Abstract
The phenazines are a class of over 150 nitrogen-containing aromatic compounds of bacterial and archeal origin. Their redox properties not only explain their activity as broad-specificity antibiotics and virulence factors but also enable them to function as respiratory pigments, thus extending their importance to the primary metabolism of phenazine-producing species. Despite their discovery in the mid-19th century, the molecular mechanisms behind their biosynthesis have only been unraveled in the last decade. Here, we review the contribution of structural biology that has led to our current understanding of phenazine biosynthesis.
Introduction
Readily observable phenomena have triggered scientific research since its early beginnings. The phenazines, whose biosynthesis is reviewed here, also owe their discovery and subsequent investigation to an observation that can be made with the naked eye: the medical literature of the 19th century reports numerous cases of “blue pus”, normally associated with drastic surgical procedures that required long periods of wound care. In 1859, Fordos described the use of chloroform to extract the blue pigment responsible for this coloration, which he named “pyocyanin” after the Greek words πύο (pus) and κυανό (cyan) [1,2]. More than 20 years later, Carl Gessard linked the occurrence of pyocyanin to the presence of a microorganism that he called Bacillus pyocyaneus [3], an organism that was also investigated by others around that time, albeit without referring to the work of Fordos. Today, B. pyocyaneus is known as Pseudomonas aeruginosa [4], and the name still reflects its capacity to produce pigments: aerugo is Latin for verdigris, the blue–green coating on copper that develops after long exposure to air or seawater.
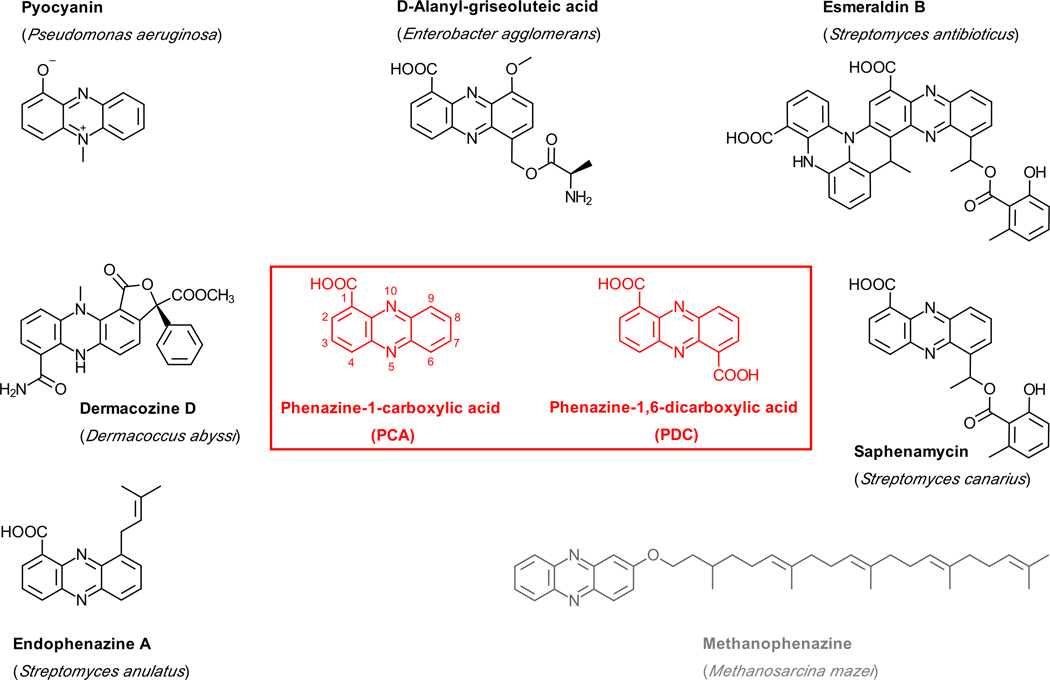
In 1924 Wrede and Strack showed that pyocyanin (PYO) is a phenazine derivative [5], and the chemical structure was established as 5-N-methyl-1-hydroxyphenazinium betaine by Hillemann in 1938 [6]. Phenazine derivatives have now been isolated from several Gram-positive and -negative bacteria as well as from archeal Methanosarcina species, and the number of known phenazine natural products has grown to over 150 (Dictionary of Natural Products, Taylor & Francis Group, available at dnp.chemnetbase.com; examples are shown in Fig. 1). PYO is still by far the best-studied representative of this family, largely because P. aeruginosa is an important opportunistic and nosocomial pathogen and also a major contributor to the early mortality observed in patients with cystic fibrosis [7–9]. In addition, PYO production provides an easy readout for assessing quorum sensing in P. aeruginosa, which is intensely studied in many laboratories worldwide.
Fig. 1.
