Abstract
Osteoporosis is a disease of excess skeletal fragility that results from estrogen loss and aging. Age related bone loss has been attributed to both elevated bone resorption and insufficient bone formation. We developed a hybrid compound, LLP2A-Ale in which LLP2A has high affinity for the α4β1 integrin on mesenchymal stem cells (MSCs) and alendronate that has high affinity for bone. When LLP2A-Ale was injected into mice, the compound directed MSCs to both trabecular and cortical bone surfaces and increased bone mass and bone strength. Additional studies are underway to further characterize this hybrid compound, LLP2A-Ale, and how it can be utilized for the treatment of bone loss resulting from hormone deficiency, aging, inflammation and to augment bone fracture healing.
Osteoporosis is a syndrome of excessive skeletal fragility that results from a combination of a reduction in bone mass and bone strength. The two most significant determinants of osteoporosis are estrogen deficiency and aging. Estrogen loss leads to a reduction in trabecular bone mass and an irreversible alteration of the trabecular bone structure. The decline of trabecular bone structure secondary to estrogen deficiency is suppressed by treatment with anti-resorptive agents (estrogen, bisphosphonates, calcitonin and selective estrogen receptor modulators [1, 2]. These agents are hypothesized to work by reducing the activation of new bone multicellular units (BMUs) while still allowing normal bone formation to continue in already activated BMUs. This results in a more complete secondary mineralization of basic structural units due to reduced turnover and an increased degree of bone mineralization (DMB). These agents have been associated with preservation of trabecular microarchitecture. However, an important limitation of this class of drugs is that they do not restore the lost bone structure. There is currently an anabolic agent, rhPTH, (1–34) that can stimulate new bone formation on existing trabeculae, increase trabecular bone mass, and reduce the risk of incidental vertebral fractures [3]. However the requirement of daily injections of rhPTH (1–34) for two years and no data on hip fracture risk reduction has limited the use of this medication in clinical practice.
Age related bone loss has been attributed to an increase in osteoclast driven bone resorption, with an insufficient increase in osteoblast number to drive bone formation. Over time this can then lead to an uncoupling of bone turnover and bone loss. However, a more detailed review of the bone microenvironment in preclinical and clinical studies of aging has provided additional insights. Aging is associated with a reduction in the number of mesenchymal stem cells (MSCs) that can differentiate into osteoblasts. This leads to a reduction in osteogenesis and bone formation [4–6]. However, it is not clear if age related reduction in bone formation results from a reduction in MSCs in the bone marrow due to cell death, if the MSCs are directed to differentiate into the adipocytes, or if the MSCs are unable to migrate to the bone surface due to changes in the bone microenvironment. A number of these factors may be present in the aging bone marrow that results in reduced bone formation.
Over the past few years, the idea that increasing the ability of MSCs to differentiate into osteoblasts in aged or estrogen deficient animal models to increase osteogenesis and facilitate new bone formation has been investigated. In the majority of the experiments, MSCs from a number of sources including whole bone marrow, fat, MSC enriched peripheral blood, or purified and cultured MSCs have been injected intravenously (IV) into the peripheral circulation in both animal and a few human studies and have generally failed to engraft within the bone marrow. Also, more than 90% of the intravenously transplanted MSCs became trapped in the lung microvasculature and while a small number of MSCs did engraft in the bone marrow, the residence time within the marrow was limited [7–9]. Also intravenous administration of MSCs in vivo have failed to promote an osteogenic response in bone due to the inability of MSCs to home to the bone surface unless they were genetically modified [10–14] or following bone trauma [8, 15] or fracture [8, 16]. The successful application of MSCs to bone has been limited to the repair of injuries in which the MSCs are presented by local subcutaneous implantation, intramedullary injection or combined with scaffolds within the bone. [17–20].
