Abstract
Objectives:
Obesity and other metabolic variables are associated with abnormal brain structural volumes and cognitive dysfunction in HIV-uninfected populations. Since individuals with HIV infection on combined antiretroviral therapy (CART) often have systemic metabolic abnormalities and changes in brain morphology and function, we examined associations among brain volumes and metabolic factors in the multi-site CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) cohort.
Design:
Cross-sectional study of 222 HIV-infected individuals.
Methods:
Metabolic variables included body mass index (BMI), total blood cholesterol (C), low- and high-density lipoprotein C (LDL-C and HDL-C), blood pressure, random blood glucose, and diabetes. MRI measured volumes of cerebral white matter, abnormal white matter, cortical and subcortical gray matter, and ventricular and sulcal CSF. Multiple linear regression models allowed us to examine metabolic variables separately and in combination to predict each regional volume.
Results:
Greater body mass index (BMI) was associated with smaller cortical gray and larger white matter volumes. Higher total cholesterol (C) levels were associated with smaller cortex volumes; higher LDL-C was associated with larger cerebral white matter volumes, while higher HDL-C levels were associated with larger sulci. Higher blood glucose levels and diabetes were associated with more abnormal white matter.
Conclusions:
Multiple atherogenic metabolic factors contribute to regional brain volumes in HIV-infected, CART-treated patients, reflecting associations similar to those found in HIV-uninfected individuals. These risk factors may accelerate cerebral atherosclerosis and consequent brain alterations and cognitive dysfunction.
Keywords: HIV, metabolic, neuroimaging, obesity, cholesterol
Introduction
Improved survival and consequent aging of the HIV-infected (HIV+) population in the era of combination antiretroviral therapy (CART) has revealed increased risk of cerebral atherosclerosis (Falutz, 2011). Moreover, alterations in body fat distribution, atherogenic changes in blood lipid levels and diabetes more commonly develop in the setting of CART. The metabolic syndrome of central obesity, hypertension, and atherogenic lipid and glucose abnormalities appears to cause alterations in brain structure and impaired cognition in normal aging populations (Pannacciulli et al. 2006; Walther et al. 2010). The combined effects of HIV infection and metabolic syndrome, or its components, may be associated with greater injury, contributing to the increased prevalence of persistent cognitive impairment in the CART-treated patients (Heaton et al. 2010).
Although wasting is common in advanced untreated HIV+ individuals, HIV+ individuals on CART have weight distributions similar to the general population. For example, rates of overweight (37%) and obese (9%) HIV+ US naval personnel are similar to those of HIV-uninfected personnel (Crum-Cianflone et al. 2010). In HIV+ individuals, higher body mass index (BMI) and waist circumference has been associated with greater cognitive impairment (McCutchan et al. 2012), and, in older HIV+ individuals, higher BMI and obesity is associated with frailty (Shah et al. 2012), supporting the potential negative impact of obesity and other metabolic factors on the brain.
Studies of various HIV-uninfected populations have linked metabolic indices, including BMI, waist circumference, glucose metabolism and blood lipid levels, to altered brain structure. While findings vary, several studies found that obesity or higher BMI was associated with smaller gray and larger white matter volumes (Brundel et al. 2010; Haltia et al. 2007; Kurth et al. 2012; Pannacciulli et al. 2006; Pannacciulli et al. 2007; Walther et al. 2010), as well as with impaired cognitive performance (Cavalieri et al. 2010; Reijmer et al. 2010; Walther et al. 2010; Ward et al. 2010; Wolf et al. 2004). Less gray matter has been associated with higher fasting plasma leptin levels in obese individuals, which may reflect an abnormal processing of leptin that negatively impacts neuronal integrity, either directly or indirectly through neuropeptide or other signaling molecules (Pannacciulli et al. 2007). Larger white matter volumes may reflect increased myelin with obesity, which may reverse with dieting (Haltia et al. 2007), while elevated blood glucose and a diagnosis of diabetes are often associated more white matter abnormalities (Reijmer et al. 2011).
