counts returned to baseline, after which normal homeostasis was restored. Numbers outside gold lines indicate percentage of cells inside each gate.
We observed marked elevations in IL-6, IL-8, and IFN-γ as well as increases in IL-10, tumor necrosis factor α, and IL-1β that also occurred within 2 to 4 hours after rhIL-15 administration (Figs 4A to 4C). Acute clinical toxicities such as fever, chills, or rigors and blood pressure changes occurred in a consistent temporal pattern related to the daily bolus administrations of rhIL-15, coincidental with increases in these inflammatory cytokines. The pattern of high IFN-α and IL-6 is characteristic of macrophage activation syndrome.37 It is interesting that IL-6, which showed the greatest increase above basal level, was recently shown to be the primary cytokine mediator of toxicity in patients with leukemia treated with chimeric antigen receptor effector cells.38,39 Additional reports have demonstrated administration of the IL-6R antagonist tocilizumab ablated severe AEs including fever, hypotension, and hypoxia in patients with macrophage activation syndrome and in those treated with chimeric antigen receptor cells.37,40
Ultimately, we concluded that rhIL-15 proved too difficult to administer as an intravenous bolus dose because of clinical toxicities produced by intense cytokine secretion that occurred in the first 2 hours after treatment. There were exceedingly high IL-15 Cmax levels initially after bolus infusions that were sufficient to signal through the IL-2/IL-15Rβ and γc receptor pair that IL-15 shares with IL-2, thereby contributing to toxicities observed. To reduce Cmax and increase the period of time when IL-15 is at an optimal concentration for heterotrimeric high-affinity IL-15Rs, we evaluated alternative dosing strategies in NHP rhesus macaques.30 By administering IL-15 by continuous intravenous infusions (CIVs) or subcutaneously to these NHPs, the exceedingly high Cmax observed with bolus infusions was avoided. With a bolus intravenous infusion to rhesus macaques at 20 μg/kg per day, the Cmax was 720 ng/mL4 (Appendix Fig A3, online only). In contrast, with subcutaneous infusions of 20 μg/kg per day, the Cmax was 50 ng/mL, and with continuous intravenous infusion of 20 μg/kg per day, the IL-15 Cmax was between 2 and 4 ng/mL throughout the 10-day study period.30 In contrast to the four- to eight-fold expansion of CD8 TEM cells observed with bolus administration, CIV rhIL-15 at a dose of 20 μg/kg per day for 10 days produced a massive (80- to 100-fold) expansion of circulating CD8+ TEM cells.4,30 Subcutaneous rhIL-15 at 20 or 40 μg/kg per day resulted in more modest (10-fold) expansions of CD8 T effector memory cells that was still superior to the intravenous bolus schedule.30 To translate these preclinical trials with the goal of reducing the Cmax and excess cytokine release and maintaining optimal IL-15 levels for a long period, we initiated a dose-escalation trial of CIV rhIL-15 administered to patients with metastatic malignancy. Furthermore, we joined with the Cancer Immunotherapy Trials Network in a phase I dose-escalation trial of subcutaneous rhIL-15 administered 5 days per week for 2 weeks.
It is hoped that with new dosing strategies, we can reduce toxicity and increase the expansion of lymphoid populations and thereby improve the antitumor effects of rhIL-15 in patients with metastatic malignancy. It is clear that IL-15 activates NK cells, monocytes, γδ, and memory phenotype CD8 T cells, augmenting, it is hoped, an already existing albeit inadequate immune response to a patient's tumor cells. This IL-15–mediated activation of monocytes and NK cells suggests that IL-15 may be of value in augmenting antibody-dependent cellular cytotoxicity. Major preclinical studies are under way to test that hypothesis.