However, administration of systemic MSCs in in vivo models do not find MSCs migrating to the bone surface or forming new bone. Generally the infused MSCs are found in the upper metaphysis, epiphysis, bone marrow sinusoids or Haversian systems and are usually removed from the bone marrow within a few weeks [7–9, 21]. One solution to this problem of insufficient numbers of MSCs in the bone marrow of older individuals that can differentiate into osteoblasts, would be to inject MSCs into the systemic circulation and allow the MSCs to move to the bone surface. However, the movement of MSCs from the bone marrow to the bone surface is complex. MSCs undergo osteogenic differentiation in the bone marrow and mobilization of the osteoblast progenitors to the bone surface is a crucial step for osteoblast maturation and the formation of mineralized tissue .[22–24]Bone cells at all maturation stages are dependent on cell-matrix and cell-cell interactions [25–28]. Once the osteoblast progenitors are “directed” to the bone surface, they synthesize a range of proteins including osteocalcin, osteopontin, bone sialoprotein, osteonectin, collagen-I and fibronectin that further enhance the adhesion and maturation of osteoblasts [29–31]. These interactions are largely mediated by transmembrane integrin receptors that primarily utilize an arginine-glycine-aspartate (RGD) sequence to identify and bind to specific ligands. MSCs express integrins α1, 2, 3, 4, 6, 11, CD51 (integrin αV), and CD29 (integrins β1) [32]. Integrins α1β1, α2β1, αvβ1, αvβ5, α5β1and α4β1 are expressed in the osteoblastic cells [26, 30, 31, 33]. Integrin α5 is required for MSC osteogenic differentiation [34] and overexpression of α4 Integrin on MSCs has been reported to increase homing of the MSCs to bone [25]. These studies suggest that a therapeutic strategy for bone regeneration could be directed toward the integrins on the surface of the MSCs and could bring the MSCs to the bone surface. In addition to their initial development of cell therapies for tissue regeneration and wound healing, MSC paracrine and functions have been increasingly recognized as important factors that contribute to their efficacy. [35–37].
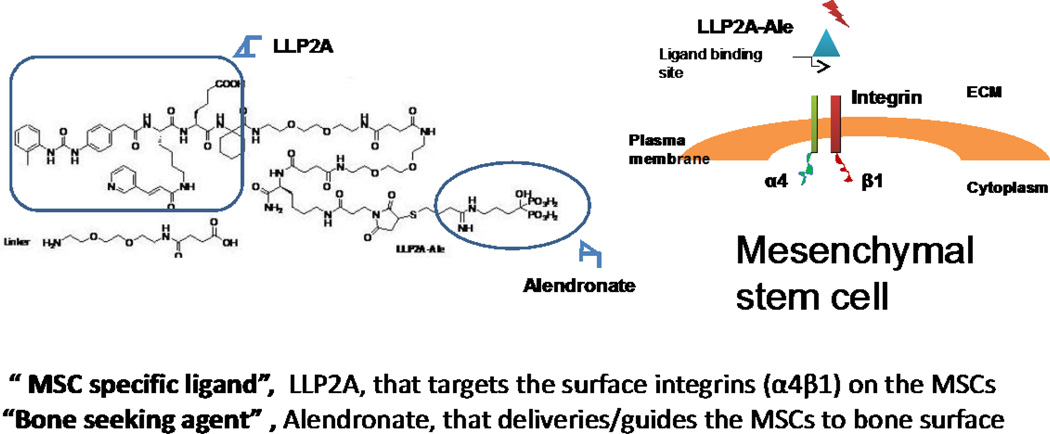
Our research team wanted to try to improve the engraftment of MSCs in the bone marrow to form new bone. To accomplish the goal of delivering the MSCs to the bone surface, we collaborated with chemists and developed a compound, LLP2A-Ale that binds to both MSCs and to bone. The scientists screened a combinatorial library for peptidomimetric ligands that were able to bind to the α4β1 integrin on the MSC surface, and LLP2A was identified as a potential ligand to bind to the integrin. Next, the chemists worked on conjugating LLP2A to a bisphosphonate so that the LLP2A would carry the bound MSCs to the bone surface. A number of bisphosphonates were attempted to bind to LLP2A, and alendronate (Ale) was the only one that could bind to LLP2A through a chemical linker and then link LLP2A with alendronate, LLP2A- Ale (Figure 1).
Figure 1.
LLP2A-Ale is a hybrid compound. It is composed of the bisphosphonate, alendronate, that has high affinity for the bone tissue. The alendronate is bound to a chemical linker that also is attached to LLP2A, a synthetic protein that has high affinity for alpha 4, beta 1 integrin that is on the surface of Mesenchymal stem cells and hematopoetic stem cells.
General in vitro and in vivo effects
Our research group performed a number of studies in young mice with a wide dose range of LLP2A-Ale and assessed weight, kidney function, liver function and calcium metabolism, and determined they were not affected by the treatment. Also, no extraskeletal calcifications were observed in the mice treated with LLP2A-Ale.