In the present study, we specifically examined the associations among metabolic factors and morphometric brain volumes in HIV+ individuals, most of whom had received CART and are at greater risk for metabolic disease, to determine whether similar brain associations exist. Based on the existing literature of HIV-uninfected individuals, we predicted smaller gray and larger white matter volumes to be associated with unhealthy levels of metabolic factors, particularly BMI, and correlations between white matter abnormalities and higher glucose levels.
Methods
Study Population
Assessments of metabolic variables and high-resolution, multi-channel MRI were acquired from 261 individuals in the CHARTER study. CHARTER is an observational cohort study examining the effects of CART on the nervous system. To focus more directly on the effects of HIV infection, we excluded cases with gross morphological abnormalities not consistent with HIV-related pathologies (e.g., cysts or masses) (n=12), and cases with severely confounding comorbid conditions (n=27) (as described in Jernigan et al. 2011). The present cross-sectional study included 222 participants with complete MRI and metabolic data.
Participants were studied at five participating CHARTER sites: University of California San Diego (n=59), Mt. Sinai School of Medicine (n=52), University of Texas Medical Branch, Galveston (n=48), Johns Hopkins University (n=35) and University of Washington, Seattle (n=28). Demographics and detailed, standardized neuromedical assessments were obtained by trained, centrally certified study personnel (Heaton et al. 2010).
Standard Protocol Approvals, Registrations, and Patient Consents
These procedures were approved by the Human Subjects Protection Committees of each institution. Written informed consent was obtained from all study participants.
Clinical and Laboratory Measurements
Medical history including CART medications and lowest (nadir) blood CD4 count, diagnosis of diabetes mellitus and use of medications for diabetes were gathered through patients’ self-report using a structured questionnaire administered by clinicians. Blood pressure was measured in the sitting position by automated sphygmomanometers. Measures of height and weight at the visit closest to their baseline neuroimaging were used to calculate BMI. Non-fasting blood samples for glucose, triglycerides, and total (TC), high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL-C) cholesterol were collected, processed, and assayed by standard methods. There were no individuals with abnormally high glucose levels that had not already self-reported a diabetes diagnosis. Likewise, CD4 lymphocyte counts were measured by flow cytometry and plasma HIV RNA concentrations were measured by ultra-sensitive (lower limit of detection <50 copies/ml) polymerase chain reaction assay (Amplicor, Roche Diagnostic Systems, Indianapolis, IN) in the CLIA-certified clinical laboratories of each institution.
Structural MRI Protocol
All imaging was done on GE 1.5 Tesla scanners (8 scanners across 5 CHARTER sites) and included four series for the morphometric analysis. Series 1 and 2 were coronal acquisitions with section thickness=2.0 mm, FOV 24 cm, matrix size 256×256: 2D T2-weighted fast spin echo (FSE) sequence with TR=5700ms, TE=90ms, ETL=16; and 2D proton density (PD) weighted FSE sequence with TR=3700ms, TE=17ms, ETL=4. Series 3 and 4 were sagittal acquisitions with section thickness=1.3mm, FOV 24 cm, matrix size 256×256×124: 3D T1-weighted SPGR sequence with TR=20ms, TE=6ms, flip angle=30; and 3D PD-weighted SPGR sequence with TR=20ms, TE=6ms, flip angle=5.
Standard morphometric analyses employed in CHARTER are described in detail elsewhere (Fennema-Notestine et al. 2013; Jernigan et al. 2011) and include image inspection, bias correction, co-registration of MRI volumes; reslicing in a standard space; skull-stripping; tissue segmentation; and anatomical segmentation (Figure 1). To identify abnormal, edematous areas in the white matter (e.g., hyperintense regions on T2), trained neuroanatomists performed a semi-automated tissue sampling procedure to obtain the signal characteristics of gray matter, white matter, and CSF. The tissue-segmented images were further processed manually to separate the cerebellum from the cerebrum, the ventricles from sulcal fluid in the subarachnoid space, and cortical from subcortical gray matter within the cerebrum (Figure 1). Abnormal white matter regions were defined as voxels within white matter that had signal values that fell in (or beyond) the distribution estimated from the gray matter sample (Figure 1 orange). In addition, a supratentorial cranial volume was computed from the sum of the cerebral gray, white and CSF compartments (excluding cerebellum). Total cerebral white matter includes both abnormal and normal-appearing white matter.