A collection of naturally occurring phenazine derivatives and their origins. With the exception of methanophenazine, for which the biosynthetic route is unclear, these molecules are synthesized from phenazine-1-carboxylic acid (PCA) or phenazine-1,6-dicarboxylic acid (PDC).
Phenazines are redox-active compounds that trigger the formation of toxic reactive oxygen species by directly reducing molecular oxygen, explaining why they are broad-specificity antibiotics and virulence factors [10–13]. While it has long been believed that the resulting competitive advantage is the physiological rationale for phenazine production, the field is currently experiencing a paradigm shift since new data indicate that phenazines also play a role in the primary metabolism of their producers. PYO, for example, can directly oxidize NADH, which may be required to sustain glycolysis in anoxic regions of biofilm [14]. The importance of PYO is corroborated by the observation that the number of surviving bacteria is greatly reduced in a mouse model of acute lung infections when PYO biosynthesis is impaired [15] and that strongly altered colony morphologies are observed in phenazine-deficient mutants of P. aeruginosa [16].
A conserved set of phenazine biosynthesis genes has been found in all bacterial phenazine producers investigated to date [17]. These genes are normally clustered in an operon that encodes five enzymes required for the generation of the “core” phenazines, phenazine-1,6-dicarboxylic acid (PDC) or phenazine-1-carboxylic acid (PCA), which are the precursors for strain-specific phenazine derivatives. Interestingly, PDC- and PCA-producers cannot be distinguished from the sequences of their “phz”-operons, an observation which is explored further below. The phz-operon is usually extended by genes needed for conversion of PDC or PCA into downstream products, genes involved in phenazine autoresistance and in delivery of precursors or regulation of the pathway.
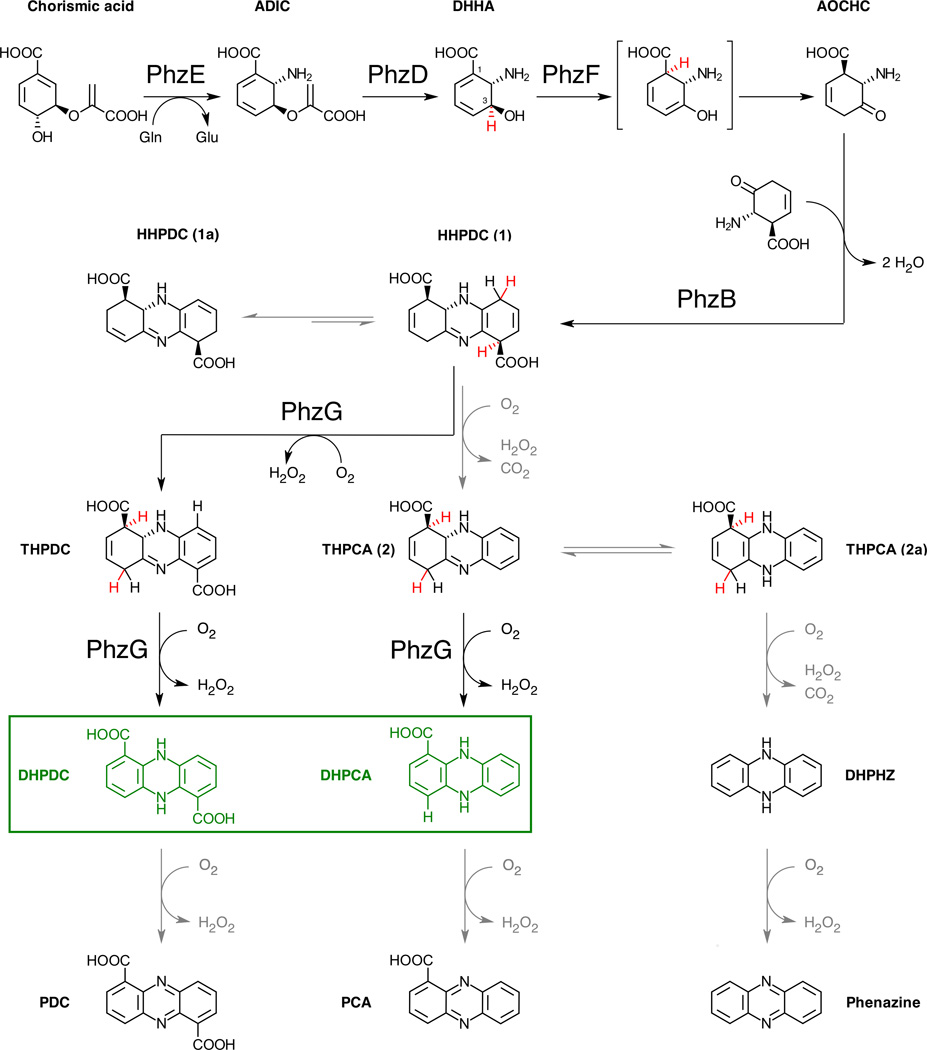
The chemistry of phenazine biosynthesis has been studied since the 1960s (reviewed in [18]), but only with the discovery of the phz-operon in the late 1990s and the subsequent application of structural biology and biochemical experiments in the last decade has it become possible to draw a detailed picture of this pathway (Figs. 2 and 3).