It was critical to determine if the synthetic peptide against α4β1 integrin, LLP2A, had affinity for MSCs that were undergoing osteoblast differentiation. We determined that the α4β1 integrin was highly expressed in the osteoprogenitor cells and had a high affinity for LLP2A[38]. In vitro studies with MSCs showed that LLP2A-Ale increased both the number of MSCs that differentiated into osteoblasts as well as the migration of the MSCs to hydroxyappetite crystals [39]. The effects of LLP2A-Ale on MSC migration appeared to be mainly chemotactic as increased chemokine levels were observed, including monocyte chemotactic protein-1 and the macrophage-inflammatory protein-1 α. Also, as MSCs can differentiate into either osteoblasts, chondrocytes or adipocytes, it was observed that treatment of MSCs with LLP2A-Ale did not increase either the chondrogenic or adipogenic phenotypes of the MSCs in the culture media.
Next, to determine if the LLP2A-Ale could direct transplanted MSCs to the bone surface two in vivo proof-of-concept studies were performed. NOD/SCID/mucopolysaccharidosis type VII (NOD/SCID/MPSVII) immune deficient mice were treated with human bone marrow (huMSCs) or with LLP2A-Ale. This mouse strain lacks the β-glucuronidase (GUSB) enzyme, which facilitates human cell detection by a simple enzymatic substrate reaction as described [40, 41]. The donor cells were detected using biochemical detection of β-glucuronidase [9, 42]. Twenty-four hours after the injections, LLP2A-Ale increased the number of huMSCs on the bone surface as compared to all the other control groups (PBS, LLP2A-Ale or huMSCs). Three weeks after a single injection of human MSCs and LLP2A-Ale, the transplanted huMSC cells were observed adjacent to the bone surface. Moreover, the transplanted huMSCs were embedded within the bone matrix in the MSC+LLP2A-Ale treated group, suggesting that the transplanted huMSCs differentiated into osteoblasts [38]. Together, these data demonstrate that LLP2A-Ale could direct the transplanted MSCs to bone surface, and lead to osteoblast differentiation in this xenotransplantation model.
Studies of LLP2A-Ale in young immunocompetent mice, estrogen deficient and aged mice
To determine whether LLP2A-Ale could augment endogenous bone formation in immunocompetent mice without MSC transplantation, two-month-old female 129/SvJ mice received two doses of LLP2A-Ale, LLP2A or Ale treatments four weeks apart. Two days after the intravenous injections, the cell populations expressing runt related transcription factor 2 (Runx2) and bromodeoxyuridine (Brdu) (a thymidine analog that is used in the detection of cell proliferation) were primarily located at the bone surface in the mice treated with LLP2A-Ale[38].
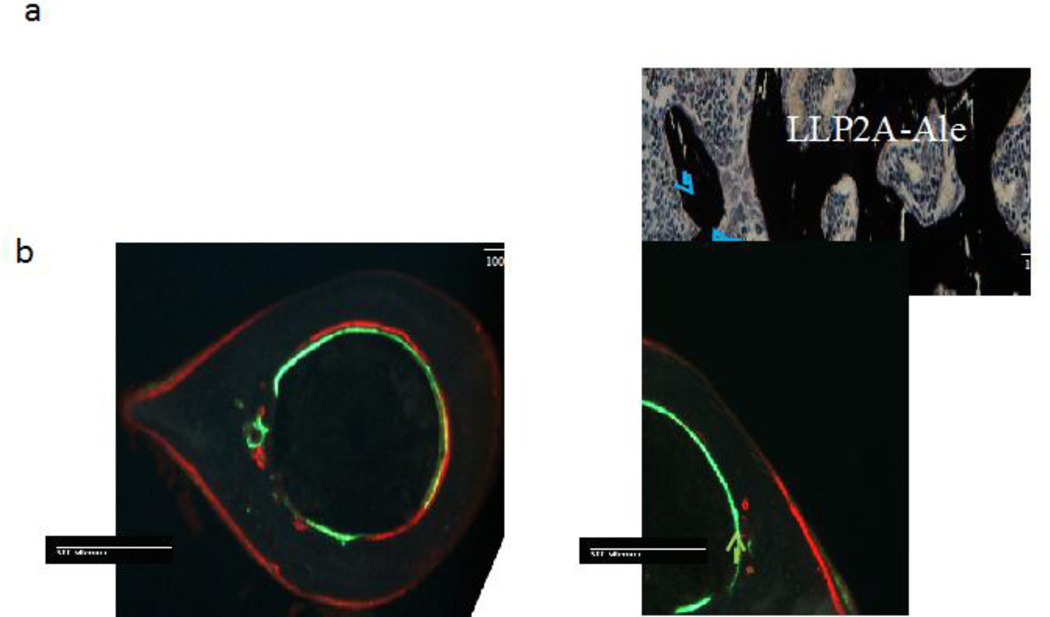
Treatment with one intravenous injection of LLP2A-Ale in young mice, 8–16 weeks of age, resulted in a higher distal femoral trabecular bone volume (measured as trabecular bone volume (BV)/tissue volume (TV)) and trabecular thickness compared to placebo-treated mice. Also, vertebral bone strength, measured by the maximum load and strength of the fifth lumbar vertebral body were significantly higher in mice treated with LLP2A-Ale compared to mice treated with PBS eight weeks after the treatment [38]. Mice treated with LLP2A-Ale had an increased number of osteoblasts on the trabecular bone surface and osteoblast bridges were observed between adjacent trabeculae (Figure 2a). LLP2A-Ale also increased periosteal bone formation at the cortical bone surface in the young growing mice (Figure 2b).