Fig. 1. Multi-channel Morphometry.
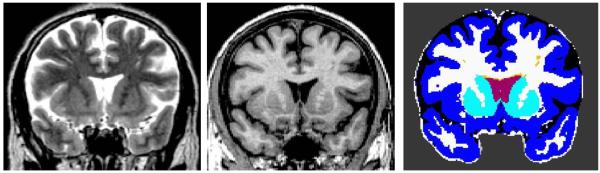
Representative coronal section of the T2 (left) and T1 (middle) images and the corresponding segmented and labeled image (right). Regions include: ventricular (red) and subarachnoid (black) CSF; total (white) and abnormal (orange) white matter; cortical (blue) and subcortical (turquoise) gray matter.
Statistical Analyses
All data reported in this study are from participants’ first CHARTER visit that captured technically adequate brain MRI images. The metabolic data is from the closest neuromedical visit that was within 30 days of imaging (average 11 days). The multivariable analyses were designed to assess the association of metabolic factors and quantitative volumetric measures of brain structure. All volumetric and metabolic variables were log transformed and CD4 measures were square-root transformed to symmetrize the distributions and stabilize the variance. Six control covariates were included in all regression analyses to account for variance in the MRI measures that were not of primary interest to this study. These included scanner, age, gender, ethnicity, supratentorial cranial volume (to control for individual differences in head size), and nadir CD4. Nadir CD4 is a biomarker of persisting effects on brain structure and function (i.e., cognitive impairment) that are a legacy of advanced immunosuppression. (Jernigan et al. 2011).
In our initial multivariate analyses, each model predicted one of the six MRI volumetric measures (e.g., total white matter volume) with the six control variables and one metabolic variable in the model. This "single metabolic variable" model examined the associations between the volumetric measures and each of these metabolic variables: BMI, total blood cholesterol (C), low- and high-density lipoprotein C (LDL-C and HDL-C), blood pressure (diastolic and systolic), random blood glucose level, and diagnosis of diabetes (categorical yes/no variable).
In our second multivariate analysis (“multiple metabolic variable model”), we examined the potential independent contributions of metabolic variables on brain volumes when all metabolic measures were included. Based on the initial analyses, five metabolic variables (BMI, LDL-C, HDL-C, blood glucose and diabetes) and the control variables described above were entered together to predict each brain volume to examine their joint impact on each brain region. Blood pressure was not included in this multiple metabolic model since it was not significantly associated with any brain volumes in the single metabolic model, and, total cholesterol was not included either, since we included its two major components, LDL-C and HDL-C.
Finally, we performed three post-hoc models to examine the potential impact of current HIV-related health status on associations between metabolic factors and brain volumes. Using the same multiple metabolic variable model described above, each of these additional variables were added one at a time: a) whether participants were on or off CART; b) whether or not HIV viral RNA was suppressed in plasma; and c) current CD4 cell count.
Results
Study population
Demographics, HIV treatment history and metabolic characteristics are summarized in Table 1. Participants were mostly male (80%), currently on CART (78%), either African American (47%) or Caucasian (41%), and had mean age of 44 years. The cohort had experienced substantial immune recovery attributable to CART, demonstrated by the difference between a median nadir CD4 count of 150 cells/mm3 and the current median CD4 count values of 462 cells/mm3. Average BMI was 25.9 (about half were overweight (BMI>25) or obese (BMI>30)), mean metabolic variables were in the normal range, and about one third (35%) of the participants were cognitively impaired as defined by HAND criteria (Antinori et al. 2007; Heaton et al. 2010; Heaton et al. 2011).
Table 1.