Fig. 2.
Current understanding of core phenazine biosynthesis. Abstracted or shifted hydrogens are shown in red, grey arrows indicate uncatalyzed steps. 5,10-Dihydro-PCA and 5,10-dihydro-PDC are the final products of the pathway (green).
Fig. 3.
A structural view of core phenazine biosynthesis. The following Protein Data Bank entries [53] have been used: PhzE, 3R75 [26]; PhzD, 1NF8 [28] and 3R77 [26]; PhzF, 1U1W [34]; PhzB, 3DZL [38] and PhzG, 4HMT [42]. The figure was generated with PyMOL [54].
Phenazine biosynthesis
Experiments in the early 1970s showed that phenazines are derived from two molecules of chorismic acid [19,20], but it was for a long time not clear what transformation chorismate underwent prior to formation of the initial phenazine tricycle. Anthranilate was logically investigated, but no significant incorporation into phenazines was observed [21]. However, anthranilate biosynthesis proceeds via 2-amino-2-desoxyisochorismic acid (ADIC), and Floss and coworkers demonstrated in a first biochemical analysis of the phz-operon that ADIC is completely incorporated into PCA, suggesting that phenazine biosynthesis branches off at this point [22].
In phenazine-producing bacteria, ADIC formation from chorismate is catalyzed by PhzE, a homodimeric enzyme closely related to anthranilate synthases (AS) but incapable of invoking the pyruvate elimination from ADIC to yield anthranilic acid. PhzE differs from the previously characterized heterotetrameric AS enzymes [23–25] in that the N-terminal chorismate-converting menaquinone, siderophore, tryptophan (MST) domain is covalently fused to the type-1 glutamine amidotransferase (GATase1) domain. Even more surprisingly, the crystal structure revealed that PhzE is an intertwined dimer in which the GATase1 domain of one chain provides ammonia to the MST domain of the other (Fig. 3). Ligand binding induces the formation of a potentially gated intramolecular channel for the diffusion of NH3 from the GATase1 to the active center of the MST domain. The channel ends at the si face at C2 of chorismate, which explains the stereochemistry of ADIC. Interestingly, the active site of PhzE’s MST domain is very similar to that of AS and reveals no indication of why AS further converts ADIC to anthranilate whereas PhzE does not. Attempts to convert PhzE into an AS via mutations yielded only inactive enzyme, leaving questions regarding differences between the two enzymes open [26].
In the following step, PhzD cleaves ADIC to (5S,6S)-6-amino-5-hydroxy-1,3-cyclohexadiene-1-carboxylic acid. This compound was first introduced into the literature as trans-2,3-dihydro-3-hydroxyanthranilic acid (DHHA) [27]. PhzD is an α/β-hydrolase but unlike structurally similar enzymes, such as nicotinamidase, PhzD is not metal-dependent nor is a covalent intermediate formed in the course of ADIC hydrolysis. Instead, PhzD employs an acid/base catalytic mechanism that involves an aspartic acid and probably a lysine residue (D38 and K122, Fig. 3) [28].
DHHA is the last stable intermediate in the pathway leading to PCA or PDC. It is the substrate of PhzF, an enzyme structurally similar to diaminopimelate epimerase [29], proline racemase [30], 2-methylaconitate isomerase [31], and 3-methylitaconate-Δ-isomerase [32]. However, whereas all of the latter require one or two cysteines as classical acid/base-catalytic residues, PhzF relies on a glutamate (E45, Fig. 3) to isomerize DHHA into 6-amino-5-oxocyclohex-2-ene-1-carboxylic acid (AOCHC) [33,34]. The reaction is thought to proceed via abstraction of a proton from C3, delocalization of the negative charge through the conjugated double bond system and reprotonation at C1, followed by tautomerization of the resulting enol to AOCHC. NMR analysis in D2O showed that the proton from C3 is fully recycled to C1 [34]. Further, crystal structures of the subsequent phenazine biosynthesis enzymes in complex with substrate analogues and in-situ-generated intermediates (see paragraphs on PhzB and PhzG) confirmed that deprotonation at C3 and reprotonation at C1 take place at the same face of the six-membered ring. Together, this indicates that the reaction catalyzed by PhzF can be classified as a suprafacial [1,5] proton shift and it is hence possible that the underlying mechanism is a sigmatropic rearrangement rather than acid/base catalysis.