Appendix
Study Objectives and Design
The primary objectives of this trial were to determine the safety, adverse event profile, dose-limiting toxicity (DLT), and maximum-tolerated dose (MTD) of intravenous bolus recombinant human interleukin-15 (rhIL-15) administered as a daily intravenous bolus infusion for 12 consecutive days in patients with metastatic malignant melanoma or metastatic renal cell cancer. Secondary objectives included4: determination of rhIL-15 pharmacokinetics, in particular the time course of decline of rhIL-15 from serum after intravenous administration31; determination of the immunogenicity of rhIL-15 in patients receiving this drug32; characterization of the biologic effects of rhIL-15 on the proportions and absolute numbers of circulating natural killer (NK) cells, CD45RO+CD8+ T cells and central and effector memory T-cell subsets based on expression of CD28, CD95, CCR7, and CD62L by flow cytometry4; and determination of the effects of IL-15 on the serum levels of proinflammatory cytokines. An additional secondary objective was to obtain preliminary information on the antitumor effects of repeated cycles of rhIL-15 in patients with metastatic malignant melanoma or metastatic renal cell cancer.
This was a single-institution, open-label, nonrandomized 3 + 3 design, phase I dose-escalation study to determine the safety and toxicity of IL-15 in patients with metastatic malignancy. The study was approved by the Institutional Review Board of the National Cancer Institute, National Institutes of Health. Written informed consent was obtained from all patients. Groups of three to six patients were scheduled to receive IL-15 daily by bolus infusions for 12 days. The initial study plan included intergroup escalating doses; however, reduced dose levels at 1.0 and 0.3 μg/kg per day were added after two of the first patients treated with 3.0 μg/kg per day experienced DLTs.
The rationale for the starting dose of 3 μg/kg was as follows: In the preclinical toxicity study in rhesus macaques, six macaques each received IL-15 10, 20, and 50 μg/kg by daily bolus infusion for 12 infusions, and a single animal received 200 μg/kg. The 200-μg/kg dose was associated with diarrhea, decreased appetite, emesis, and lethargy and was deemed to be beyond the MTD. The 10-, 20-, and 50-μg/kg bolus infusions for 12 days were well tolerated in the rhesus macaques, with the only toxicities being diarrhea in three of six animals and emesis in two of six animals in the group receiving 50 μg/kg. There was no toxicity observed with the 10-μg/kg dose. On the basis of these studies, it was predicted that the 3-μg/kg dose would be a no observed adverse effect level dose with no toxicity. However, these studies in rhesus macaques did not accurately predict the cytokine release and macrophage activation syndromes characterized by fever, rigors, and hypotension associated with marked elevations in the cytokines IL-6 and interferon gamma characteristic of these syndromes that were observed in the patients. Because of the DLTs observed at 3.0 μg/kg, a de-escalation program was initiated, with groups receiving levels of 1.0 and 3.0 μg/kg per day.
The daily ×12 intravenous dosing schedule was chosen on the basis of preclinical studies in mice and then in rhesus macaques. When daily IL-15 injections in mice were administered for over 20 days, a progressive increase in the number of circulating NK cells was noted over the first 12 to 15 days of administration. After that period, presumably by tachyphylaxis, there was a reduction to normal circulation NK numbers despite the continued administration of IL-15. There was no such tachyphylaxis observed with CD8 circulating T-cell numbers. In rhesus macaques, daily bolus intravenous subcutaneous infusions or continuous intravenous infusions for 10 to 12 days were associated with approximately six-fold elevations of NK numbers at 20 μg/kg and, depending on the dosing site (eg, bolus subcutaneous v continuous), a six- to a 100-fold increase in the number of circulating CD8 effector memory cells. However, when IL-15 was administered subcutaneously twice per week, there was not a substantial (< two-fold) increase in the numbers of any of the circulating lymphocyte subset levels. On the basis of these studies, we chose a 12-day daily bolus intravenous dosing schedule.
rhIL-15 was produced under current good manufacturing practice conditions in the Escherichia coli expression system as previously described.4 Eligibility criteria included age ≥ 18 years and pathologically confirmed metastatic malignant melanoma or metastatic renal cell cancer. Patients with metastatic renal cell cancer must either have refused treatment with, have not tolerated, or experienced progressive disease after receiving sorafenib or sunitinib and temsirolimus. The patients had to have measurable disease and then an absolute granulocyte count of at least 1,500/μL and platelet count of at least 75,000/μL.