Participant Characteristics.
| n | Range | ||
|---|---|---|---|
| Age in yearsa | 44.2 (8.0) | 222 | 23-67 |
| Education a | 13.0 ( 2.4) | 222 | 7-18 |
| WRAT Reading a | 95.7 (13.5) | 222 | 55-118 |
| Gender - Male | 80.2% | 178 | |
| Ethnicity African American Caucasian Hispanic Other |
46.8% 41.0% 9.5% 2.7% |
104 91 21 6 |
|
| Neurocognitively Impaired c | 35.1% | 78 | |
| Median CD4 Nadir (per mm3) b | 158 [34,300] | 222 | 0-1500 |
| Median Current CD4 (per mm3) b | 462 [291,622] | 222 | 7-1447 |
| CART History Current Past Never |
77.5% 12.1% 10.4% |
172 27 23 |
|
| Detectable Plasma Viral Load | 49.0% | 109 | |
| BMIa (kg/m2) | 25.9 (4.6) | 222 | 17.3-48.4 |
| Total Cholesterola | 179.5 (39.8) | 222 | 92-311 |
| C-LDLa (mM) | 99.9 (35.4) | 222 | 29-198 |
| C-HDLa (mM) | 48.6 (20.6) | 222 | 17-149 |
| Systolic BPa (mm Hg) | 125.3 (15.2) | 222 | 91-187 |
| Diastolic BPa (mm Hg) | 76.4 (10.8) | 222 | 46-110 |
| Blood glucosea (mM) | 95.8 (24.1) | 222 | 57-233 |
| Diabetes | 7.2% | 16 |
Mean (standard deviation) or percentage (%) of the cohort are reported for each variable.
= Mean (std. dev.);
= Median [Inter-quartile range];
Neurocognitive impairment status as defined in Heaton et al. 2010.
Abbreviations: WRAT=Wide range achievement test, BMI=body mass index, C-LDL= low-density lipoprotein, C-HDL= high-density lipoprotein, BP=blood pressure.
Metabolic correlates of brain morphometry
On examining each metabolic variable separately to predict brain volumes (Table 2, Single metabolic variable (S) models), greater BMI was associated with smaller cortical gray (p<.0001; Figure 2a) and larger total cerebral white matter volumes (p<.0001; Figure 2b). Higher total cholesterol levels also were associated with smaller cortical gray matter volumes (p=.024). Higher LDL-C was associated with larger cerebral white matter volumes (p=.012), while higher HDL-C levels were associated with more sulcal CSF. Neither cholesterol component was significantly associated with cortical gray matter volume directly. The observed higher amounts of sulcal CSF between the cortical gyri may result from loss of cortical gray matter (post-hoc Pearson’s correlation, p=. 016). Higher glucose levels (p=.007) and diagnosis of diabetes (p=.021) were associated with larger volumes of abnormal white matter (Table 2; Figure 2c). Diabetes also was associated with larger ventricular size (p=.016). Systolic and diastolic blood pressures were not significantly associated with any brain volume measures.
Table 2.
Associations (t valuesa) between metabolic factors and brain volumes within single (S) and multiple (M) metabolic variable models.
| Abnormal White |
Total White |
Cortical Gray |
Subcortical Gray |
Ventricular Fluid |
Sulcal Fluid |
|
|---|---|---|---|---|---|---|
| BMI (S) | −0.58 | 4.23**** | −4.09**** | −0.53 | −1.43 | −0.64 |
| BMI (M) | −0.90 | 3.76*** | −4.16**** | −0.43 | −1.55 | −0.08 |
| Cholesterol (S) | 0.10 | 1.29 | −2.27* | −1.81 | 1.20 | 0.33 |
| LDL-C (S) | −0.15 | 2.53* | −1.33 | −1.05 | −0.17 | −1.67+ |
| LDL-C (M) | −0.09 | 1.67 | −0.44 | −0.96 | 0.17 | −1.59 |
| HDL-C (S) | 0.09 | −1.71 | −1.25 | −1.79 | 1.80 | 2.42* |
| HDL-C (M) | −0.21 | −1.50 | −1.68 | −1.95 | 2.01* | 2.41* |
| Glucose (S) | 2.71** | −0.27 | −0.02 | −0.57 | 1.33 | 0.71 |
| Glucose (M) | 1.91 | 0.06 | −0.01 | −0.12 | 0.25 | 0.51 |
| Diabetes (S) | 2.32* | 1.10 | 0.66 | 0.63 | 2.43* | 0.58 |
| Diabetes (M) | 1.39 | 1.72 | 0.87 | 0.71 | 2.51* | 0.54 |
Parameter estimates shown are t values from the multivariable regression models, and statistically significant associations are indicated in bold with p values linked to the number of asterisks
=p<.05,
=p<.01,
=p<.001
All models included control variables of scanner, age, gender, ethnicity, CD4 nadir, and supratentorial cranial vault. Multiple metabolic variable models (M) also included BMI, HDL-C, LDL-C, Glucose, Diabetes. Abbreviations: BMI=body mass index, C-LDL= low-density lipoprotein, C-HDL= high-density lipoprotein.