The aminoketone AOCHC is highly reactive and cannot be isolated. One of the reactions it undergoes spontaneously is a twofold condensation with a second molecule of itself. Such a diagonal-symmetrical pairing as a central step in phenazine biosynthesis was already established in the 1970s, albeit without knowing the chemical structure of the pairing intermediate [35–37]. The self-condensation of AOCHC may be aided by a structural feature of PhzF: the enzyme is a “face-to-face” homodimer that adopts a closed conformation with a central cavity upon ligand binding [34]. The likelihood of two AOCHC molecules meeting each other would be increased if one assumes that both active centers release product simultaneously through this cavity. The kinetic properties of PhzF have, however, not been studied in sufficient detail as yet to determine to what extent PhzF facilitates AOCHC condensation.
Since the condensation of two AOCHC molecules is a bimolecular reaction, it is enhanced when PhzF acts at high concentrations of DHHA. However, because AOCHC probably also reacts with other amines e.g. on proteins, it likely is toxic to the cell such that its accumulation must be prevented. Indeed, the condensation reaction is significantly accelerated by PhzB, a dimeric enzyme of the ketosteroid isomerase / nuclear transport factor 2 family. Each monomer provides a large cavity for the binding of two AOCHC molecules, even if the chain contains only approx. 160 amino acids. The crystal structure of PhzB from Burkholderia lata 383 in complex with two molecules of the AOCHC-analogue 3-oxocyclohexanecarboxylic acid revealed that the relative orientation of the substrate molecules is secured through interaction of their carboxylates with two arginine residues, R41 and R160* from the C-terminus of the other monomer (Fig. 3, [38]). This C-terminus was found disordered in a ligand-free crystal form of PhzB, indicating that it acts as a lid that covers the active site by arm exchange. It is therefore likely that the AOCHC molecule that interacts with R160* binds second and PhzB hence catalyzes a sequential ordered bi uni mechanism, even if both substrate molecules are identical. Catalysis of the first condensation reaction is achieved by E140, which protonates the oxyanion intermediate emerging during the attack of the amino group from the second AOCHC molecule. Similar to related enzymes that also carry an acidic residue at this position [39], a hydrophobic environment increases the pKa of E140 such that it will be protonated at the onset of the reaction. The second condensation probably is catalyzed by H73 and S77, which may protonate the second tetrahedral intermediate.
Interestingly, pseudomonads, which are the most prolific phenazine producers, carry a second copy of the phzB gene termed phzA immediately upstream in their phz-operons. Even though PhzA is approx. 70% identical to PhzB, it is inactive in an AOCHC condensation assay, which may be attributable to mutations at the positions equivalent to H73 and S77 of PhzB to leucine [38]. PhzA nevertheless plays a role in phenazine biosynthesis of pseudomonads: McDonald et al. observed that PCA production was reduced to 25% of the wildtype level when PhzA was absent [22]. The molecular basis for this observation is not clear at present.
Using HPLC-coupled NMR spectroscopy, the product of PhzB was detected as hexahydrophenazine-1,6-dicarboxylic acid (HHPDC) isomer 1a [38], which likely arises through spontaneous conjugation of the double bonds in isomer 1 (Fig. 2). HHPDC is not stable and undergoes rapid oxidative decarboxylation to tetrahydrophenazine-1,6-carboxylic acid (THPCA). Because this reaction is uncatalyzed, it explains why the asymmetric PCA is always observed as a major product of phenazine biosynthesis, even if the investigated strain uses PDC as a precursor for strain-specific phenazine derivatives [40].
The final step of “core” phenazine biosynthesis is catalyzed by PhzG, a homodimeric flavin enzyme related to PdxH, a pyridoxine-5’-phosphate oxidase . The FMN cofactor and substrate binding site of PhzG are formed by residues from both chains, and the flexible N-terminus seems to act as a lid that is involved in substrate binding [41,42]. Surprisingly, trapping experiments with crystals of PhzG from P. fluorescens 2–79, which is a PCA-only producing organism and hence THPCA is expected to be its PhzG’s substrate, led to the observation of HHPDC tightly bound to the active center. When diffraction data were collected after several days, however, THPCA was found instead, indicating that PhzG can act on different tricyclic intermediates and thereby contribute to the generation of both PDC and PCA. Together, this suggests that the final oxidative steps in phenazine biosynthesis can follow different routes of enzyme-catalyzed oxidation and oxidative decarboxylation or both: for PDC, PhzG oxidizes HHPDC in two consecutive steps, whereas one oxidative decarboxylation and one PhzG-catalyzed oxidation lead to PCA. Two oxidative decarboxylations would generate unsubstituted phenazine, which was indeed detected in reaction mixtures of PhzF turning over DHHA in the absence or presence of PhzG [42]. The difference between PDC- and PCA-producing strains therefore does not seem to be a consequence of principal differences of their Phz-enzymes but rather rooted in different relative activities of the PhzF, PhzB and PhzG enzymes and the availability of oxygen, as was also corroborated in a recent study by Rui et al. [43].
