Abstract
Purpose
Women with abnormal cervical cancer screening results are referred to colposcopy and biopsy for diagnosis of cervical cancer precursors (high-grade squamous intraepithelial lesions [HSILs]). Colposcopy with a single biopsy can miss identification of HSILs. No systematic study has quantified the improved detection of HSIL by taking multiple lesion-directed biopsies.
Methods
The Biopsy Study was an observational study of 690 women referred to colposcopy after abnormal cervical cancer screening results. Up to four directed biopsies were taken from distinct acetowhite lesions and ranked by colposcopic impression. A nondirected biopsy of a normal-appearing area was added if fewer than four directed biopsies were taken. HSIL identified by any biopsy was the reference standard of disease used to evaluate the incremental yield and sensitivity of multiple biopsies.
Results
In the overall population, sensitivities for detecting HSIL increased from 60.6% (95% CI, 54.8% to 66.6%) from a single biopsy to 85.6% (95% CI, 80.3% to 90.2%) after two biopsies and to 95.6% (95% CI, 91.3% to 99.2%) after three biopsies. A significant increase in sensitivity of multiple biopsies was observed in all subgroups. The highest increase in yield of HSIL was observed for women with a high-grade colposcopic impression, HSIL cytology, and human papillomavirus (HPV) type 16 positivity. Only 2% of all HSILs diagnosed in the participants were detected by biopsies of normal-appearing transformation zone.
Conclusion
Collection of additional lesion-directed biopsies during colposcopy increased detection of histologic HSIL, regardless of patient characteristics. Taking additional biopsies when multiple lesions are present should become the standard practice of colposcopic biopsy.
INTRODUCTION
Women with abnormal cervical cancer screening results are referred to colposcopy for diagnosis and management. Each year in the United States, about three million women are evaluated with colposcopic procedures by gynecologic oncologists, gynecologists, family physicians, and nurse practitioners.1,2 Typically, colposcopy results are characterized by a colposcopic impression and by selection of the worst-appearing site for biopsy.3 The biopsy result determines whether treatment is required by excision of the transformation zone. In addition, women with persistent abnormalities after colposcopy and women undergoing surveillance after treatment are managed with colposcopy.4
Despite its importance in the management of cervical abnormalities, there has been no primary evaluation of colposcopy-biopsy performance in the United States. Secondary analyses from screening and vaccination trials have suggested that colposcopic impression and biopsy placement are poorly reproducible5,6 and fail to detect 30% to 50% of prevalent high-grade squamous intraepithelial lesions (HSILs).7,8 These data also suggest that taking more biopsies increases the detection of HSILs. Some investigators have proposed that taking biopsies of normal-appearing cervix may identify up to 30% more HSILs.9,10
The considerable variability of colposcopy protocols demonstrates uncertainty about standard practice and performance of colposcopy. Although most commonly a single biopsy from the worst-appearing area on the cervix is taken, some centers have adopted an extended biopsy protocol with four-quadrant biopsies.10 In clinical trials, multiple-biopsy protocols have been used to maximize disease ascertainment.8,11 We designed the present investigation to quantify the benefit of taking multiple lesion-directed biopsies and an additional biopsy of normal-appearing cervix in a US colposcopy population.
METHODS
Population
Women age 18 years and older with abnormal cervical cancer screening results were enrolled and treated at the University of Oklahoma Health Sciences Center (Oklahoma City, OK) between February 2009 and September 2012. Exclusion criteria included previous surgical treatment for cervical disease, prior chemotherapy or radiation treatment for cervical neoplasia, pregnancy, or known HIV infection. Of 2,270 women with appointments at the colposcopy clinic, 897 were found not eligible for enrollment. A total of 690 (50.3%) of 1,373 eligible women agreed to participate in the study and provided written informed consent at the time of enrollment. Institutional review board approval for the study was provided by University of Oklahoma Health Sciences Center and the National Cancer Institute.
Colposcopy and Biopsy Protocol
Referral to colposcopy was based on the 2007 American Society for Colposcopy and Cervical Pathology guidelines.4 Six colposcopists performed between 60 and 179 colposcopic examinations each. Before colposcopy, cervical specimens for cytology and human papillomavirus (HPV) testing were collected using a Wallach broom and transferred to PreservCyt solution (Hologic, Marlborough, MA). An extended biopsy protocol involved digital photographic documentation of colposcopic impression and biopsy sites. Up to four lesion-directed biopsies were obtained from distinct areas of epithelium that turned white on application of 5% acetic acid (acetowhite lesions) in the cervical transformation zone using sharp Tischler or baby Tischler biopsy forceps. When less than four directed biopsies were taken, a biopsy was added targeting normal-appearing cervical transformation zone epithelium. The colposcopist ranked all biopsies by order of severity at the time of colposcopy. For each biopsy, hematoxylin and eosin–stained tissue sections were evaluated by a study pathologist for clinical management. In addition, an adjacent tissue section was stained with p16 (CINtec; Roche mtm Laboratories, Mannheim, Germany) and evaluated to establish biomarker-adjudicated HSIL end points.12 All colposcopic images from study participants were evaluated by an expert colposcopist blinded to the clinical diagnosis. For a sensitivity analysis of nondirected biopsies, we only considered those biopsies evaluated as normal by both the clinic colposcopist and the external reviewer to be nondirected.
Cytology and HPV Testing
Referral cytology was community based; results were reported using the Bethesda nomenclature, including the categories normal for intraepithelial lesion or malignancy; atypical squamous cells of undetermined significance (ASCUS); low-grade squamous intraepithelial lesion (LSIL); atypical squamous cells, favor high grade; and HSIL.13 HPV genotyping was conducted from liquid-based cytology specimens collected at enrollment using the Linear Array HPV Genotyping Test (Roche Molecular Diagnostics, Branchburg, NJ) according to the manufacturer's instructions with slight modifications.14,15
Definition of Histologic End Points
Histologic end points were defined based on the worst result from all biopsies taken at colposcopy. We adopted the Lower Anogenital Squamous Terminology guidelines,16 which dichotomize histology results into LSIL and HSIL. All patients with cervical intraepithelial neoplasia (CIN) grade 2 (CIN 2) that stained diffusely positive for p16 were considered as having HSIL, along with all patients with CIN grade 3 (CIN 3) regardless of p16 staining. Additional ancillary analyses were conducted to evaluate CIN 2+ and CIN 3 end points. Patients with histologically confirmed CIN 3 and most patients with CIN 2 were referred for treatment according to clinical guidelines.4
Statistical Analysis
The population was stratified by referral cytology result (normal for intraepithelial lesion or malignancy; ASCUS; LSIL; or HSIL, including atypical squamous cells, favor high grade), colposcopy impression (normal, acetowhitening, low-grade lesion, or high-grade lesion), HPV-16 status (negative or positive), and age group (21 to 24, 25 to 29, or ≥ 30 years). We considered the following two related measures to evaluate the incremental benefit of multiple biopsies: relative sensitivity and absolute disease yield. Sensitivities for one, two, three, and all four biopsies are the percentages found to have HSIL among all women eventually diagnosed with HSIL. Yield has the same numerator as sensitivity, but the denominator is all women with one, two, three, or four biopsies. Because the colposcopists ranked the biopsies that were taken in terms of severity, we were able to directly measure the marginal increase in sensitivity and yield for each additional biopsy.