Fig. 2. Association between metabolic factors and structural volumes.
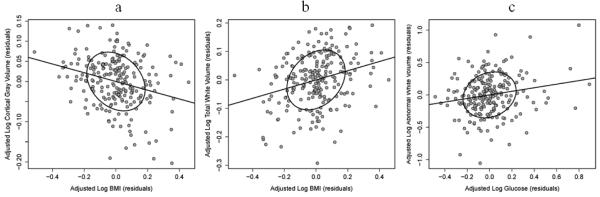
Partial correlation scatterplots (plots of partial residuals) show the relationship between metabolic variables and log-transformed volumes, after adjusting for all other control variables in the single metabolic variable regression models. a) BMI and cortical gray matter; b) BMI and total white matter; and c) glucose levels and abnormal white matter.
The multiple metabolic variable model (M) included five metabolic factors (Table 2 gray (M) rows). Most of the metabolic correlates of brain morphometry identified by the single metabolic models remained significant in the multiple model, supporting independent associations. Smaller cortical gray (p<.0001) and larger total white matter (p=.0002) volumes remained highly correlated with higher BMI. Glucose levels and diabetes diagnosis, however, were no longer significantly associated with higher abnormal white matter volume, although the same underlying patterns remained. Higher ventricular CSF volume remained associated with diabetes (p=.013), and higher sulcal (p=.046) and ventricular CSF (p=.017) correlated with higher HDL-C.
In the post-hoc models that examined the potential moderating effects of current HIV-related health status on associations between metabolic factors and brain volumes, the patterns of reported associations remained the same. Current CART status, plasma HIV RNA viral suppression, and CD4 cell count did not contribute significantly to any models.
Discussion
This study examined the relationships between metabolic factors and regional brain morphometric volumes in HIV+ patients attending five US academic HIV clinics. All analyses were adjusted for demographic and MRI-related technical variables and a measure of maximum HIV-related immunosuppression (CD4 nadir), a biomarker of brain damage caused by maximal HIV-mediated immunosuppression (Jernigan et al. 2011). We found that elevated BMI, total cholesterol, LDL-C, HDL-C, blood glucose and diagnosis of diabetes were associated with altered brain volumes in multivariable models; elevated BMI, HDL-C, and diagnosis of diabetes demonstrated independent contributions in the multiple metabolic models.
Higher BMI was associated with larger total white and smaller cortical gray volumes, in agreement with a number of prior studies in HIV-uninfected individuals. In HIV-uninfected individuals, obesity has been similarly associated with smaller cortical gray matter volume and density and larger white matter volumes (Debette et al. 2010; Haltia et al. 2007; Kurth et al. 2012; Pannacciulli et al. 2006; Walther et al. 2010). In a study of 95 women (aged 52-92), higher BMI predicted smaller volumes in a number of cortical gray matter regions, including frontal cortices, alongside larger volumes of white matter in frontal, temporal and parietal lobes (Walther et al. 2010). In addition, obese volunteers have shown less gray matter density in frontal cortex and putamen compared to lean controls (Pannacciulli et al. 2006), and smaller gray matter volumes correlated with higher BMI or waist circumference in 115 healthy adults (Kurth et al. 2012). In a study comparing obese to lean participants, obesity was associated with greater white matter in the temporal lobe, brain stem and cerebellum, although not with differences in gray matter (Haltia et al. 2007). Of clinical relevance, the initially larger white matter volumes were reduced after six weeks of dieting in the obese group, supporting the potential reversal of the effects of obesity (Haltia et al. 2007). Thus, in HIV+ patients, most of whom are on CART, the consequences of being overweight or obese appear to produce brain structural abnormalities similar to those seen in HIV-uninfected individuals.