Because FMN is a two-electron acceptor, the routes laid out above lead to PDC or PCA in their dihydro form, the reduced state of the phenazine electron shuttle. DHPDC and DHPCA are physiologically relevant derivatives and therefore they, and not the fully aromatic PDC and PCA normally shown in the literature, are very likely the end product of core phenazine biosynthesis. This also explains why the phz-operon contains only one oxidase and is further supported by the finding that enzymes that convert PDC or PCA to strain-specific derivatives possess higher activity towards the reduced substrate [44].
Conclusions and perspectives
Arriving at an understanding of the chemistry of phenazine biosynthesis has, for a long time, been hampered by the difficulty of isolating the unstable intermediates summarized above. The identification of phenazine biosynthesis genes has alleviated this situation by enabling biochemical and structural experiments that now provide us with a clear picture of the pathway. Its hallmarks are the two unique activities of PhzF and PhzB, which create the tricyclic phenazine scaffold from two identical precursor molecules. While some details of the “core” pathway still require investigation, e.g. the potentially pericyclic nature of the reaction catalyzed by PhzF or the role of the PhzB-doppelganger PhzA in pseudomonads, structural biology is currently turning its focus towards other aspects of phenazine biochemistry such as phenazine-modifying enzymes [45–48] and phenazine autoresistance proteins [49]. Because research with the Pseudomonas aeruginosa phenazine pyocyanin has shown that phenazines can also modulate host eukaryotic cells, it is to be hoped that these interactions will also be investigated in more detail in the future. The recent structure of a complex with glutathione reductase in a work aimed at understanding the anti-malarial properties of pyocyanin can be regarded as a starting point for such studies [50].
Another unresolved problem is the biosynthesis of phenazines in Methanosarcina spp., which utilize the membrane-bound methanophenazine (Fig. 1) as a substitute for ubiquinone in their electron transport chain [51]. The genomes of these species do not contain a phz-operon [52], suggesting that their phenazine biosynthesis follows a different route. It will be interesting to see if principles of the bacterial pathway are also conserved in these archea.
Highlights.
Phenazines are bacterial and archeal virulence factors and respiratory pigments
Their biosynthesis from chorismate involves five enzymes encoded in the phz-operon
Structural biology was instrumental in finding the unique activities of PhzF and PhzB
The tricyclic phenazine scaffold forms from two identical but unstable aminoketones
Unrefined handling of reactive intermediates impacts pathway end-product ratios
Acknowledgement
WB thanks his collaborators, coworkers and colleagues for their contributions to the various aspects of phenazine structural biology investigated in his group. The research reviewed here was supported by the Deutsche Forschungsgemeinschaft (BL587/1). Access to synchrotron facilities at BESSYII (Berlin, Germany), DESY (Hamburg, Germany), ESRF (Grenoble, France), NSLS (Brookhaven, USA) and SLS (Villigen, Switzerland) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Fordos MJ. Recherches sur la matière colorante des suppurations bleues: pyocyanine. Rec. Trav. Soc. d’Émul. Sci. Pharm. 1859;3:30. [Google Scholar]
- 2.Fordos MJ. Recherches sur la matière colorante des suppurations blue: pyocyanine. C. R. Hebd. Seances Acad. Sci. 1860;51:215–217. [Google Scholar]
- 3.Gessard C. Thèse pour le Doctorat en Médicine: De la pyocyanine et de son microbe. Colorations qui en dépendant dans les liquides organiques (pus et sérosités, sueur, liquides des culture) Faculté de Médicine de Paris. 1882 [Google Scholar]
- 4.Migula W. Pseudomonas aeruginosa (Schröter) Mig. System der Bakterien. Handbuch der Morphologie, Entwicklungsgeschichte und Systematik der Bakterien. Gustav Fischer. 1900:884–885. [Google Scholar]
- 5.Wrede F, Strack E. Über das Pyocyanin, den blauen Farbstoff des Bacillus pyocyaneus. II. Hoppe Seylers Z. Physiol. Chem. 1924;142:103–119. [Google Scholar]
- 6.Hillemann H. Position of the methyl groups in pyocyanine and attempts to synthesize isopyocyanine. Ber. Dtsch. Chem. Ges. B. 1938;71:46–52. [Google Scholar]
- 7.Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, Grimwood K. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J. Pediatr. 2001;138:699–704. doi: 10.1067/mpd.2001.112897. [DOI] [PubMed] [Google Scholar]
- 8.Courtney JM, Bradley J, Mccaughan J, O’Connor TM, Shortt C, Bredin CP, Bradbury I, Elborn JS. Predictors of mortality in adults with cystic fibrosis. Pediatr. Pulmonol. 2007;42:525–532. doi: 10.1002/ppul.20619. [DOI] [PubMed] [Google Scholar]