Figure 2.
a. Representative sections of the trabecular bone of young adult mice treated with either Placebo (PBS) or LLP2A- Ale, with three monthly injections. LLP2A treatments significantly increased the number of osteoblasts on the trabecular bone surface and new bone bridges were formed between the trabeculae (blue arrows). Figure 2B is a coronal bone section of the proximal tibial metaphyses from an osterix mCherry red reporter mouse. Treatment with LLP2A- Ale increased osteoblast number and activity on the periosteal surface (yellow arrow) and this was not seen in the PBS treated mice. Figure 3C is a cross-section of the mid- tibial shaft. Treatment with LLP2A- Ale resulted in new bone formation on the periosteal surface (green arrow) and this was not observed in the PBS treated mice.
To determine if LLP2A- Ale could prevent age related bone loss, C57BL/6 mice received one injection of LLP2A-Ale and age-related trabecular bone loss was not observed for 8 weeks after the injection. Also, histomorphometric parameters of bone formation including, at both the distal femur and lumbar vertebrae were much higher than the placebo treated mice. Again osteoblast bridges were observed at both of these trabecular bone sites in LLP2A-Ale–treated mice.
To determine whether LLP2A-Ale could prevent bone loss in a model that is relevant to the clinical disease osteoporosis, LLP2A-Ale, LLP2A, parathyroid hormone fragments PTH (1–34), or placebo was studied in mice that had been ovariectomized and allowed to develop osteopenia. In this study, the group treated with LLP2A-Ale had higher values for osteoblast surface and mineralizing surface, as well a higher bone-formation rate per total bone surface at the fifth lumbar vertebral body compared to the groups treated with PBS, Ale or LLP2A [39]. However, the novel finding in this experiment was that treatment with either LLP2A-Ale or PTH resulted in bone formation on the endocortical surface. While, none of the treatments changed either cortical bone thickness or cortical bone strength, it is possible that a longer study duration and additional treatments may, over time, reveal changes in these parameters.
Effects of LLP2A-Ale +/− MSC on aged mice
Since aging is associated with the reduced number of the MSCs in bone marrow and reduced bone formation [43, 44], we evaluated if LLP2A-Ale and transplanted MSCs, LLP2A-Ale alone, MSCs alone or PTH treatment could augment bone formation in the skeleton of aged (24-month-old) female C57BL/6 mice. We observed MSCs within the bone marrow only in the animals that were treated with MSCs alone.
In contrast, the animals were treated with both MSCs + LLP2A-Ale had transplanted MSCs both on the t trabecular bone surface and within bone matrix as osteocytes. Animals treated with either LLP2A-Ale or PTH alone had a non-significant increase in surface-based bone formation parameters. However, the LLP2A-Ale and MSC combinational treatment significantly increased surface based bone formation and vertebral and cortical bone strength in these extremely aged mice [39].
In Summary
MSCs are precursors of osteoblasts. However, MSCs do not readily migrate to the bone, and this creates a major obstacle for the use of MSCs for bone regeneration. Our research group developed a ligand, LLP2A, which targets integrin α4β1, a protein that is highly expressed by MSCs undergoing osteoblast differentiation and attached it to a bisphosphonate (Ale) to guide the MSCs to the bone surface. We found that treatment of both young and old mice and estrogen deficient mice with LLP2A-Ale alone increased trabecular and cortical bone mass and strength compared to controls with an effect that was similar to hPTH (1–34). Interestingly, treatment of aged or estrogen deficient mice with the combination of LLP2A-Ale with MSCs resulted in significant gains in both trabecular and cortical bone mass and strength. These preliminary studies will need to be confirmed with longer duration studies and additional toxicity studies to determine if this novel treatment to bring MSCs to bone surface to augment bone formation will have potential therapeutic value.