DLTs were defined as follows:
any grade 3 or 4 toxicity (PVI Common Terminology Criteria for Adverse Events [version 4.0]), if deemed possibly, probably, or definitely related to the study drug by the principal investigator during the first cycle of treatment, with the following exceptions:
Hematologic exceptions:
grade 3 or 4 lymphopenia (rhIL-15 was to be continued in the event of asymptomatic grade 3 or 4 lymphopenia, unless there were clinical signs of significant infection [persistent fevers, labile blood pressure, localized complaints or findings on physical examination, hypoxia, or organ dysfunction]); grade 3 granulocytopenia (rhIL-15 was to be continued in the event of grade 3 granulocytopenia unless there were clinical signs indicating a significant infection, as listed for grade 3 or 4 lymphopenia); and grade 3 leukocytosis (WBC > 100,000/μL; in the absence of signs of leukostasis or other toxicities possibly related to the expansion of activated cells).
Nonhematologic exceptions:
transient (< 24 hours) grade 3 hypoalbuminemia, hypokalemia, hypomagnesemia, hyponatremia, or hypophosphatemia responding to medical intervention (rhIL-15 was to be continued while the metabolic abnormalities were corrected by intravenous and oral supplementation) and transient (< 24 hours) grade 3 liver function test (ALT, AST, alkaline phosphatase, or total or direct bilirubin) abnormalities in the absence of clinical signs of hepatic dysfunction (lethargy, confusion, anorexia, pruritus, or tremor; rhIL-15 was to be continued for up to 24 hours while medications that could potentially contribute to these abnormalities [eg, acetaminophen] were adjusted or discontinued).
Hematology, Clinical Chemistry, and Fluorescence-Activated Cell-Sorting Analyses
Hematologic tests performed after infusion included WBC, RBC, hemoglobin, hematocrit, mean corpuscular volume, platelet counts, and percentage and absolute numbers of peripheral blood lymphocytes, monocytes, neutrophils, and eosinophils. Bone marrow aspirates were obtained on day 8. The following clinical chemistry tests were also performed: serum Na, K, Cl, glucose, phosphorus, alkaline phosphatase, ALT, AST, total and direct bilirubin, BUN, creatinine, total protein, albumin, troponin T, cholesterol, triglycerides, magnesium, amylase, lactate dehydrogenase, and uric acid. The following coagulation tests were performed: prothrombin time, partial thromboplastin time, and fibrinogen. Assays for soluble serum IL-2 receptor alpha and IL-18 concentrations were assessed on these samples using enzyme-linked immunosorbent assay (ELISA) procedures.4 Plasma IL-6, IL-8, IL-10, IL-12 interferon gamma, IL-1β, and tumor necrosis factor alpha concentrations were performed using a mesoscale method.4
Flow Cytometry Analyses
Polychromatic flow cytometry analysis was performed as described previously on heparinized blood obtained preinfusion and at various times after the first infusion, with analysis of multiple lymphocyte populations including naïve, central memory, and effector memory CD4 and CD8 T cells31,32; B, NK, and NK T cells; T regulatory cells; and plasmocytoid and myeloid dendritic cells. Proliferating cells were detected by the analysis of intracellular Ki-67 on T-cell subsets.
All unconjugated antibodies were purchased from BD Biosciences (San Jose, CA). Nonfluorescent antibodies were conjugated according to the protocols reported (http://www.drmr.com/abcon). Frozen peripheral blood mononuclear cells were thawed in RPMI-1640 medium containing benzonase nuclease (EMD Biochemicals, San Diego, CA), extensively washed and stained immediately with the violet or aqua viability dye (Life Technologies, Carlsbad, CA) in phosphate-buffered saline (PBS) for 15 minutes at room temperature. Cells were then washed and stained with a panel of monoclonal antibodies for 20 minutes at room temperature. Alternatively, CCR5 and CCR7 were detected by staining for 20 minutes at 37°C to improve surface detection. For the analysis of intracellular Ki-67, cells were fixed and permeabilized with the Cytofix/Cytoperm buffer (BD Biosciences) according to the manufacturer's instructions and subsequently incubated with Ki-67 antibody for 20 minutes at 4°C. For the analysis of NK T cells, we incubated cells with the PBS57-loaded CDld tetramer (National Institutes of Health Tetramer Core Facility, Atlanta, GA) conjugated to allophycocyanin or phycoerythrin for 10 minutes at room temperature. Labeled cells were fixed with 1% paraformaldehyde and analyzed using a modified LSR II, equipped to detect up to 18 fluorescences. Flow cytometric data were compensated and analyzed with FlowJo software (version 9.7; http://www.flowjo.com). Data were presented with SPICE software.32
Pharmacokinetic Procedures
The rhIL-15 concentrations in serum were assessed using a human IL-15–specific ELISA kit from R&D Systems (Minneapolis, MN) according to manufacturer's directions. Noncompartmental pharmacokinetic analysis of data was undertaken using the software package WINNonlin (version 5.0; Scientific Consultant, Apex, NC; Pharsight, Mountain View, CA). Maximum serum concentrations were reported as back-extrapolated values. The area under the plasma concentration versus time curve was calculated using the linear trapezoidal method from time 0 (at drug administration) to time of last sample with measurable drug concentration for each individual. The inferred area under the curve value was calculated by extrapolation by dividing the last measurable drug concentration by the rate constant at the terminal phase. All statistical analyses were carried out using NCSS 2004 software (http://www.ncss.com). P < .05 was considered to be statistically significant in paired t tests.