When fewer than four biopsies were taken, we could not directly measure the yield of four biopsies. The major results were confirmed for the biopsies actually taken, but for more detailed analyses, we imputed HSIL outcomes for biopsies that were not performed, bracketing the range of plausible estimates. One model imputed the unobserved yields and sensitivities based on an assumption that additional biopsies would not have detected any additional HSIL (ie, that yield of additional biopsies would have been zero), which is the implicit assumption of current clinical practice that takes only one biopsy. Imputation in the second model was based on the assumption that each additional biopsy would have had the same marginal yield as among women for whom the additional directed biopsies were actually performed. As an intermediate, we estimated that additional biopsies had the same yield of HSIL as additional biopsies from normal-appearing sites. All three reasonable assumptions gave similar answers; for data presentation, we showed the third, intermediate imputation approach when estimating yield and sensitivity of multiple biopsies. We calculated 95% CIs for sensitivity estimates using the bootstrap approach. In brief, 1,000 repeated random samples of the same size equaling the overall study population or individual strata were drawn with replacement from the data. The 95% CIs were based on the range of estimates from the 2.5 to the 97.5 percentiles of the ranked random samples. All analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC).
To calculate adjusted yields, we fit multinomial logistic regression models adjusting for colposcopy impression, cytology, HPV status, and age. We used the computer software package SUDAAN (RTI International, Research Triangle Park, NC) to calculate the predictive margins (adjusted proportions) based on the entire analytic sample. SUDAAN produced predictive margins for each category of each covariate in the model. We multiplied predictive margins by the totals from each category to get the expected counts. This method directly standardizes the crude proportions to the distribution of the covariates for the entire sample of study participants17 to obtain the adjusted proportions.
RESULTS
Study Population and Colposcopy Procedures
The median age of women enrolled onto the study was 26 years (range, 18 to 67 years) (Table 1). All women had at least one biopsy; 54.6% of women had four biopsies, 26.6% of women had three biopsies, and 18.8% of women had less than three biopsies. As expected, the number of biopsies increased with increasingly severe colposcopic impression. The median numbers of biopsies were one, three, and four among women with normal colposcopic impression, acetowhitening, and low-grade or high-grade colposcopic impression, respectively. Among 285 women who had endocervical sampling performed, only four women with CIN 2 and three women with CIN 3 were detected in addition to disease that was found with colposcopic biopsies; we did not consider endocervical sampling further in the analysis. All women with CIN 3 or cancer and 90% of women with CIN 2 were positive for p16 in histology (Table 1). We combined p16-positive CIN 2 and CIN 3 into an end point of HSIL.16 Four cancers were included in this group.
Table 1.
Clinical Characteristics of Study Population
| Characteristic | Total No. of Patients | Histologic Diagnosis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| < CIN 1 |
CIN 1 |
CIN 2 |
CIN 3+* |
||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| Visit age, years | 677† | ||||||||
| Median | 27 | 26 | 25 | 26 | |||||
| Range | 19-67 | 18-67 | 19-50 | 19-66 | |||||
| Referral result | 667† | 151 | 100 | 244 | 100 | 196 | 100 | 76 | 100 |
| NILM | 2 | 2 | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ASCUS | 159 | 49 | 32.5 | 73 | 29.9 | 26 | 13.3 | 11 | 14.5 |
| LSIL | 287 | 66 | 43.7 | 126 | 51.6 | 77 | 39.3 | 18 | 23.7 |
| HSIL | 209 | 34 | 22.5 | 44 | 18.0 | 89 | 45.4 | 42 | 55.3 |
| Previous biopsy | 10 | 0 | 0 | 1 | 0.4 | 4 | 2.0 | 5 | 6.6 |
| HPV status | 680† | 161 | 100 | 247 | 100 | 196 | 100 | 76 | 100 |
| HPV negative | 57 | 28 | 17.4 | 26 | 10.5 | 3 | 1.5 | 0 | 0 |
| Noncarcinogenic HPV | 86 | 25 | 15.5 | 51 | 20.6 | 9 | 4.6 | 1 | 1.3 |
| Carcinogenic HPV, no HPV-16 | 348 | 78 | 48.4 | 134 | 54.3 | 110 | 56.1 | 26 | 34.2 |
| HPV-16 | 189 | 30 | 18.6 | 36 | 14.6 | 74 | 37.8 | 49 | 64.5 |
| Colposcopic impression | 678† | 159 | 100 | 248 | 100 | 197 | 100 | 74 | 100 |
| Normal | 48 | 27 | 17.0 | 19 | 7.7 | 2 | 1.0 | 0 | 0 |
| Acetowhitening | 71 | 31 | 19.5 | 31 | 12.5 | 7 | 3.6 | 2 | 2.7 |
| Low-grade lesion | 339 | 76 | 47.8 | 142 | 57.3 | 97 | 49.2 | 24 | 32.4 |
| High-grade lesion | 215 | 25 | 15.7 | 56 | 22.6 | 91 | 46.2 | 43 | 58.1 |
| Invasive cancer | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 6.8 |
| No. of biopsies | 683† | ||||||||
| Median | 3 | 3 | 4 | 4 | |||||
| Range | 1-4 | 1-4 | 1-4 | 2-4 | |||||
| p16 immunohistochemistry | 682† | 160 | 100 | 249 | 100 | 197 | 100 | 76 | 100 |
| Negative | 282 | 127 | 79.4 | 136 | 54.6 | 19 | 9.6 | 0 | 0 |
| Positive | 400 | 33 | 20.6 | 113 | 45.4 | 178 | 90.4 | 76 | 100 |
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy.
Including four cancers.
Missing values are excluded.