Higher levels of high-density lipoprotein cholesterol (HDL-C) were associated with more sulcal fluid; ventricular fluid volumes were similarly associated, although only significant in the multiple metabolic model. Since higher levels of HDL-C are associated with decreased risk of atherosclerotic cardiovascular disease, atherosclerosis does not appear to account for the inferred loss of brain tissue that would lead to compensatory expansion of cerebrospinal fluid volume. In contrast, higher levels of low-density lipoprotein cholesterol (LDL-C) were associated with larger total white matter volume in our single metabolic model. This latter observation is consistent with studies of HIV-uninfected individuals (Bokura et al. 2008; Choi et al. 2009; Segura et al. 2009). However, a recent study of 183 HIV-uninfected cognitively-normal individuals reported that higher levels of HDL-C were associated with larger temporal gray matter volume and no significant associations with LDL-C (Ward et al. 2010). A recent study suggests that CART affects cholesterol efflux (Piconi et al. 2013), thus, relationships of cholesterol fractions with regional brain morphology may be more complex.
Hyperglycemia and diabetes were associated with larger volumes of abnormal white matter (e.g., hyperintensities on T2), despite the relatively small number of individuals with these conditions (n=16), suggesting that the mechanism for these changes may be associated with ischemia from cerebral atherosclerosis. In HIV-uninfected individuals, elevated blood glucose and a diagnosis of diabetes are associated with both greater brain atrophy over 4 years and more white matter abnormalities similar to those we found in this cross-sectional study (Reijmer et al. 2011). Lower cortical gray matter volumes have also been reported in patients with type 2 diabetes (Brundel et al. 2010) and in older persons with hyperglycemia (Leritz et al. 2011). Although our findings do not support an effect of glucose dysregulation on gray matter volume in HIV+ individuals, our power to detect these effects may have been limited by the cross-sectional design and limited number of participants with these conditions. Furthermore the use of non-fasting glucose levels may have limited our ability to detect accurate levels of insulin resistance or unreported diabetes.
Most of our findings are congruent with those in HIV-uninfected individuals and persisted in our analyses when controlling for HIV-related factors, thus, these effects may not be HIV-related. Nonetheless, several HIV-related factors are known to influence brain volumes. In our analysis of the clinical correlates of brain volumes in the larger CHARTER cohort (Jernigan et al. 2011), longer exposure to CART was associated with lower white matter and higher sulcal CSF volumes. Higher current CD4 counts and detectable plasma HIV RNA levels were associated with white matter abnormalities and increased variability in subcortical gray matter volumes cross-sectionally and over time (Jernigan et al. 2011; Fennema-Notestine et al. 2013). Since we did not find that the plasma viral suppression, status of CART, or current CD4 levels further modified the reported metabolic-brain volume relationships, our findings may represent the sum of both HIV-mediated and HIV-independent processes. The functional implications of structural and metabolic abnormalities that we have identified in this cohort have been presented elsewhere (Jernigan et al. 2011; McCutchan et al. 2012; Fennema-Notestine et al. 2013).
The mechanisms by which metabolic factors interact with other HIV- and age-related neurodegenerative conditions to injure the brain are unclear. Several components of the metabolic syndrome (e.g., obesity and higher LDL-C) are associated with loss of gray matter and diabetes may contribute to increases in abnormal white matter (Brundel et al. 2010; Choi et al. 2009). Both observations are consistent with brain damage mediated by cerebrovascular atherosclerosis. While atherosclerosis or other cerebral vasculopathies (e.g. arteriolar sclerosis or amyloid angiopathy) may contribute to these findings, expanded sulcal CSF spaces in those with higher levels of HDL-C, which protect against atherosclerosis, appear to be inconsistent with an atherosclerotic process. Thus, metabolic factors may mediate brain damage by a variety of tissue-specific mechanisms.
White matter volumes may increase with obesity because of expanded myelin content that is reversible with dieting (Haltia et al. 2007). An additional mechanism, inflammation from the immune recovery inflammatory syndrome (IRIS), may be relevant in this HIV-infected cohort. IRIS occurs when CART improves immune function leading to inflammation, usually in response to opportunistic infections, but potentially to HIV in the brain as well. This inflammation may contribute to the larger volumes of total or abnormal white matter in our participants most of whom were taking CART, as suggested by increasing abnormal white matter in HIV+ individuals during immune recovery (Fennema-Notestine et al. 2013). Thus, CART could accelerate the onset and increase the severity of brain injury in HIV infection by either promoting atherosclerosis, inducing IRIS, or through direct drug toxicity (Ciccarelli et al. 2011).