ELISA Method for Detection of Host Production of Antibody Directed Toward Infused rhIL-15
Serum samples were analyzed for the in vivo production of antibodies to rhIL-15 at days 14, 21, 28, and 42 after initial IL-15 infusion using an ELISA method developed in our laboratory. This two-arm capture ELISA procedure achieved a limit of quantitation of 156 ng/mL in undiluted serum and 470 ng/mL when the test sample was diluted at a ratio of 1:3. The study material, rhIL-15, was used as the antigenic ligand. rhIL-15 100 μL at a concentration of 100 ng/mL was added to wells of a 96-well microliter plate; the plate was sealed and incubated at 37°C for 3 hours. The plates were then washed three times with PBS using a plate washer. To eliminate any remaining active plate sites, PBS 300 μL/3% bovine serum albumin blocking buffer was added to all wells, and the plate was incubated at 37°C for 1 hour followed by washing as described. A commercial affinity purified goat antihuman IL-15 (R&D Catalog No. AF315) was used to form a standard curve for antibody quantitation by serially diluting the antibody from 100 to 9.8 ng/mL. Test serum samples were diluted at a ratio of 1:3 and added concomitantly to the appropriate IL-15–coated wells, along with positive and negative controls. After overnight incubation at 4°C, the plates were washed three times with PBS, followed immediately by addition of 100 μL of biotinylated rhIL-15 at a final concentration determined by prior optimal titration. The plates were sealed and incubated for 2 hours at 37°C and then washed three times with PBST using a plate washer. 100 μL of streptavidin-alkaline phosphatase, diluted in PBS plus 1% bovine serum albumin according to manufacturer's instructions, was added to each well, and the plate was incubated for 2 hours at 37°C followed by three washes with PBS. Substrate p-nitrophenyl phosphate, freshly dissolved in diethanolamine buffer, was added to all wells for color development. The plate was incubated at 37°C for 1 hour, and the resultant color was detected at 405-nm absorbance using the SpectraMax Plus 384 microplate reader (Molecular Devices, Sunnyvale, CA). Mathematic construction of the standard curve and determination of anti–rhIL-15 values for the serum unknowns were accomplished using SoftMax Pro computational software (version 4.8; Molecular Devices).
Table A1.
Patient Demographic and Clinical Characteristics (N = 18)
| Characteristic | No. of Patients |
|---|---|
| Sex | |
| Male | 9 |
| Female | 9 |
| Age, years | |
| Median | 55 |
| Range | 21-83 |
| > 65 | 6 |
| Primary disease | |
| Metastatic melanoma | 11 |
| Metastatic renal cell cancer | 7 |
| No. of prior anticancer regimens | |
| Median | 3 |
| Range | 0-5 |
Table A2.