Detection of HSIL Using Lesion-Directed Biopsies
We directly measured the detection of HSIL by increasing numbers of lesion-directed biopsies, separately for women with one, two, three, and four biopsies taken (Fig 1). The yield of HSIL for the first biopsy increased from 12% in women with one directed biopsy to 34% in women with four lesion-directed biopsies, reflecting the increasing severity of the cases. When examining the marginal gain of taking more than one biopsy, the greatest increase in yield was observed for the second biopsy, with decreasing increments for additional biopsies. A second biopsy led to a 6% increase in yield in women with two biopsies and to a 15% increase in yield in women with four biopsies. A third biopsy increased the yield of HSIL by 5% in women with three biopsies and by 6% in women with four biopsies. A fourth biopsy increased the yield of HSIL by 2%.
Fig 1.
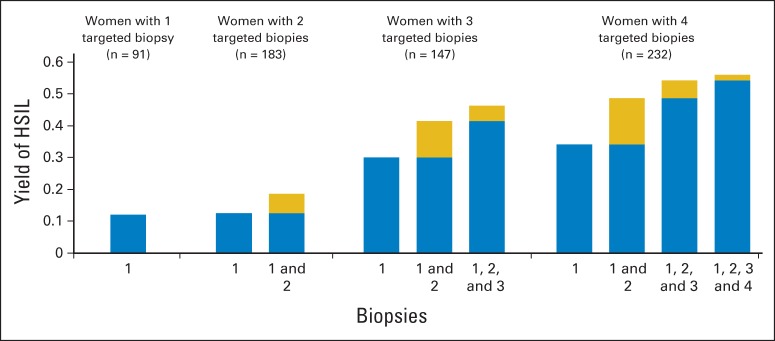
Yield of high-grade squamous intraepithelial lesions (HSILs) by number of biopsies. Bars show the incremental yield of HSILs with increasing numbers of biopsies in women with one, two, three, and four biopsies. All biopsies were targeted at acetowhite or worse-appearing areas in the transformation zone. The gold bars show the incremental yield of each additional biopsy. The blue and gold bars combined show the total yield for the respective number of biopsies.
Detection of HSIL Using Biopsies Targeting Normal-Appearing Areas
In 446 (65%) of 690 women with fewer than four biopsies targeting acetowhite areas, a nondirected biopsy was added. In 30 women, the nondirected biopsy was the only biopsy taken, whereas 90, 181, and 145 women had the nondirected biopsy taken as the second, third, or fourth biopsy, respectively, after lesion-directed biopsies (Table 2). Overall, only 10 additional HSILs were detected by nondirected biopsies (4% of all HSILs in the study). Only one (3%) of 30 nondirected biopsies in women without colposcopic abnormalities yielded HSIL. Among women with one directed biopsy, adding the nondirected biopsy increased the yield of HSIL from 12% to 15%. Among women with two directed biopsies, the yield increased from 19% to 21%, and among women with three directed biopsies, the yield of HSIL increased from 46% to 48%. In models adjusted for colposcopy impression, cytology, and HPV status, we found similar yields of HSIL for additional random biopsies taken (Appendix Table A1, online only). In an ancillary analysis that considered a biopsy as nondirected only if both the clinic review and the external review called the colposcopic impression at the biopsy site normal, only five additional HSILs were detected (2% of all HSIL in the study).
Table 2.
Detection of HSIL by Biopsies Targeting an Additional Normal-Appearing Area, by Number of Biopsies Targeting Acetowhite Areas
| No. of Biopsies Targeting Acetowhite Areas | No. of Patients | Yield of HSILs Based on Biopsies Targeting Acetowhite Areas |
Additional Yield of Biopsy Targeting Normal-Appearing Area |
||
|---|---|---|---|---|---|
| No. of HSILs | % | No. of HSILs | % | ||
| None | 30 | NA | 1 | 3.3 | |
| 1 | 90 | 11 | 12.2 | 3 | 3.3 |
| 2 | 181 | 34 | 18.8 | 4 | 2.2 |
| 3 | 145 | 68 | 46.9 | 2 | 1.4 |
Abbreviations: HSIL, high-grade squamous intraepithelial lesion; NA, not applicable.
Cumulative Sensitivity and Yield of HSIL With Increasing Number of Biopsies
Figure 2 shows the incremental sensitivity for HSIL of taking one to four lesion-directed biopsies. We constructed this summary figure using the three imputation approaches, all of which gave similar results. The sensitivity of the first biopsy was 60.6% (95% CI, 54.8% to 66.6%) and increased to 85.6% (95% CI, 80.3% to 90.2%) for two biopsies and 95.6% (95% CI, 91.3% to 99.2%) for three biopsies (Table 3). The estimated proportion of women who would have an HSIL diagnosis in the overall population increased from 0.24 for taking one biopsy to 0.39 for taking four biopsies. In stratified analyses, the highest yields of HSIL were observed for women with a high-grade colposcopic impression (0.60), HSIL cytology (0.61), and HPV-16 positivity (0.64). In all strata (except for the few women with normal colposcopic impression), the absolute increase in sensitivity from the first to the second biopsy was statistically significant and ranged from 19% to 50%. In women with high-grade colposcopy impression, HSIL cytology, and HPV-16 positivity, we also observed a statistically significant increase of sensitivity from the second to the third biopsy, ranging from 7% to 11%. In models adjusted for colposcopy impression, cytology, HPV status, and age, we found similar marginal yields of HSIL for additional biopsies taken (Appendix Table A2, online only). In sensitivity analyses, results for conventional histologic end points CIN 2 or greater and CIN 3 or greater were similar (Appendix Table A3, online only). We observed similar results for each of the six colposcopists who performed at least 60 colposcopies, and we did not observe any temporal changes during the course of the study (Appendix Table A4, online only).
Fig 2.
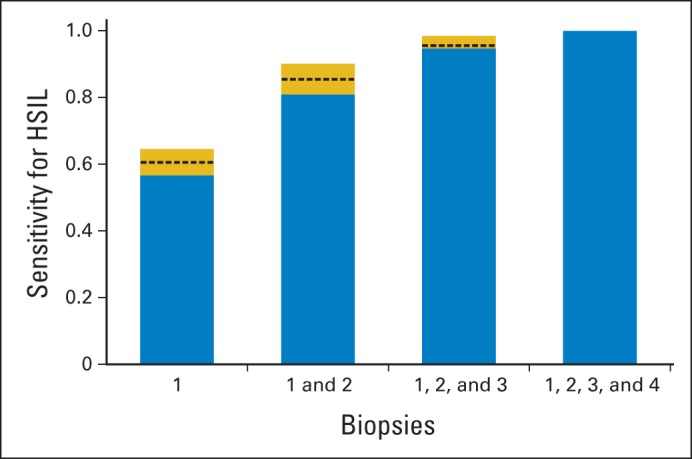
Sensitivity to detect high-grade squamous intraepithelial lesions (HSILs) with increasing numbers of biopsies. Blue bars indicate minimum estimate for sensitivity, with imputation based on the assumption that additional biopsies had the same yield as among women for whom four lesion-directed biopsies were performed. Blue plus gold bars indicate maximum estimate for sensitivity, with imputation based on the assumption that additional biopsies did not detect additional HSILs. Dashed line indicates intermediate estimate for sensitivity, assuming that additional biopsies had the same yield of HSILs as additional biopsies from normal-appearing sites in the transformation zone.