- 9.Hauser AR, Jain M, Bar-Meir M, McColley SA. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin. Microbiol. Rev. 2011;24:29–70. doi: 10.1128/CMR.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Haas D, Defago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 12.Pierson LS, 3rd, Pierson EA. Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010;86:1659–1670. doi: 10.1007/s00253-010-2509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rada B, Leto TL. Pyocyanin effects on respiratory epithelium: relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013;21:73–81. doi: 10.1016/j.tim.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price-Whelan A, Dietrich LE, Newman DK. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 2007;189:6372–6381. doi: 10.1128/JB.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau GW, Ran H, Kong F, Hassett DJ, Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 2004;72:4275–4278. doi: 10.1128/IAI.72.7.4275-4278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich LE, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321:1203–1206. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mavrodi DV, Peever TL, Mavrodi OV, Parejko JA, Raaijmakers JM, Lemanceau P, Mazurier S, Heide L, Blankenfeldt W, Weller DM, et al. Diversity and evolution of the phenazine biosynthesis pathway. Appl. Environ. Microbiol. 2010;76:866–879. doi: 10.1128/AEM.02009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mentel M, Ahuja EG, Mavrodi DV, Breinbauer R, Thomashow LS, Blankenfeldt W. Of two make one: the biosynthesis of phenazines. Chembiochem. 2009;10:2295–2304. doi: 10.1002/cbic.200900323. [DOI] [PubMed] [Google Scholar]
- 19.Calhoun DH, Carson M, Jensen RA. The branch point metabolite for pyocyanine biosynthesis in Pseudomonas aeruginosa. J. Gen. Microbiol. 1972;72:581–583. doi: 10.1099/00221287-72-3-581. [DOI] [PubMed] [Google Scholar]
- 20.Longley RP, Halliwell JE, Campbell JJ, Ingledew WM. The branchpoint of pyocyanine biosynthesis. Can. J. Microbiol. 1972;18:1357–1363. doi: 10.1139/m72-210. [DOI] [PubMed] [Google Scholar]
- 21.Millican RC. Biosynthesis of Pyocyanine - Incorporation of [14c]Shikimic Acid. Biochim. Biophys. Acta. 1962;57 407-&. [Google Scholar]
- 22. McDonald M, Mavrodi DV, Thomashow LS, Floss HG. Phenazine biosynthesis in Pseudomonas fluorescens: branchpoint from the primary shikimate biosynthetic pathway and role of phenazine-1,6-dicarboxylic acid. J. Am. Chem. Soc. 2001;123:9459–9460. doi: 10.1021/ja011243+. The first systematic biochemical analysis of the phz-operon. Although the methods employed in this work could not reveal all details of phenazine biosynthesis, this paper laid the basis for subsequent structural studies.
- 23.Knochel T, Ivens A, Hester G, Gonzalez A, Bauerle R, Wilmanns M, Kirschner K, Jansonius JN. The crystal structure of anthranilate synthase from Sulfolobus solfataricus: Functional implications. Proc. Nat. Acad. Sci. U. S. A. 1999;96:9479–9484. doi: 10.1073/pnas.96.17.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morollo AA, Eck MJ. Structure of the cooperative allosteric anthranilate synthase from Salmonella typhimurium. Nat. Struct. Biol. 2001;8:243–247. doi: 10.1038/84988. [DOI] [PubMed] [Google Scholar]
- 25.Spraggon G, Kim C, Nguyen-Huu X, Yee MC, Yanofsky C, Mills SE. The structures of anthranilate synthase of Serratia marcescens crystallized in the presence of (i) its substrates, chorismate and glutamine, and a product, glutamate, and (ii) its end-product inhibitor, L-tryptophan. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6021–6026. doi: 10.1073/pnas.111150298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Q-A, Mavrodi DV, Thomashow LS, Roessle M, Blankenfeldt W. Ligand binding induces an ammonia channel in 2-amino-2-desoxyisochorismate (ADIC) synthase PhzE. J. Biol. Chem. 2011;286:18213–18221. doi: 10.1074/jbc.M110.183418. This first structure of ADIC synthase PhzE revealed an unexpected intertwined dimer and principles of the formation of an intramolecular channel for the diffusion of ammonia.
- 27.McCormick JRD, Reichenthal J, Hirsch U, Sjolander NO. Biosynthesis of the Tetracyclines. III.1 A New Amino Acid from Streptomyces aureofaciens: (+)-trans-2,3-Dihydro-3-hydroxyanthranilic Acid2,3. J. Am. Chem. Soc. 1962;84:3711–3714. [Google Scholar]
- 28. Parsons JF, Calabrese K, Eisenstein E, Ladner JE. Structure and mechanism of Pseudomonas aeruginosa PhzD, an isochorismatase from the phenazine biosynthetic pathway. Biochemistry. 2003;42:5684–5693. doi: 10.1021/bi027385d. The first structure of an isochorismatase. This study illustrated them mechanism of DHAA formation and more generally, enzyme-catalyzed vinyl ether hydrolysis.
