Abstract
Alzheimer's disease (AD) is the most common age-related neurodegenerative disorder. Amyloid-β peptide (Aβ) deposition in the brain is one of its hallmarks and the measure of plasma Aβ is considered to be a biomarker for anti-amyloid drug efficacy in animal models of AD. However, age-associated plasmatic Aβ modulation in animal models is practically never addressed in the literature. Mouse lemur primates are used as a model of normal and AD-like cerebral aging. Here, we studied the effect of age on plasmatic Aβ in 58 mouse lemurs aged from 1 to 10 years. A subset of animals presented high plasmatic Aβ and the proportion of animals with high plasmatic Aβ was higher in aged animals as compared to young ones. Histological evaluation of the brain of some of these animals was carried out to assess extracellular and intracellular amyloid load. In aged lemurs, plasmatic Aβ was negatively correlated with the density of neurons accumulating deposits of Aβ.
Keywords: Amyloid, Alzheimer, Intracellular amyloid, Cerebral aging, Lemur, Microcebus murinus, Plasma
1. INTRODUCTION
Alzheimer's disease (AD) is the most common age-related neurodegenerative disorder. It is characterized by two main microscopic lesions: senile plaques and neurofibrillary tangles. Senile plaques are mainly constituted of aggregated extracellular deposition of amyloid-β (Aβ) peptides. These latter come from the proteolytic processing of the transmembrane protein APP (amyloid-β precursor protein) into mainly two types of Aβ peptides: Aβ40 and Aβ42. Neurofibrillary tangles are constituted of intraneuronal accumulation of abnormally phosphorylated tau proteins. The amyloid cascade hypothesis is currently the dominant explanation of AD aetiology. It suggests that a chronic imbalance between production and clearance of Aβ peptides results in intracerebral accumulation of Aβ. This leads to a cascade of events that cause AD (Hardy and Selkoe, 2002; Sperling et al., 2011). In addition to senile plaques, Aβ is also present as soluble toxic oligomeric forms (Lacor et al., 2004; Selkoe, 2008) as well as intracellular Aβ deposits (Gouras et al., 2000; Gyure et al., 2001). The latter form occurs before plaque formation (Gyure et al., 2001; Wirths et al., 2001) and is also toxic for the brain (Wirths et al., 2004).
In humans, biomarkers of Aβ are used to facilitate AD diagnosis (Blennow et al., 2010; Jack et al., 2009) and evaluate the efficacy of anti-AD therapies (Relkin et al., 2009). The most widely used biochemical marker of Aβ is the measurement of Aβ42 level in cerebrospinal fluid (CSF). Indeed, the concentration of Aβ42 in CSF is lower in AD patients than in healthy controls (Andreasen et al., 1999). The decrease in CSF Aβ42 in AD has been attributed, at least in part, to deposition of Aβ in plaques in the brain (Motter et al., 1995; Strozyk et al., 2003). Although measures of Aβ42 in the CSF are widely used in the clinic, it is interesting to outline that Aβ40 is the main Aβ component in the brain and in peripheric fluids and the concentration of Aβ40 in the CSF is assumed to reflect the total amount of Aβ proteins in the brain (Wiltfang et al. 2007). Also, recent studies suggested that including measurement of CSF Aβ40 levels in a decision tree can help to better discriminate patients with AD in ambiguous clinical diagnosis cases (Slaets et al., 2013; Sauvee et al., 2014).
As plasma sampling is simpler and less invasive than a lumbar puncture, several authors have investigated the ability to use plasmatic Aβ as an alternative to measurements in CSF. The published data on plasma Aβ from single cross-sectional studies in humans are however still conflicting: plasma Aβ of AD patients have been reported to be higher, lower, or unchanged as compared to controls (Buerger et al., 2009; Roher et al., 2009; Sobow et al., 2005). However, a meta-analysis recently suggested higher baseline plasma Aβ40 and Aβ42 in cognitively normal subjects who converted to AD (Song et al., 2011). Then when AD develops, plasmatic Aβ42 declines (Song et al., 2011). Also, direct evaluation of relationships between plasma Aβ loads and intracerebral amyloid load measures by imaging methods have revealed a positive relationship between plasma Aβ40 levels and cerebral amyloid load and a negative relationship between Aβ42/Aβ40 ratio and cerebral amyloid load (Devanand et al., 2011). In addition to AD, other factors such as aging have been reported to increase baseline plasma Aβ in humans (Lopez et al., 2008). In addition to its potential use for AD diagnosis, one possible application of measures of plasmatic Aβ is the evaluation of target engagements for therapies modulating Aβ synthesis. For example, treatments with γ-secretase inhibitors are shown to reduce the level of Aβ40 in the plasma (Fleisher et al., 2008).