Table 3.
Sensitivity for and Yield of HSIL With Increasing Number of Lesion-Directed Biopsies
| Biopsy* | Cumulative Sensitivity (%) | 95% CI (%) | No. of HSILs Detected | Cumulative HSIL Yield |
|---|---|---|---|---|
| All (N = 653) | ||||
| Biopsy 1 | 60.6 | 54.8 to 66.6 | 157 | 0.24 |
| Biopsies 1-2 | 85.6 | 80.3 to 90.2 | 222 | 0.34 |
| Biopsies 1-3 | 95.6 | 91.3 to 99.2 | 246 | 0.38 |
| Biopsies 1-4 | 100 | 100 to 100 | 252 | 0.39 |
| Colposcopy impression normal (n = 20) | ||||
| Biopsy 1 | 0 | 0 to 0 | 0 | 0.00 |
| Biopsies 1-2 | 100 | 100 to 100 | 1 | 0.05 |
| Biopsies 1-3 | 100 | 100 to 100 | 1 | 0.05 |
| Biopsies 1-4 | 100 | 100 to 100 | 1 | 0.05 |
| Colposcopy impression acetowhitening (n = 69) | ||||
| Biopsy 1 | 37.5 | 37.5 to 37.5 | 3 | 0.04 |
| Biopsies 1-2 | 87.5 | 87.5 to 87.5 | 7 | 0.10 |
| Biopsies 1-3 | 100 | 100 to 100 | 8 | 0.12 |
| Biopsies 1-4 | 100 | 100 to 100 | 8 | 0.12 |
| Colposcopy impression low grade (n = 339) | ||||
| Biopsy 1 | 58.5 | 47.65 to 69 | 67 | 0.20 |
| Biopsies 1-2 | 83 | 74.55 to 91.5 | 95 | 0.28 |
| Biopsies 1-3 | 91.5 | 83.1 to 99 | 104 | 0.31 |
| Biopsies 1-4 | 100 | 100 to 100 | 109 | 0.32 |
| Colposcopy impression high grade (n = 220) | ||||
| Biopsy 1 | 65 | 56.8 to 73.5 | 86 | 0.39 |
| Biopsies 1-2 | 88.4 | 82.7 to 93.8 | 117 | 0.53 |
| Biopsies 1-3 | 99.2 | 97.5 to 100 | 131 | 0.60 |
| Biopsies 1-4 | 100 | 100 to 100 | 132 | 0.60 |
| Cytology ASCUS (n = 152) | ||||
| Biopsy 1 | 50 | 31.5 to 67.8 | 16 | 0.11 |
| Biopsies 1-2 | 87.5 | 75 to 100 | 28 | 0.18 |
| Biopsies 1-3 | 100 | 100 to 100 | 32 | 0.21 |
| Biopsies 1-4 | 100 | 100 to 100 | 32 | 0.21 |
| Cytology LSIL (n = 269) | ||||
| Biopsy 1 | 54.3 | 43.1 to 65.4 | 48 | 0.18 |
| Biopsies 1-2 | 83.7 | 73 to 93.2 | 74 | 0.28 |
| Biopsies 1-3 | 92.8 | 82.5 to 100 | 82 | 0.30 |
| Biopsies 1-4 | 100 | 100 to 100 | 85 | 0.32 |
| Cytology HSIL (n = 208) | ||||
| Biopsy 1 | 67.8 | 59.15 to 75.6 | 86 | 0.41 |
| Biopsies 1-2 | 89 | 83.3 to 94.3 | 113 | 0.54 |
| Biopsies 1-3 | 98.4 | 96 to 100 | 124 | 0.60 |
| Biopsies 1-4 | 100 | 100 to 100 | 126 | 0.61 |
| HPV-16 negative (n = 465) | ||||
| Biopsy 1 | 50.5 | 41.8 to 59.25 | 70 | 0.15 |
| Biopsies 1-2 | 80.7 | 71.85 to 88.9 | 112 | 0.24 |
| Biopsies 1-3 | 92.3 | 84.1 to 99.1 | 127 | 0.27 |
| Biopsies 1-4 | 100 | 100 to 100 | 132 | 0.28 |
| HPV-16 positive (n = 185) | ||||
| Biopsy 1 | 72.6 | 63.75 to 80.3 | 87 | 0.47 |
| Biopsies 1-2 | 91.8 | 85.95 to 96.7 | 110 | 0.59 |
| Biopsies 1-3 | 99.2 | 97.2 to 100 | 118 | 0.64 |
| Biopsies 1-4 | 100 | 100 to 100 | 119 | 0.64 |
| Age 21-24 years (n = 240) | ||||
| Biopsy 1 | 55.5 | 45.45 to 65.6 | 54 | 0.23 |
| Biopsies 1-2 | 87.4 | 78.4 to 94.95 | 85 | 0.35 |
| Biopsies 1-3 | 96.6 | 89.15 to 100 | 94 | 0.39 |
| Biopsies 1-4 | 100 | 100 to 100 | 95 | 0.40 |
| Age 25-29 years (n = 202) | ||||
| Biopsy 1 | 58.7 | 47.6 to 69.35 | 51 | 0.25 |
| Biopsies 1-2 | 80.6 | 70.15 to 90 | 70 | 0.35 |
| Biopsies 1-3 | 93.4 | 84.05 to 100 | 80 | 0.40 |
| Biopsies 1-4 | 100 | 100 to 100 | 83 | 0.41 |
| Age ≥ 30 years (n = 185) | ||||
| Biopsy 1 | 65 | 53.15 to 77.2 | 42 | 0.23 |
| Biopsies 1-2 | 88.2 | 79.75 to 96.35 | 57 | 0.31 |
| Biopsies 1-3 | 96.9 | 92.1 to 100 | 62 | 0.34 |
| Biopsies 1-4 | 100 | 100 to 100 | 64 | 0.35 |
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Results include imputed biopsy results. Biopsy 1 = HSIL found with the first biopsy only. Biopsies 1-2 = HSIL found with the first two biopsies. Biopsies 1-3 = HSIL found with the first three biopsies. Biopsies 1-4 = HSIL found with all four biopsies.