Although our findings are similar to studies of HIV-uninfected cohorts, this study is limited by the absence of controls that enable direct comparisons to an HIV-uninfected group. Furthermore, these cross-sectional observations cannot address time-dependent alterations in brain structure that may be related to changes in factors such as CART regimens, obesity, or treatments for metabolic conditions (cholesterol, diabetes, etc.). Longitudinal studies within HIV+ participants and comparisons of HIV+ cohorts to matched uninfected controls could elucidate the possible roles of these mechanisms.
As HIV-infected individuals age we expect that metabolic factors will affect their brains similarly to HIV-uninfected persons and that similar clinical interventions are indicated. A recent longitudinal study of brain structure found that 51 HIV+ patients had accelerated loss of frontal, parietal and temporal cortical volumes with age compared to 61 HIV-controls (Pfefferbaum et. al (2014). Longitudinal study of aging HIV+ patients undergoing treatment for various components of the metabolic syndrome is needed to further elucidate how their responses resemble or differ from those of HIV-uninfected persons.
These findings are especially interesting in light of our recent findings that BMI and more specifically central obesity (ie, waist circumference) are associated with neurocognitive impairment in another substudy of this cohort (McCutchan et al. 2012). In addition, greater BMI and central obesity may be associated with frailty in older HIV individuals (Shah et al. 2012). The brain abnormalities associated with BMI, that is, smaller cortical gray and larger white matter volumes, could reflect pathological processes that contribute to cognitive impairment and frailty. For example, lower gray matter volume could indicate cortical atrophy and higher white matter volume could result from inflammation. Understanding the mechanisms and causal pathways that underlie the relationships of HIV clinical and metabolic factors to brain damage could suggest interventions to prevent or reverse HIV-associated brain damage. Interventions to reduce the effects of metabolic abnormalities in HIV+ individuals such as treatment with statin drugs have been studied and are widely practiced (Grunfeld 2010). Our findings that components of the metabolic syndrome appear to affect brain structure emphasize the need for their systematic monitoring and treatment in ART-treated patients. Additional longitudinal and interventional studies incorporating biomarkers (e.g., of inflammation) and/or imaging (e.g., carotid intima-media thickness, MRI angiography, or MRI diffusion imaging) are needed to assess if onset of morphometric changes and consequent cognitive impairment are accelerated and to identify the vascular and other mechanisms of the underlying brain damage.
Acknowledgements
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study is supported by awards N01 MH22005, HHSN271201000027C, and HHSN271201000030C from the National Institutes of Health. The present study also was supported by National Institutes of Health grants P30 MH62512 (HIV Neurobehavioral Research Center, HNRC) and R01 MH79752.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government. Some aspects of this study were presented at the Fourth International Meeting on HIV Infection and the Central Nervous System: Treating the Brain in the HAART Era. Frascati (Rome), Italy, July 2011.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Iijima K, Nagai A, Oguro H. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke. 2008;39:1607–9. doi: 10.1161/STROKEAHA.107.508630. [DOI] [PubMed] [Google Scholar]
- Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ. Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci. 2010;299:126–30. doi: 10.1016/j.jns.2010.08.048. [DOI] [PubMed] [Google Scholar]
- Cavalieri M, Ropele S, Petrovic K, Pluta-Fuerst A, Homayoon N, Enzinger C, Grazer A, Katschnig P, Schwingenschuh P, Berghold A, Schmidt R. Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care. 2010;33:2489–95. doi: 10.2337/dc10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Cho YM, Kang JH, Shin CS, Park KS, Lee HK. Cerebral white matter hyperintensity is mainly associated with hypertension among the components of metabolic syndrome in Koreans. Clin Endocrinol (Oxf) 2009;71:184–8. doi: 10.1111/j.1365-2265.2008.03444.x. [DOI] [PubMed] [Google Scholar]