- 29.Cirilli M, Zheng R, Scapin G, Blanchard JS. Structural symmetry: the three-dimensional structure of Haemophilus influenzae diaminopimelate epimerase. Biochemistry. 1998;37:16452–16458. doi: 10.1021/bi982138o. [DOI] [PubMed] [Google Scholar]
- 30.Buschiazzo A, Goytia M, Schaeffer F, Degrave W, Shepard W, Grégoire C, Chamond N, Cosson A, Berneman A, Coatnoan N, et al. Crystal structure, catalytic mechanism, and mitogenic properties of Trypanosoma cruzi proline racemase. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1705–1710. doi: 10.1073/pnas.0509010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garvey GS, Rocco CJ, Escalante-Semerena JC, Rayment I. The three-dimensional crystal structure of the PrpF protein of Shewanella oneidensis complexed with trans-aconitate: insights into its biological function. Protein Sci. 2007;16:1274–1284. doi: 10.1110/ps.072801907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velarde M, Macieira S, Hilberg M, Bröker G, Tu S-M, Golding BT, Pierik AJ, Buckel W, Messerschmidt A. Crystal structure and putative mechanism of 3-methylitaconate-delta-isomerase from Eubacterium barkeri. J. Mol. Biol. 2009;391:609–620. doi: 10.1016/j.jmb.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 33. Parsons JF, Song F, Parsons L, Calabrese K, Eisenstein E, Ladner JE. Structure and function of the phenazine biosynthesis protein PhzF from Pseudomonas fluorescens 2–79. Biochemistry. 2004;43:12427–12435. doi: 10.1021/bi049059z. These two independent structure determinations showed that PhzF is related to diaminopimelate epimerase but uses a principally different catalytic mechanism that proceeds via a [1,5]-proton shift. Both studies also showed that PhzF is sufficient to generate PCA from DHHA, hinting at the instability of intermediates occurring downstream of DHHA.
- 34. Blankenfeldt W, Kuzin AP, Skarina T, Korniyenko Y, Tong L, Bayer P, Janning P, Thomashow LS, Mavrodi DV. Structure and function of the phenazine biosynthetic protein PhzF from Pseudomonas fluorescens. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16431–16436. doi: 10.1073/pnas.0407371101. These two independent structure determinations showed that PhzF is related to diaminopimelate epimerase but uses a principally different catalytic mechanism that proceeds via a [1,5]-proton shift. Both studies also showed that PhzF is sufficient to generate PCA from DHHA, hinting at the instability of intermediates occurring downstream of DHHA.
- 35.Hollstein U, McCamey DA. Biosynthesis of phenazines. II. Incorporation of (6–14C)-D-shikimic acid into phenazine-1-carboxylic acid and iodinin. J. Org. Chem. 1973;38:3415–3417. doi: 10.1021/jo00959a041. [DOI] [PubMed] [Google Scholar]
- 36.Herbert RB, Holliman FG, Sheridan JB. Biosynthesis of Microbial Phenazines - Incorporation of Shikimic Acid. Tetrahedron Lett. 1976;17:639–642. [Google Scholar]
- 37.Hollstein U, Mock DL, Sibbitt RR, Roisch U, Lingens F. Incorporation of Shikimic Acid into Iodinin. Tetrahedron Lett. 1978;19:2987–2990. [Google Scholar]
- 38. Ahuja EG, Janning P, Mentel M, Graebsch A, Breinbauer R, Hiller W, Costisella B, Thomashow LS, Mavrodi DV, Blankenfeldt W. PhzA/B catalyzes the formation of the tricycle in phenazine biosynthesis. J. Am. Chem. Soc. 2008;130:17053–17061. doi: 10.1021/ja806325k. This study defined the role of PhzB as the enzyme that generates the tricyclic phenazine scaffold and showed how two identical substrate molecules are condensed in a diagonal-symmetrical fashion, corroborating results of biochemical studies in the 1970s (references [35–37]).