DISCUSSION
Recent evidence-based updates of cervical cancer screening have led to major changes in screening and management algorithms but have not addressed colposcopy practice.3,18 The lack of consensus about colposcopic practice in the United States is reflected by a wide variation of colposcopy-biopsy procedures.10,19
In the Biopsy Study, we demonstrated that adding a second biopsy, targeting even minimally abnormal-looking areas of the cervical transformation zone, increased the sensitivity to detect a prevalent HSIL significantly from 61% to 86%. Adding a third biopsy increased the sensitivity further to 96%. The incremental benefit of taking multiple biopsies was present regardless of referral cytology, HPV-16 status, and colposcopic impression; for example, even when there was a high-grade colposcopic impression, a single biopsy did not identify a prevalent HSIL in 35% of the women. Importantly, the absolute increase in disease detected by additional biopsies differed across risk strata. In women with increased prior risk of HSIL, related to more severe referral cytology, HPV status, and colposcopic impression, additional biopsies found more disease. Our results were consistent across six well-trained and experienced colposcopists.
Our results extend the evidence from previous secondary analyses from clinical trials suggesting that a single biopsy can fail to detect HSIL in 30% to 50% of patients.7,8 Unlike the study by Gage et al,7 we stratified our results by colposcopic impression, which is related both to number of biopsies and severity of disease, and we provide similar estimates of yields when accounting for colposcopic impression, cytology, HPV-16 status, and age. Unlike the study by Stoler et al,8 which was conducted in young women enrolled onto a vaccination trial, our findings are more generalizable to a US colposcopy population.
Our study identified additional benefit from directed biopsies of visible lesions but limited benefit from additional nondirected biopsies. In studies conducted in China and the United States, multiple nondirected biopsies were taken, and up to 30% of CIN3 lesions were found by these biopsies.9,10 In our study, multiple lesion-directed biopsies were taken at a lower threshold for abnormality (acetowhitening), possibly explaining the low yield from subsequent nondirected biopsies.
Our study has several other strengths. We used digital documentation of colposcopy impression; all images were re-evaluated by an experienced colposcopist. Although we used a low threshold of abnormality for targeted biopsies, more than 95% of HSILs were identified in women with at least low-grade colposcopy impression, supporting that most HSILs are found in colposcopically abnormal areas. This is also corroborated by a prospective evaluation that showed a low risk of HSIL in women with normal colposcopic findings when re-examined several years later.20
One unavoidable limitation of our study is the lack of a perfect gold standard. Misclassification can occur when the worst lesion is not sampled. However, achieving unbiased estimates of HSIL would require loop electrosurgical excision of all women, independent of biopsy result, which is not ethical. We minimized verification bias by taking multiple biopsies and by imputing additional biopsy results for women with fewer than four biopsies. We observed a plateauing yield of HSIL with increasing number of biopsies, suggesting that few additional HSILs were missed in our study.
More sensitive screening and biopsy approaches may preferentially detect lesions with a low risk of progression to cervical cancer. This is a challenge in all studies evaluating cervical precancer end points. We used a biomarker-enhanced end point of HSIL to achieve higher specificity for cervical cancer precursors.16
Our study was highly efficient, because each considered intervention was evaluated in each woman. Despite the additional biopsies required, the participation rate of our study was 50.3%, comparable to similar studies, such as the ASCUS-LSIL Triage Study.21 Our results are generalizable because we have provided risk and sensitivity estimates stratified by and adjusted for important clinical criteria, such as cytology, HPV status, and colposcopic impression, that can be applied to other populations.
For clinical recommendations, the benefits and harms of taking additional biopsies need to be considered. Taking more biopsies and increasing the sensitivity for HSIL will allow earlier management decisions and provide greater reassurance for women with negative results. Currently, women with negative biopsies undergo repeated colposcopies until HSIL is detected or screening tests turn negative,4 which is burdensome, emotionally stressful for women, and costly. Potential harms of taking multiple biopsies are discomfort and increased cost of pathology processing and evaluation. In our study, taking additional biopsies with sharp high-quality biopsy forceps was well tolerated. No adverse events occurred in our study; we previously demonstrated that taking more biopsies did not increase the risk of subsequent HPV infections.22
The efficiency data presented in our study represent the essential first step to move to consideration of population effectiveness of standardized colposcopy-biopsy protocols. The optimal number of biopsies depends on prior risk, as determined by cytology status, colposcopy impression, and HPV type status.23 In almost all patients, a second biopsy appears particularly useful. A recent study of endocervical curettage in more than 13,000 women found a yield of only 1% for endocervical sampling,24 much lower compared with the 10% yield of a second biopsy observed in our study.
Analogously, positive prostate cancer screening results are followed up with biopsies. Studies have demonstrated that conventional biopsy protocols miss up to 30% of prostate cancers, which has led to recommendations of more biopsies.25
In summary, our findings reaffirm the importance of colposcopy and lesion-directed biopsy and have important implications for clinical practice—taking multiple directed biopsies during colposcopy can increase detection of HSIL. The full benefit of earlier detection of HSIL by screening using HPV testing3 will depend on improvement and standardization of colposcopy. Rather than sampling only the worst-appearing site, at least two or three biopsies should be taken when distinct abnormal sites, including acetowhitening as abnormal, are present.
Appendix
Table A1.