Summary of Clinical Features of Patients Receiving rhIL-15 3.0, 1.0, and 0.3 μg/kg per Day
| Feature | Patient Age (years) | Sex | No. of Doses | Maximum-Fold Increase ALC From Baseline | Platelets (× 103/mm3) |
Absolute Neutrophil Count |
Maximum ALT/AST |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | Nadir | Start | Nadir | Days < 1,000/mm3 | Days < 1,500/mm3 | ALT | AST | |||||
| 3 μg/kg | ||||||||||||
| Melanoma | 83 | F | 1 | NC | NC | NC | 0 | NC | NC | |||
| Ocular melanoma | 43 | M | 12 | 1.8 | 568 | 187 | 9,270 | 2,750 | 0 | 52 | 49 | |
| Melanoma | 53 | M | 10 | 4.2 | 672 | 157 | 4,130 | 2,370 | 0 | 99* | 120* | |
| Ocular melanoma | 57 | F | 12 | 2.4 | 296 | 97 | 4,560 | 1,360 | 0 | 36 | 118 | |
| Melanoma | 34 | M | 6 | 3.8 | 176 | 44† | 3,940 | 1,020 | 0 | 29 | 38 | |
| 1 μg/kg | ||||||||||||
| Renal cell | 57 | M | 4 | 2.3 | 207 | 129 | 5,520 | 1,430 | 0 | 232† | 128† | |
| Renal cell | 67 | M | 12 | 1.9 | 265 | 166 | 4,680 | 1,270 | 0 | 49 | 59 | |
| Melanoma | 21 | F | 12 | 2.2 | 617 | 239 | 4,810 | 1,110 | 0 | 1 | 19 | 41 |
| Mucosal melanoma | 50 | M | 4 | 1.3 | 312 | 120 | 6,420 | 590‡ | 1 | 2 | 465† | 399† |
| 0.3 μg/kg | ||||||||||||
| Melanoma | 68 | F | 12 | 1.5 | 338 | 167 | 4,630 | 930‡ | 2 | 6 | 41 | 48 |
| Melanoma | 66 | F | 12 | 1.4 | 251 | 110 | 2,680 | 870‡ | 4 | 11 | 102* | 85* |
| 6 | NC | 206 | 127 | 2,530 | 930‡ | 1 | 5 | 67 | 52 | |||
| Renal cell | 71 | M | 12 | 3.2 | 177 | 92 | 3,500 | 930‡ | 0 | 9 | 82 | 64 |
| 12 | 1.3 | 155 | 95 | 2,760 | 930‡ | 0 | 3 | 68 | 48 | |||
| Renal cell | 43 | M | 12 | 1.7 | 199 | 118 | 5,450 | 1,930 | 0 | 38 | 27 | |
| Renal cell | 47 | M | 12 | 1.3 | 176 | 108 | 3,970 | 2,230 | 0 | 51 | 40 | |
| 12 | 1.8 | 173 | 111 | 4,520 | 2,680 | 0 | 36 | 33 | ||||
| Renal cell | 61 | F | 12 | 1.4 | 168 | 111 | 2,860 | 560‡ | 8 | 190* | 241* | |
| 12 | 1.3 | 176 | 128 | 1,620 | 460‡ | 11 | 118* | 121* | ||||
| Melanoma | 74 | F | 12 | 2.5 | 288 | 145 | 8,750 | 1,850 | 0 | 27 | 28 | |
| 12 | 1.1 | 294 | 191 | 3,450 | 2,260 | 0 | 22 | 23 | ||||
| Renal cell | 33 | F | 12 | 2.1 | 204 | 122 | 2,580 | 750‡ | 1 | 7 | 20 | 53 |
| 6 | NC | 219 | 132 | 3,890 | 1,110 | 0 | 4 | 19 | 16 | |||
| Mucosal melanoma | 44 | F | 12 | 1.3 | 317 | 148 | 5,060 | 1,790 | 0 | 58 | 57 | |
Abbreviations: ALC, absolute lymphocyte count; NC, no change; rhIL-15, recombinant human interleukin-15.
Notable transaminase result.
Dose-limiting toxicity.
Notable neutrophil count.
Table A3.