- Ciccarelli N, Fabbiani M, Di Giambenedetto S, Fanti I, Baldonero E, Bracciale L, Tamburrini E, Cauda R, De Luca A, Silveri MC. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76:1403–9. doi: 10.1212/WNL.0b013e31821670fb. [DOI] [PubMed] [Google Scholar]
- Crum-Cianflone N, Roediger MP, Eberly L, Headd M, Marconi V, Ganesan A, Weintrob A, Barthel RV, Fraser S, Agan BK. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5:e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Beiser A, Hoffmann U, Decarli C, O'Donnell CJ, Massaro JM, Au R, Himali JJ, Wolf PA, Fox CS, Seshadri S. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68:136–44. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Ellis RJ, Archibald SL, Jernigan TL, Letendre SL, Notestine RJ, Taylor MJ, Theilmann RJ, Julaton MD, Croteau DJ, Wolfson T, Heaton RK, Gamst AC, Franklin DR, Jr., Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I. Increases in brain white matter abnormalities and subcortical gray matter are linked to CD4 recovery in HIV infection. J Neurovirol. 2013;19:393–401. doi: 10.1007/s13365-013-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C. Dyslipidemia and its Treatment in HIV Infection. Top HIV Med. 2010;18:112–8. [PMC free article] [PubMed] [Google Scholar]
- Haltia LT, Viljanen A, Parkkola R, Kemppainen N, Rinne JO, Nuutila P, Kaasinen V. Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab. 2007;92:3278–84. doi: 10.1210/jc.2006-2495. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR, Jr., Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Levitt JG, Phillips OR, Luders E, Woods RP, Mazziotta JC, Toga AW, Narr KL. Relationships between gray matter, body mass index, and waist circumference in healthy adults. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Fischl B, McGlinchey RE, Milberg WP. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54:2659–71. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, Wolfson T, Rosario D, Alexander TJ, Marra C, Ances BM, Grant I. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78:485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–25. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Pannacciulli N, Le DS, Chen K, Reiman EM, Krakoff J. Relationships between plasma leptin concentrations and human brain structure: a voxel-based morphometric study. Neurosci Lett. 2007;412:248–53. doi: 10.1016/j.neulet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Zahr NM, Sullivan EV. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging. 2013;35:1755–68. doi: 10.1016/j.neurobiolaging.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconi S, Parisotto S, Rizzardini G, Passerini S, Meraviglia P, Schiavini M, Niero F, Biasin M, Bonfanti P, Ricci ED, Trabattoni D, Clerici M. Atherosclerosis is associated with multiple pathogenic mechanisms in HIV-infected antiretroviral-naive or treated individuals. AIDS. 2013;27:381–9. doi: 10.1097/QAD.0b013e32835abcc9. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, de Bresser J, Kessels RP, Kappelle LJ, Algra A, Biessels GJ. Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab Res Rev. 2011;27:195–202. doi: 10.1002/dmrr.1163. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26:507–19. doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- Segura B, Jurado MA, Freixenet N, Falcon C, Junque C, Arboix A. Microstructural white matter changes in metabolic syndrome: a diffusion tensor imaging study. Neurology. 2009;73:438–44. doi: 10.1212/WNL.0b013e3181b163cd. [DOI] [PubMed] [Google Scholar]
- Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A New Frailty Syndrome: Central Obesity and Frailty in Older Adults with the Human Immunodeficiency Virus. J Am Geriatr Soc. 2012 doi: 10.1111/j.1532-5415.2011.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–64. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MA, Bendlin BB, McLaren DG, Hess TM, Gallagher CL, Kastman EK, Rowley HA, Asthana S, Carlsson CM, Sager MA, Johnson SC. Low HDL Cholesterol is Associated with Lower Gray Matter Volume in Cognitively Healthy Adults. Front Aging Neurosci. 2010:2. doi: 10.3389/fnagi.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H, Hensel A, Arendt T, Kivipelto M, Winblad B, Gertz HJ. Serum lipids and hippocampal volume: the link to Alzheimer's disease? Ann Neurol. 2004;56:745–8. doi: 10.1002/ana.20289. [DOI] [PubMed] [Google Scholar]