Observed and Adjusted Marginal Yields of HSIL for Targeted and Random Biopsies
| Biopsy | No. of Patients | No. of HSILs |
||||
|---|---|---|---|---|---|---|
| Observed Counts | Marginal Yield | Adjusted Counts | Adjusted Marginal Yield (predictive margins) | 95% CI | ||
| All | 419 | |||||
| Targeted | 108 | 25.78 | 108 | 25.78 | 21.8 to 30.2 | |
| Random | 8 | 1.91 | 8 | 1.91 | 0.96 to 3.78 | |
| Colposcopy impression normal/acetowhitening | 101 | |||||
| Targeted | 6 | 5.94 | 11.9 | 11.79 | 5.56 to 23.26 | |
| Random | 3 | 2.97 | 2.6 | 2.55 | 0.8 to 7.88 | |
| Colposcopy impression low grade | 235 | |||||
| Targeted | 63 | 26.81 | 62.7 | 26.68 | 21.67 to 32.38 | |
| Random | 5 | 2.13 | 5.8 | 2.48 | 1.01 to 5.97 | |
| Colposcopy impression high grade | 83 | |||||
| Targeted | 39 | 46.99 | 26.3 | 31.63 | 23.61 to 40.92 | |
| Random | 0 | 0 | 0 | 0 | 0 to 0 | |
| Cytology ASCUS | 110 | |||||
| Targeted | 17 | 15.45 | 19.7 | 17.91 | 11.92 to 26.04 | |
| Random | 0 | 15.45 | 0 | 0 | 0 to 0 | |
| Cytology LSIL | 190 | |||||
| Targeted | 37 | 19.47 | 43.7 | 23.01 | 17.57 to 29.53 | |
| Random | 3 | 1.58 | 2.7 | 1.41 | 0.45 to 4.31 | |
| Cytology HSIL | 119 | |||||
| Targeted | 54 | 45.38 | 41.8 | 35.12 | 27.58 to 43.47 | |
| Random | 5 | 4.2 | 6.8 | 5.75 | 2.39 to 1.32 | |
| HPV-16 negative | 316 | |||||
| Targeted | 58 | 18.35 | 65.2 | 20.64 | 1.64 to 25.63 | |
| Random | 5 | 1.58 | 5.1 | 1.61 | 0.67 to 3.82 | |
| HPV-16 positive | 103 | |||||
| Targeted | 50 | 48.54 | 40.3 | 39.15 | 30.82 to 48.15 | |
| Random | 3 | 2.91 | 3.1 | 2.98 | 0.99 to 8.67 | |
| No targeted biopsy | 26 | |||||
| Targeted | NA | NA | NA | NA | ||
| Random | 1 | 3.85 | 1.9 | 7.47 | 0.94 to 40.81 | |
| One targeted biopsy | 83 | |||||
| Targeted | 11 | 13.25 | 14.8 | 17.86 | 10.65 to 28.39 | |
| Random | 3 | 3.61 | 2.8 | 3.33 | 1.14 to 9.29 | |
| Two targeted biopsies | 172 | |||||
| Targeted | 32 | 18.6 | 33.4 | 19.42 | 14.21 to 25.96 | |
| Random | 3 | 1.74 | 2.9 | 1.69 | 0.54 to 5.17 | |
| Three targeted biopsies | 138 | |||||
| Targeted | 65 | 47.1 | 51.9 | 37.58 | 30.0 to 45.83 | |
| Random | 1 | 0.72 | 1.1 | 0.78 | 0.1 to 6.06 | |
NOTE. Observed count and yield are based on biopsy results without imputation. Adjusted count and yield are based on predictive margins from multinomial logistic regression, mutually adjusted for colposcopy impression, cytology result, HPV-16 status, and number of targeted biopsies. Differences in numbers between Table 2 and Appendix Table A1 are related to missing covariates, which leads to exclusion of patients for this analysis.
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NA, not applicable.
Table A2.
Observed and Adjusted Yield of HSIL With Increasing Number of Biopsies
| Biopsy | No. of Patients | No. of HSILs |
||||
|---|---|---|---|---|---|---|
| Observed Counts | Marginal Yield | Adjusted Counts | Adjusted Marginal Yield (predictive margins) | 95% CI | ||
| All | 624 | |||||
| Biopsy 1 | 152 | 24.36 | 152 | 24.36 | 21.14 to 27.89 | |
| Biopsy 2 | 60 | 9.62 | 60 | 9.62 | 7.53 to 12.20 | |
| Biopsy 3 | 18 | 2.88 | 18 | 2.88 | 1.82 to 4.54 | |
| Biopsy 4 | 4 | 0.64 | 4 | 0.64 | 0.24 to 1.70 | |
| Colposcopy impression normal/acetowhitening | 86 | |||||
| Biopsy 1 | 3 | 3.49 | 7.2 | 8.41 | 3.14 to 20.63 | |
| Biopsy 2 | 3 | 3.49 | 5.1 | 5.94 | 1.97 to 16.54 | |
| Biopsy 3 | 1 | 1.16 | 1.3 | 1.51 | 0.22 to 9.78 | |
| Biopsy 4 | 0 | 0 | 0 | 0 | 0 to 0 | |
| Colposcopy impression low grade | 326 | |||||
| Biopsy 1 | 64 | 19.63 | 76.3 | 23.42 | 19.16 to 28.29 | |
| Biopsy 2 | 27 | 8.28 | 28.2 | 8.66 | 6.02 to 12.32 | |
| Biopsy 3 | 6 | 1.84 | 5.9 | 1.81 | 0.78 to 4.14 | |
| Biopsy 4 | 3 | 0.92 | 3.4 | 1.04 | 0.31 to 3.46 | |
| Colposcopy impression high grade | 212 | |||||
| Biopsy 1 | 85 | 40.09 | 63.4 | 29.89 | 24.74 to 35.61 | |
| Biopsy 2 | 30 | 14.15 | 27.6 | 13.03 | 9.19 to 18.17 | |
| Biopsy 3 | 11 | 5.19 | 11.8 | 5.55 | 2.89 to 10.40 | |
| Biopsy 4 | 1 | 0.47 | 0.9 | 0.42 | 0.05 to 3.5 | |
| Cytology ASCUS | 146 | |||||
| Biopsy 1 | 16 | 10.96 | 24.8 | 17.02 | 1.22 to 23.24 | |
| Biopsy 2 | 12 | 8.22 | 10.2 | 6.98 | 4.02 to 11.84 | |
| Biopsy 3 | 4 | 2.74 | 4.5 | 3.10 | 1.31 to 7.16 | |
| Biopsy 4 | 0 | 0 | 0.8 | 0.53 | 0.07 to 3.70 | |
| Cytology LSIL | 253 | |||||
| Biopsy 1 | 46 | 18.18 | 48.7 | 19.26 | 13.79 to 26.23 | |
| Biopsy 2 | 24 | 9.49 | 32.8 | 12.98 | 8.51 to 19.30 | |
| Biopsy 3 | 8 | 3.16 | 10.6 | 4.19 | 1.88 to 9.08 | |
| Biopsy 4 | 2 | 0.79 | 3.8 | 1.49 | 0.37 to 5.79 | |
| Cytology HSIL | 202 | |||||