Summary of AEs After Bolus rhIL-15
| AE | 3 μg/kg (n = 5) |
1 μg/kg (n = 4) |
0.3 μg/kg (n = 9) |
|||
|---|---|---|---|---|---|---|
| Grade 1 or 2 | Grade 3 or 4 | Grade 1 or 2 | Grade 3 or 4 | Grade 1 or 2 | Grade 3 or 4 | |
| Fever | 3 | 1 | 3 | 3 | ||
| Chills/rigors | 4 | 4 | 7 | |||
| Capillary leak syndrome | 4 | 3 | ||||
| Nausea | 4 | 2 | 4 | |||
| Vomiting | 2 | 1 | 1 | |||
| Hypotension | 3 | 2* | 1 | 4 | ||
| Fatigue | 3 | 2 | 8 | |||
| Edema | 4 | 2 | 1 | |||
| Pain | 2 | 2 | 5 | |||
| Diarrhea | 1 | 1 | ||||
| Hypoxia | 3† | |||||
| Pleural effusion | 2 | 2 | ||||
| Albumin | 5 | 4 | 1 | |||
| AST | 5 | 2 | 1‡ | 3 | 1 | |
| ALT | 1 | 2 | 2‡ | 5 | ||
| Alkaline phosphatase | 4 | 3 | ||||
| Bilirubin | 2 | 1 | ||||
| Leukopenia | 1 | 2 | 5 | 1 | ||
| Lymphopenia | 3 | 2 | 3 | 3 | 3 | |
| Thrombocytopenia | 2 | 1‡ | 1 | 8 | ||
| Neutropenia | 3 | 3 | 5 | |||
| Anemia | 2 | 1 | 2 | 6 | 1 | |
| Calcium | 1 | |||||
| Phosphate | 2 | 1 | 1 | |||
| Creatinine | 1 | 1 | ||||
Abbreviations: AE, adverse event; DLT, dose-limiting toxicity; rhIL-15, recombinant human interleukin-15.
One event was asymptomatic, transient, and not DLT. Other event was symptomatic, recurrent, and assessed as DLT.
Transient asymptomatic decrease in transcutaneous pulse oximeter reading.
DLT at this dose level.
Fig A1.
Identification of natural killer (NK) and T-cell subsets. (A) Peripheral blood mononuclear cells from representative patient were stained with panel of monoclonal antibodies. Total NK cells were identified. Combination of CD16 and CD56 expression on total NK cells identified three subsets: CD56+CD16−, CD56+CD16dim, and CD56dimCD16bright. Numbers outside gold lines indicate percentage of cells inside each gate. (B) Flow cytometric assay identified multiple subsets of naïve (TN) and memory cells. CD8 T cells are shown (depicted in black in background). Naïve-like T cells (red) were first identified as CD45RO−CCR7+, further selected as CD45RA+CD27+, and finally selected as CD95−. T stem-cell memory (TSCM) has same phenotype but expresses CD95. Central memory (TCM) is CD45RO+CCR7+, transitional memory (TTM) is CD45RO+CCR7−CD27+, effector memory (TEM) is CD45RO+CCR7−CD27−, and terminal effector (TTE) is CD45RO−CCR7−CD27−. Same strategy applied to CD4 T cells.
Fig A2.
Dose effect on lymphocyte dynamics during and after daily interleukin-15 (IL-15) infusions. All data shown as mean for administration of IL-15 3 μg/kg (n = 4) or 0.3 μg (n = 9). All fold-change values were computed by individual relative to baseline (Pre). Light blue bars indicate IL-15 infusion time. Error bars were omitted for clarity. (A) Representation within natural killer (NK) cells and (B) proliferation state of differentiation stages of NK cells. (C) Absolute CD4 and CD8 T-cell counts. (D) Absolute counts of CD8 differentiation stages. (E) Absolute counts of γδ T-cell lineages. Activation and proliferation states of (F) CD8 and (G) CD4 cells based on any of four markers. (H) Absolute counts and (I) representation (frequency within total CD4 T cells) of T regulatory cells.
Fig A3.