- 39.Kim SW, Cha SS, Cho HS, Kim JS, Ha NC, Cho MJ, Joo S, Kim KK, Choi KY, Oh BH. High-resolution crystal structures of delta5-3-ketosteroid isomerase with and without a reaction intermediate analogue. Biochemistry. 1997;36:14030–14036. doi: 10.1021/bi971546+. [DOI] [PubMed] [Google Scholar]
- 40.Giddens SR, Feng Y, Mahanty HK. Characterization of a novel phenazine antibiotic gene cluster in Erwinia herbicola Eh1087. Mol. Microbiol. 2002;45:769–783. doi: 10.1046/j.1365-2958.2002.03048.x. [DOI] [PubMed] [Google Scholar]
- 41. Parsons JF, Calabrese K, Eisenstein E, Ladner JE. Structure of the phenazine biosynthesis enzyme PhzG. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2110–2113. doi: 10.1107/S0907444904022474. The first crystal structure of PhzG, showing its similarity to pyridoxine-5’-phosphate oxidase PdxH
- 42. Xu N, Ahuja EG, Janning P, Mavrodi DV, Thomashow LS, Blankenfeldt W. Trapped intermediates in crystals of the FMN-dependent oxidase PhzG provide insight into the final steps of phenazine biosynthesis. Acta Crystallogr. D Biol. Crystallogr. 2013;69:1403–1413. doi: 10.1107/S0907444913008354. Crystal structures of PhzG in complex with unstable tricyclic phenazine biosynthesis intermediates reveal that PhzG is a promiscuous terminal oxidase and that its specificity is not responsible for the difference between PDC- and PCA-producing bacteria. This study also suggests that dihydro-PDC and -PCA, and not their fully aromatized counterparts, are the final products of “core” phenazine biosynthesis.
- 43. Rui Z, Ye M, Wang S, Fujikawa K, Akerele B, Aung M, Floss HG, Zhang W, Yu T-W. Insights into a divergent phenazine biosynthetic pathway governed by a plasmid-born esmeraldin gene cluster. Chem. Biol. 2012;19:1116–1125. doi: 10.1016/j.chembiol.2012.07.025. This biochemical study indicates that the difference between PDC- and PCA-producing microorganisms is routed in the relative activities of Phz-enzymes and not in their specificities.
- 44.Saleh O, Gust B, Boll B, Fiedler H-P, Heide L. Aromatic prenylation in phenazine biosynthesis: dihydrophenazine-1-carboxylate dimethylallyltransferase from Streptomyces anulatus. J. Biol. Chem. 2009;284:14439–14447. doi: 10.1074/jbc.M901312200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parsons JF, Greenhagen BT, Shi K, Calabrese K, Robinson H, Ladner JE. Structural and functional analysis of the pyocyanin biosynthetic protein PhzM from Pseudomonas aeruginosa. Biochemistry. 2007;46:1821–1828. doi: 10.1021/bi6024403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenhagen BT, Shi K, Robinson H, Gamage S, Bera AK, Ladner JE, Parsons JF. Crystal structure of the pyocyanin biosynthetic protein PhzS. Biochemistry. 2008;47:5281–5289. doi: 10.1021/bi702480t. [DOI] [PubMed] [Google Scholar]
- 47.Bera AK, Atanasova V, Gamage S, Robinson H, Parsons JF. Structure of the D-alanylgriseoluteic acid biosynthetic protein EhpF, an atypical member of the ANL superfamily of adenylating enzymes. Acta Crystallogr. D Biol. Crystallogr. 2010;66:664–672. doi: 10.1107/S0907444910008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zocher G, Saleh O, Heim JB, Herbst DA, Heide L, Stehle T. Structure-based engineering increased the catalytic turnover rate of a novel phenazine prenyltransferase. PLoS ONE. 2012;7:e48427. doi: 10.1371/journal.pone.0048427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu S, Vit A, Devenish S, Mahanty HK, Itzen A, Goody RS, Blankenfeldt W. Atomic resolution structure of EhpR: phenazine resistance in Enterobacter agglomerans Eh1087 follows principles of bleomycin / mitomycin C resistance in other bacteria. BMC Struct. Biol. 2011;11:33. doi: 10.1186/1472-6807-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasozi DM, Gromer S, Adler H, Zocher K, Rahlfs S, Wittlin S, Fritz-Wolf K, Schirmer RH, Becker K. The bacterial redox signaller pyocyanin as an antiplasmodial agent: comparisons with its thioanalog methylene blue. Redox Rep. 2011;16:154–165. doi: 10.1179/174329211X13049558293678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abken HJ, Tietze M, Brodersen J, Baumer S, Beifuss U, Deppenmeier U. Isolation and characterization of methanophenazine and function of phenazines in membrane-bound electron transport of Methanosarcina mazei Gol. J. Bacteriol. 1998;180:2027–2032. doi: 10.1128/jb.180.8.2027-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deppenmeier U, Johann A, Hartsch T, Merkl R, Schmitz RA, Martinez-Arias R, Henne A, Wiezer A, Baumer S, Jacobi C, et al. The genome of Methanosarcina mazei: Evidence for lateral gene transfer between bacteria and archaea. J. Mol. Microbiol. Biotechnol. 2002;4:453–461. [PubMed] [Google Scholar]
- 53.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schrödinger L. The PyMOL Molecular Graphics System. 2010 [Google Scholar]