| Biopsy 1 | 83 | 41.09 | 105.6 | 52.29 | 43.47 to 60.98 | |
| Biopsy 2 | 24 | 11.88 | 33.5 | 16.58 | 11.10 to 24.04 | |
| Biopsy 3 | 6 | 2.97 | 6.1 | 3.02 | 1.16 to 7.65 | |
| Biopsy 4 | 2 | 0.99 | 1.6 | 0.81 | 0.12 to 5.24 | |
| HPV-16 negative | 444 | |||||
| Biopsy 1 | 68 | 15.32 | 84 | 18.93 | 15.41 to 23.02 | |
| Biopsy 2 | 39 | 8.78 | 43.9 | 9.88 | 7.30 to 13.23 | |
| Biopsy 3 | 13 | 2.93 | 14.1 | 3.18 | 1.88 to 5.33 | |
| Biopsy 4 | 3 | 0.68 | 3 | 0.69 | 0.23 to 2.00 | |
| HPV-16 positive | 180 | |||||
| Biopsy 1 | 84 | 46.67 | 62.8 | 34.86 | 28.99 to 41.24 | |
| Biopsy 2 | 21 | 11.67 | 18.5 | 10.26 | 6.72 to 15.35 | |
| Biopsy 3 | 5 | 2.78 | 4.7 | 2.59 | 1.11 to 5.92 | |
| Biopsy 4 | 1 | 0.56 | 1.1 | 0.59 | 0.1 to 3.44 | |
| Age 21-24 years | 233 | |||||
| Biopsy 1 | 52 | 22.32 | 54.3 | 23.29 | 18.66 to 28.67 | |
| Biopsy 2 | 30 | 12.88 | 30.7 | 13.18 | 9.46 to 18.05 | |
| Biopsy 3 | 8 | 3.43 | 8.5 | 3.63 | 1.82 to 7.1 | |
| Biopsy 4 | 0 | 0 | 0 | 0 | 0 to 0 | |
| Age 25-29 years | 195 | |||||
| Biopsy 1 | 50 | 25.64 | 47 | 24.10 | 19.36 to 29.58 | |
| Biopsy 2 | 18 | 9.23 | 16.4 | 8.40 | 5.34 to 12.97 | |
| Biopsy 3 | 6 | 3.08 | 5.3 | 2.73 | 1.25 to 5.88 | |
| Biopsy 4 | 2 | 1.03 | 1.9 | 0.96 | 0.24 to 3.85 | |
| Age ≥ 30 years | 176 | |||||
| Biopsy 1 | 41 | 23.3 | 41.4 | 23.53 | 18.26 to 29.78 | |
| Biopsy 2 | 12 | 6.82 | 12.7 | 7.23 | 4.20 to 12.16 | |
| Biopsy 3 | 4 | 2.27 | 4.2 | 2.39 | 0.89 to 6.28 | |
| Biopsy 4 | 2 | 1.14 | 2.5 | 1.43 | 0.34 to 5.73 | |
NOTE. Observed count and yield are based on biopsy results without imputation. Adjusted count and yield are based on predictive margins from multinomial logistic regression, mutually adjusted for colposcopy impression, cytology result, HPV-16 status, and age group. Biopsies 1-4 = marginal yield of HSIL found with biopsies 1, 2, 3, or 4. Differences in numbers between Table 3 and Appendix Table A2 are related to missing covariates, which leads to exclusion of patients for this analysis.
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Table A3.
Sensitivity for and Yield of CIN 2/CIN 3 and CIN 3 Alone With Increasing Number of Lesion-Directed Biopsies
| Biopsy | Cumulative Sensitivity (%) | 95% CI (%) | No. of CIN 2 and CIN 3 Detected | Cumulative Yield |
|---|---|---|---|---|
| All (N = 653), CIN 2/CIN 3 end point | ||||
| Biopsy 1 | 62.4 | 55.8 to 68.6 | 174 | 0.27 |
| Biopsies 1-2 | 86.1 | 80.4 to 90.8 | 240 | 0.37 |
| Biopsies 1-3 | 95.9 | 91.4 to 99.3 | 267 | 0.41 |
| Biopsies 1-4 | 100 | 100 to 100 | 278 | 0.43 |
| All (N = 653), CIN 3 end point | ||||
| Biopsy 1 | 65.4 | 54.4 to 76.4 | 50 | 0.08 |
| Biopsies 1-2 | 90.2 | 83.1 to 96.1 | 69 | 0.11 |
| Biopsies 1-3 | 96.1 | 91.4 to 100 | 73 | 0.11 |
| Biopsies 1-4 | 100 | 100 to 100 | 76 | 0.12 |
Abbreviation: CIN, cervical intraepithelial neoplasia.
Table A4.
Sensitivity for and Yield of HSIL With Increasing Number of Lesion-Directed Biopsies by Colposcopist
| Biopsy | No. of Patients | Cumulative Sensitivity (%) | 95% CI (%) | No. of HSILs Detected | Cumulative Yield |
|---|---|---|---|---|---|
| Colposcopist 1 | 172 | ||||
| Biopsy 1 | 58.1 | 45.8 to 70.0 | 36 | 0.21 | |
| Biopsies 1-2 | 83.9 | 75 to 92.3 | 52 | 0.30 | |
| Biopsies 1-3 | 96.8 | 91.6 to 100 | 60 | 0.35 | |
| Biopsies 1-4 | 100 | 100 to 100 | 62 | 0.36 | |
| Colposcopist 2 | 84 | ||||
| Biopsy 1 | 63.3 | 45.7 to 80 | 19 | 0.23 | |
| Biopsies 1-2 | 90.0 | 78.3 to 100 | 27 | 0.32 | |
| Biopsies 1-3 | 93.3 | 82.4 to 100 | 28 | 0.33 | |
| Biopsies 1-4 | 100 | 100 to 100 | 30 | 0.36 | |
| Colposcopist 3 | 68 | ||||
| Biopsy 1 | 32.3 | 14.2 to 62.1 | 12 | 0.18 | |
| Biopsies 1-2 | 56.6 | 29.1 to 93.5 | 21 | 0.31 | |
| Biopsies 1-3 | 68.5 | 36.5 to 100 | 25 | 0.37 | |
| Biopsies 1-4 | 100 | 100 to 100 | 37 | 0.54 | |
| Colposcopist 4 | 72 | ||||
| Biopsy 1 | 71 | 53.1 to 87.5 | 22 | 0.31 | |
| Biopsies 1-2 | 93.5 | 83.9 to 100 | 29 | 0.40 | |
| Biopsies 1-3 | 100 | 100 to 100 | 31 | 0.43 | |
| Biopsies 1-4 | 100 | 100 to 100 | 31 | 0.43 | |
| Colposcopist 5 | 175 | ||||
| Biopsy 1 | 69.2 | 56.8 to 80.3 | 50 | 0.29 | |
| Biopsies 1-2 | 91.3 | 81.2 to 98.7 | 66 | 0.38 | |
| Biopsies 1-3 | 95.4 | 85.5 to 100 | 69 | 0.39 | |
| Biopsies 1-4 | 100 | 100 to 100 | 72 | 0.41 | |
| Colposcopist 6 | 53 | ||||
| Biopsy 1 | 53.3 | 32.4 to 75 | 12 | 0.23 | |
| Biopsies 1-2 | 88.9 | 73.2 to 100 | 20 | 0.38 | |
| Biopsies 1-3 | 100 | 100 to 100 | 22 | 0.42 | |
| Biopsies 1-4 | 100 | 100 to 100 | 22 | 0.42 |
Abbreviation: HSIL, high-grade squamous intraepithelial lesion.