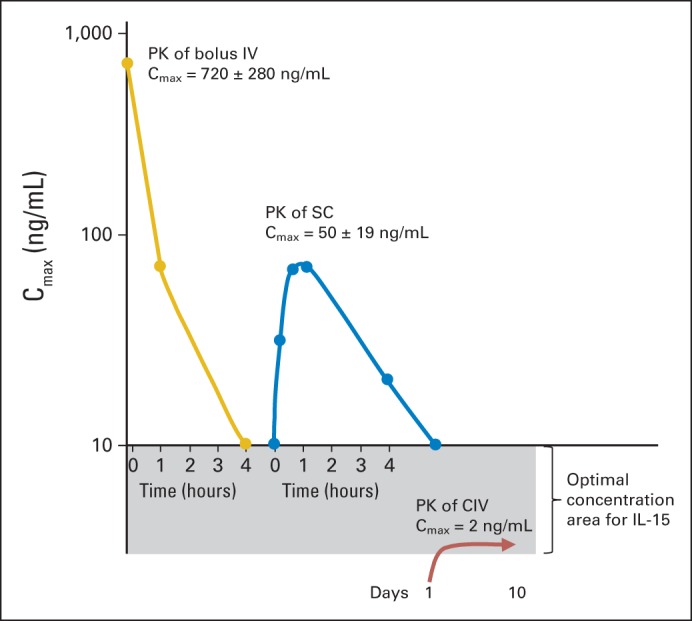
Comparative pharmacokinetics (PKs) for 20-μg dose at different dosing strategies in cynomolgus macaques. In our previous publications,4,30 we reported that with bolus intravenous (IV) infusion to rhesus macaques at 20 μg/kg per day, there was exceedingly high maximum serum concentration (Cmax) of 720 ± 280 ng/mL. With identical subcutaneous (SC) dose of 20 μg/kg, Cmax was 10-fold less, at 50 ± 19 ng/mL, and with continuous IV (CIV) infusion of 20 μg/kg per day, it was 2 to 4 ng/mL, maintained throughout 10-day study period. Initial high peak and rapid decline with IV bolus dosing was analogous to PKs seen in our phase I patient clinical trial. Clinical trials in patients with metastatic malignancy with interleukin-15 (IL-15) administered SC or by CIV infusions have been initiated to reduce peak IL-15 effects on cytokine release and infusional toxicities and maintain IL-15 at optimal concentration for high-affinity IL-15 receptor.
Footnotes
Support information appears at the end of this article.
Clinical trial information: NCT01021059.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Kevin C. Conlon, Enrico Lugli, Mario Roederer, Thomas A. Waldmann
Financial support: Mario Roederer, Thomas A. Waldmann
Administrative support: Mario Roederer, Thomas A. Waldmann
Provision of study materials or patients: Kevin C. Conlon, Steven A. Rosenberg, Antonio Tito Fojo, Tat'Yana A. Worthy, Jason L. Yovandich, Stephen P. Creekmore, Thomas A. Waldmann
Collection and assembly of data: Kevin C. Conlon, Enrico Lugli, Hugh C. Welles, Thomas A. Fleisher, Carolyn K. Goldman, Bonita R. Bryant, Jing Chen, William D. Figg Sr, Cody J. Peer, Mario Roederer, Thomas A. Waldmann
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported by the Intramural Programs of the Center for Cancer Research, National Cancer Institute, and the Vaccine Research Center (National Institute of Allergy and Infectious Disease), National Institutes of Health (NIH; Bethesda, MD). SAIC-Frederick (McLean, VA) and the NIH Biopharmaceutical Development Program, National Cancer Institute Frederick (Frederick, MD), provided the recombinant human interleukin-15 used in this study.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Redistribution, Hyperproliferation, Activation of Natural Killer Cells and CD8 T Cells, and Cytokine Production During First-in-Human Clinical Trial of Recombinant Human Interleukin-15 in Patients With Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Kevin C. Conlon
No relationship to disclose
Enrico Lugli
No relationship to disclose
Hugh C. Welles
No relationship to disclose
Steven A. Rosenberg
No relationship to disclose
Antonio Tito Fojo
No relationship to disclose
John C. Morris
Speakers' Bureau: Boehringer-Ingelheim
Travel, Accommodations, Expenses: Amgen, VentiRx
Thomas A. Fleisher
No relationship to disclose
Sigrid P. Dubois
No relationship to disclose
Liyanage P. Perera
No relationship to disclose
Donn M. Stewart
No relationship to disclose
Carolyn K. Goldman
No relationship to disclose
Bonita R. Bryant
No relationship to disclose
Jean M. Decker
No relationship to disclose
Jing Chen
No relationship to disclose
Tat'Yana A. Worthy
No relationship to disclose
William D. Figg Sr
No relationship to disclose
Cody J. Peer
No relationship to disclose
Michael C. Sneller
No relationship to disclose
H. Clifford Lane
Patents, Royalties, Other Intellectual Property: Patent royalties from US Government–held patent for use of interleukin-2 in HIV infection
Jason L. Yovandich
No relationship to disclose
Stephen P. Creekmore
No relationship to disclose
Mario Roederer
No relationship to disclose
Thomas A. Waldmann
No relationship to disclose
REFERENCES
- 1.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- 2.Lenardo MJ. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–724. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann TA, Lugli E, Roederer M, et al. Safety (toxicity), pharmacokinetics, immunogenicity and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117:4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton JD, Bamford RN, Peters C, et al. A lymphokine, provisionally designated interleukin-T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer-cells. Proc Natl Acad Sci U S A. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 7.Bamford RN, Grant AJ, Burton JD, et al. The interleukin IL(2) receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldmann TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nature Reviews Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 9.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugamura K, Asao H, Kondo M, et al. The interleukin-2 receptor gamma chain: Its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Nakamura Y, Russell SM, et al. Interleukin-2 receptor gamma chain: A functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 12.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: Implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 14.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: Immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 15.Sadlack B, Kühn R, Schorle H, et al. Development and proliferation of lymphocytes in mice deficient for both interleukins-2 and-4. Eur J Immunol. 1994;24:281–284. doi: 10.1002/eji.1830240144. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks-Koncalik J, Dubois S, Losi JM, et al. IL-2 induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubois S, Mariner J, Waldmann TA, et al. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 19.Burkett PR, Koka R, Chien M, et al. Coordinate expression and trans presentation of interleukin (IL)-15R alpha and IL-15 supports natural killer cell and memory CD8(+) T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandau MM, Schluns KS, Lefrancois L, et al. Cutting edge: Transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R by the same cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi H, Dubois S, Sato N, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 22.Munger W, DeJoy SQ, Jeyaseelan R, Sr, et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: Comparison with interleukin-2. Cell Immunol. 1995;165:289–293. doi: 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]
- 23.Evans R, Fuller JA, Christianson G, et al. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: The potential role of NK cell subpopulations. Cell Immunol. 1997;179:66–73. doi: 10.1006/cimm.1997.1132. [DOI] [PubMed] [Google Scholar]
- 24.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8(+) T cell expansion and function. J Exper Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker TC, Wherry EJ, Boone D, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo-Saito C, Wansley EK, Gruys ME, et al. Combination therapy of an orthotopic renal cell carcinoma model using intratumoral vector-mediated costimulation and systemic interleukin-2. Clin Cancer Res. 2007;13:1936–1946. doi: 10.1158/1078-0432.CCR-06-2398. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Ju W, Yao Z, et al. Interleukin-15(IL-15) combined with an agonistic anti-CD40 antibody that augments IL-15Rα expression leads to regression of established TRAMP-C2 tumors in mice. J Immunol. 2012;188:6156–6164. doi: 10.4049/jimmunol.1102604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu P, Steel JC, Zhang M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A. 2012;109:6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sneller MC, Kopp WC, Kory J, et al. IL-15 administered by continuous intravenous infusion to rhesus macaques induces massive expansion of the CD8+ T effector memory population in peripheral blood. Blood. 2011;118:6845–6848. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lugli E, Goldman CK, Perera LP, et al. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood. 2010;116:3238–3248. doi: 10.1182/blood-2010-03-275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roederer M, Nozzi JL, Nason MC. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+CD25hi Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei S, Kryczek l, Edwards RP, et al. Interleukin-2 administration alters the CD4+FoxP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67:7487–7494. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- 35.Choo Sim G, Martin-Orozco N, Jin L, et al. IL-2 therapy promotes suppressive ICOS+ T reg expansion in melanoma patients. J Clin Invest. 2014;124:99–110. doi: 10.1172/JCI46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cesana GC, DeRaffele G, Cohen S, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 37.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krüttgen A, Rose-John S. Interleukin-6 in sepsis and capillary leak syndrome. J Interferon Cytokine Res. 2012;32:60–65. doi: 10.1089/jir.2011.0062. [DOI] [PubMed] [Google Scholar]