Acknowledgments
The compound LLP2A-Ale is patented, 76916-787324/206400US This work was supported in part by NIH grants R21AR057515 (WY), R01AR061366 (WY) R01AR43052(NEL), R01AR04884(NEL), and the California Institute of Regenerative Medicine DR2A-05302 (NEL and WY). Endowment for Aging Research at U. C. Davis(NEL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cummings SR, Cosman F, Eastell R, Reid IR, Mehta M, Lewiecki EM. Goal-directed treatment of osteoporosis. J Bone Miner Res. 2013;28:433–438. doi: 10.1002/jbmr.1854. [DOI] [PubMed] [Google Scholar]
- 2.Diab DL, Watts NB. Postmenopausal osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2013;20:501–509. doi: 10.1097/01.med.0000436194.10599.94. [DOI] [PubMed] [Google Scholar]
- 3.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 4.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mechanisms of Ageing and Development. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Katsara O, Mahaira LG, Iliopoulou EG, Moustaki A, Antsaklis A, Loutradis D, Stefanidis K, Baxevanis CN, Papamichail M, Perez SA. Effects of Donor Age, Gender, and In Vitro Cellular Aging on the Phenotypic, Functional, and Molecular Characteristics of Mouse Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2011 doi: 10.1089/scd.2010.0280. [DOI] [PubMed] [Google Scholar]
- 6.Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci U S A. 2003;100:5840–5845. doi: 10.1073/pnas.1036475100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho SW, Sun HJ, Yang JY, Jung JY, An JH, Cho HY, Choi HJ, Kim SW, Kim SY, Kim D, Shin CS. Transplantation of mesenchymal stem cells overexpressing RANK-Fc or CXCR4 prevents bone loss in ovariectomized mice. Mol Ther. 2009;17:1979–1987. doi: 10.1038/mt.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granero-Molto F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP, Spagnoli A. Regenerative Effects of Transplanted Mesenchymal Stem Cells in Fracture Healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyerrose TE, De Ugarte DA, Hofling AA, Herrbrich PE, Cordonnier TD, Shultz LD, Eagon JC, Wirthlin L, Sands MS, Hedrick MA, Nolta JA. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25:220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutwald R, Haberstroh J, Kuschnierz J, Kister C, Lysek DA, Maglione M, Xavier SP, Oshima T, Schmelzeisen R, Sauerbier S. Mesenchymal stem cells and inorganic bovine bone mineral in sinus augmentation: comparison with augmentation by autologous bone in adult sheep. Br J Oral Maxillofac Surg. 2010;48:285–290. doi: 10.1016/j.bjoms.2009.06.226. [DOI] [PubMed] [Google Scholar]
- 11.Vertenten G, Lippens E, Girones J, Gorski T, Declercq H, Saunders J, Van den Broeck W, Chiers K, Duchateau L, Schacht E, Cornelissen M, Gasthuys F, Vlaminck L. Evaluation of an injectable, photopolymerizable, and three-dimensional scaffold based on methacrylate-endcapped poly(D,L-lactide-co-epsilon-caprolactone) combined with autologous mesenchymal stem cells in a goat tibial unicortical defect model. Tissue Eng Part A. 2009;15:1501–1511. doi: 10.1089/ten.tea.2008.0367. [DOI] [PubMed] [Google Scholar]
- 12.Longobardi L, Granero-Molto F, O'Rear L, Myers TJ, Li T, Kregor PJ, Spagnoli A. Subcellular localization of IRS-1 in IGF-I-mediated chondrogenic proliferation, differentiation and hypertrophy of bone marrow mesenchymal stem cells. Growth Factors. 2009;27:309–320. doi: 10.1080/08977190903138874. [DOI] [PubMed] [Google Scholar]
- 13.Halleux C, Sottile V, Gasser JA, Seuwen K. Multi-lineage potential of human mesenchymal stem cells following clonal expansion. J Musculoskelet Neuronal Interact. 2001;2:71–76. [PubMed] [Google Scholar]
- 14.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 15.Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E, Trompier F, Dudoignon N, Germain C, Mazurier C, Aigueperse J, Borneman J, Gorin NC, Gourmelon P, Thierry D. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5:1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 16.Dreger T, Watson JT, Akers W, Molligan J, Achilefu S, Schon LC, Zhang Z. Intravenous application of CD271-selected mesenchymal stem cells during fracture healing. J Orthop Trauma. 2014;28(Suppl 1):S15–S19. doi: 10.1097/BOT.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, Lieberman JR. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda R, Matsumoto T, Niikura T, Kawakami Y, Fukui T, Lee SY, Mifune Y, Kawamata S, Fukushima M, Asahara T, Kawamoto A, Kurosaka M. Local transplantation of granulocyte colony stimulating factor-mobilized CD34+ cells for patients with femoral and tibial nonunion: pilot clinical trial. Stem Cells Transl Med. 2014;3:128–134. doi: 10.5966/sctm.2013-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Liao X, Luo E, Chen W, Bao C, Xu HH. Mesenchymal stem cells systemically injected into femoral marrow of dogs home to mandibular defects to enhance new bone formation. Tissue Eng Part A. 2014;20:883–892. doi: 10.1089/ten.tea.2012.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahney CS, Hu DP, Taylor AJ, Ferro F, Britz HM, Hallgrimsson B, Johnstone B, Miclau T, Marcucio RS. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res. 2014;29:1269–1282. doi: 10.1002/jbmr.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jürg A, Gasser LCC, Lynne K, Kamibayashi Intravenously Administered Mesenchymal OVX-Induced Bone Loss in Distribution of Labelled MSCs. J Bone Miner Res. 1999;14:1. [Google Scholar]
- 22.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 24.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118:491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994;9:487–496. doi: 10.1002/jbmr.5650090408. [DOI] [PubMed] [Google Scholar]
- 27.Vukicevic S, Luyten FP, Kleinman HK, Reddi AH. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990;63:437–445. doi: 10.1016/0092-8674(90)90176-f. [DOI] [PubMed] [Google Scholar]
- 28.Mbalaviele G, Shin CS, Civitelli R. Cell-cell adhesion and signaling through cadherins: connecting bone cells in their microenvironment. J Bone Miner Res. 2006;21:1821–1827. doi: 10.1359/jbmr.060811. [DOI] [PubMed] [Google Scholar]
- 29.Gronthos S, Akintoye SO, Wang CY, Shi S. Bone marrow stromal stem cells for tissue engineering. Periodontol 2000. 2006;41:188–195. doi: 10.1111/j.1600-0757.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- 30.Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28:174–181. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- 31.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res. 1997;12:1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- 32.Brooke G, Tong H, Levesque JP, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008 doi: 10.1089/scd.2007.0156. [DOI] [PubMed] [Google Scholar]
- 33.Cowles EA, Brailey LL, Gronowicz GA. Integrin-mediated signaling regulates AP-1 transcription factors and proliferation in osteoblasts. J Biomed Mater Res. 2000;52:725–737. doi: 10.1002/1097-4636(20001215)52:4<725::aid-jbm18>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 34.Hamidouche Z, Fromigue O, Ringe J, Haupl T, Vaudin P, Pages JC, Srouji S, Livne E, Marie PJ. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci U S A. 2009;106:18587–18591. doi: 10.1073/pnas.0812334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolbe M, Xiang Z, Dohle E, Tonak M, Kirkpatrick CJ, Fuchs S. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng Part A. 2011;17:2199–2212. doi: 10.1089/ten.TEA.2010.0474. [DOI] [PubMed] [Google Scholar]
- 38.Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, Ritchie RO, Lane NE. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18:456–462. doi: 10.1038/nm.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao W, Guan M, Jia J, Dai W, Lay YA, Amugongo S, Liu R, Olivos D, Saunders M, Lam K, Nolta J, Olvera D, Ritchie RO, Lane NE. Reversing Bone Loss by Directing Mesenchymal Stem Cells to the Bone. Stem Cells. 2013 doi: 10.1002/stem.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hess DA, Craft TP, Wirthlin L, Hohm S, Zhou P, Eades WC, Creer MH, Sands MS, Nolta JA. Widespread nonhematopoietic tissue distribution by transplanted human progenitor cells with high aldehyde dehydrogenase activity. Stem Cells. 2008;26:611–620. doi: 10.1634/stemcells.2007-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyerrose T, De Ugarte D, Hofling A, Herrbrich PE, Cordonnier TD, Shultz LD, Eagon JC, Wirthlin L, Sands MS, Hedrick MA, Nolta JA. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25:220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyerrose TE, Roberts M, Ohlemiller KK, Vogler CA, Wirthlin L, Nolta JA, Sands MS. Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells. 2008;26:1713–1722. doi: 10.1634/stemcells.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 2007;3:226–237. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]