Footnotes
Listen to the podcast by Dr Horowitz at www.jco.org/podcasts
Supported by the Intramural Research Program of the National Cancer Institute, Bethesda, MD.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
The funding source had no role in design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Nicolas Wentzensen, Joan L. Walker, Michael A. Gold, Mark Schiffman
Collection and assembly of data: Nicolas Wentzensen, Joan L. Walker, Michael A. Gold, Katie M. Smith, Rosemary E. Zuna, Cara Mathews, S. Terence Dunn, Roy Zhang, Katherine Moxley, Erin Bishop, Meaghan Tenney, Elizabeth Nugent, Mark Schiffman
Data analysis and interpretation: Nicolas Wentzensen, Barry I. Graubard, Sholom Wacholder, Mark Schiffman
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Multiple Biopsies and Detection of Cervical Cancer Precursors at Colposcopy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Nicolas Wentzensen
No relationship to disclose
Joan L. Walker
No relationship to disclose
Michael A. Gold
No relationship to disclose
Katie M. Smith
No relationship to disclose
Rosemary E. Zuna
No relationship to disclose
Cara Mathews
No relationship to disclose
S. Terence Dunn
No relationship to disclose
Roy Zhang
No relationship to disclose
Katherine Moxley
No relationship to disclose
Erin Bishop
No relationship to disclose
Meaghan Tenney
No relationship to disclose
Elizabeth Nugent
No relationship to disclose
Barry I. Graubard
No relationship to disclose
Sholom Wacholder
No relationship to disclose
Mark Schiffman
No relationship to disclose
REFERENCES
- 1.Davey DD, Neal MH, Wilbur DC, et al. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004;128:1224–1229. doi: 10.5858/2004-128-1224-BIARRP. [DOI] [PubMed] [Google Scholar]
- 2.Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 3.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright TC, Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 5.Jeronimo J, Massad LS, Castle PE, et al. Interobserver agreement in the evaluation of digitized cervical images. Obstet Gynecol. 2007;110:833–840. doi: 10.1097/01.AOG.0000281665.63550.8f. [DOI] [PubMed] [Google Scholar]
- 6.Massad LS, Jeronimo J, Schiffman M. Interobserver agreement in the assessment of components of colposcopic grading. Obstet Gynecol. 2008;111:1279–1284. doi: 10.1097/AOG.0b013e31816baed1. [DOI] [PubMed] [Google Scholar]
- 7.Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264–272. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 8.Stoler MH, Vichnin MD, Ferenczy A, et al. The accuracy of colposcopic biopsy: Analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer. 2011;128:1354–1362. doi: 10.1002/ijc.25470. [DOI] [PubMed] [Google Scholar]
- 9.Pretorius RG, Zhang WH, Belinson JL, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004;191:430–434. doi: 10.1016/j.ajog.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 10.Pretorius RG, Belinson JL, Azizi F, et al. Utility of random cervical biopsy and endocervical curettage in a low-risk population. J Low Genit Tract Dis. 2012;16:333–338. doi: 10.1097/LGT.0b013e3182480c18. [DOI] [PubMed] [Google Scholar]
- 11.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 12.Bergeron C, Ordi J, Schmidt D, et al. Conjunctive p16INK4a testing significantly increases accuracy in diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin Pathol. 2010;133:395–406. doi: 10.1309/AJCPXSVCDZ3D5MZM. [DOI] [PubMed] [Google Scholar]
- 13.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 14.Wentzensen N, Schiffman M, Dunn ST, et al. Grading the severity of cervical neoplasia based on combined histopathology, cytopathology, and HPV genotype distribution among 1,700 women referred to colposcopy in Oklahoma. Int J Cancer. 2009;124:964–969. doi: 10.1002/ijc.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wentzensen N, Schiffman M, Dunn T, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125:2151–2158. doi: 10.1002/ijc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16:205–242. doi: 10.1097/LGT.0b013e31825c31dd. [DOI] [PubMed] [Google Scholar]
- 17.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 18.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(suppl 1):S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 19.Mayeaux EJ, Cox JT. Modern Colposcopy Textbook and Atlas. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 20.Kelly RS, Walker P, Kitchener H, et al. Incidence of cervical intraepithelial neoplasia grade 2 or worse in colposcopy-negative/human papillomavirus-positive women with low-grade cytological abnormalities. BJOG. 2012;119:20–25. doi: 10.1111/j.1471-0528.2011.02970.x. [DOI] [PubMed] [Google Scholar]
- 21.Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study: Design, methods and characteristics of trial participants. Acta Cytol. 2000;44:726–742. doi: 10.1159/000328554. [DOI] [PubMed] [Google Scholar]
- 22.Castle PE, Wentzensen N, Wheeler CM, et al. Effect of the number of biopsies on the subsequent acquisition of new human papillomavirus infections. Obstet Gynecol. 2009;114:1057–1062. doi: 10.1097/AOG.0b013e3181bb5632. [DOI] [PubMed] [Google Scholar]
- 23.Wentzensen N, Wacholder S. From differences in means between cases and controls to risk stratification: A business plan for biomarker development. Cancer Discov. 2013;3:148–157. doi: 10.1158/2159-8290.CD-12-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gage JC, Duggan MA, Nation JG, et al. Detection of cervical cancer and its precursors by endocervical curettage in 13,115 colposcopically guided biopsy examinations. Am J Obstet Gynecol. 2010;203:481–489. doi: 10.1016/j.ajog.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjurlin MA, Carter HB, Schellhammer P, et al. Optimization of initial prostate biopsy in clinical practice: Sampling, labeling and specimen processing. J Urol. 2013;189:2039–2046. doi: 10.1016/j.juro.2013.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]