Animal models are widely used to discover fundamental mechanisms associated to AD pathology and to evaluate new drugs against AD. Transgenic mouse models of amyloidosis expressing mutant forms of human APP and presenilin 1 (PS1) (Duyckaerts et al., 2008) and spontaneous models of brain aging such as primates (Picq et al., 2012) are the most widely used models to study various aspects of AD pathology and therapy. As in humans, Aβ modulation in animal models can be evaluated in the CSF (Das et al., 2011; Liu and Duff, 2008). In small animals, Aβ can be measured more easily in the plasma. These measurements are used to evaluate the effects of various biological factors such as APOE genotype on Aβ clearance (Sharman et al., 2010) or to assess therapeutic effects of potential drugs. For example, studies in transgenic mouse models of AD have shown that treatments with γ- or β-secretase inhibitors reduce plasmatic Aβ42 and Aβ40 (Chang et al., 2004; Kounnas et al., 2010; Lanz et al., 2010). Modulation of plasmatic Aβ can also be used to follow-up the effects of anti-Aβ immunotherapies in mice (Lemere, 2009; Wang et al., 2011; Yamada et al., 2009). Studies of therapeutic interventions in primates also revealed changes in plasmatic Aβ. For example, increased plasmatic Aβ is detected following active immunotherapy in primates such as the mouse lemur (Trouche et al., 2009; Joseph-Mathurin et al., 2013) and the caribbean vervet (Lemere et al., 2004).
Few studies in animal models have focused on the modulation of plasmatic Aβ during aging. Studies in the Tg2576 transgenic mice, a model that develops Aβ plaques at 12 months, suggested constant plasma Aβ until 12 months of age, after what, an age-associated decrease in plasma Aβ was detected in coincidence with the marked cerebral deposition of Aβ (Kawarabayashi et al., 2001). Another study in dogs showed higher plasmatic Aβ in young animals as compared to old cognitively unimpaired animals and higher Aβ in old animals with mild cognitive impairment as compared to either cognitively unimpaired or severely affected dogs (Gonzalez-Martinez et al., 2011). To the best of our knowledge, age-associated modulation of plasmatic Aβ has never been addressed in non-human primates. The aim of the current study was thus to evaluate the effect of age on plasmatic Aβ in the mouse lemur primate (Microcebus murinus). This small primate (70–150g) has a short lifespan of approximately 12 years. A subcategory of aged lemurs can develop both extracellular (Bons et al., 1991) and intracellular (Joseph-Mathurin et al., 2013; Mestre-Frances et al., 2000) Aβ deposits and Tau pathologies (Kraska et al., 2011). Our results suggest increased plasmatic Aβ levels in a subpopulation of aged lemurs. We also show that a high plasmatic Aβ in aged animals is associated to a low level of intraneuronal material detected by the 4G8 monoclonal antibody and that corresponds to Aβ accumulation.
2. MATERIALS AND METHODS
2.1 Animals
Fifty eight mouse lemurs were involved in the current study (n=25 young animals (1–5.5 years) and n=33 aged animals (5.5–10 years)). They were all born in a laboratory breeding colony (Brunoy, France, Agreement n°E91-114-1). All experiments were carried out in accordance with European Communities Council directive (86/609/EEC) under the authorization number 91–326 from the “Direction Départementale des Services Vétérinaires de l’Essonne” and the Internal Review Board of the URA CEA CNRS 2210.
2.2 Blood collection, pre-treatment and plasmatic Aβ detection
Blood was collected from the saphenous vein. The vein was pricked with a needle at a 45° angle and blood was collected in small heparini sed capillaries (60µl). Every blood sampling was done at the same time in the morning. All blood samples were kept on ice and centrifuged (2000g; 10 min) at 4°C in the 15 min following the collection. The plasma layer was then collected in 200µl polypropylene tubes. A cocktail of proteases inhibitors (Complete Mini; Roche, Meylan, France) was added to each plasma sample at a final concentration of 1X. The samples were then allowed to freeze in a −80°C freezer and were then kept at −80°C until their analysis.
Plasmatic level of Aβ40 was assessed with enzyme-linked immunosorbent assay (ELISA) kits “Human β amyloid 1–40” (Invitrogen, Saint Aubin, France). The ELISA were carried out following the manufacturer’s protocol and executed using non-diluted plasma samples.
2.3 Brain tissue processing and immunohistochemical staining
The brains of seven aged mouse lemurs with known concentration of plasmatic Aβ were removed and fixed in 4% formalin after the natural death of animals. Brains were plunged in a 15% sucrose solution for 24 hours and then in a 30% sucrose solution for cryoprotection. They were then frozen and sliced into 40-µm-thick coronal sections on a freezing microtome. Slices were then stored at −20°C in a storage solution (gl ycerol 30%, ethylene glycol 30% and phosphate buffer 0.1M).
Brains sections from all studied animals were first immunostained using the 4G8 antibody directed against the residues 17–24 of Aβ. The 4G8 antibody is routinely used to detect amyloid deposits (Alafuzoff et al., 2008). Sections were pretreated with hydrogen peroxide (0.3%), then incubated in a phosphate buffered (0.1M) saline (0.9%) and triton (0.2%) solution (PBS+Tx) with normal goat serum (NGS;4.5%). Sections were then incubated for two days into PBS+Tx solution with NGS (3%) and the primary antibody mouse monoclonal 4G8 antibody; 1/500; Covance Signet Antibodies, Debham, MA, USA). Sections were incubated for one hour into a PBS+Tx solution with NGS (3%) and the secondary antibody (biotinylated goat anti-mouse antibody; 1/1000; Vector Laboratories, Burlingame, CA, USA). The signal was amplified using an avidin-biotin complex for one hour (Vectastain, Vector Laboratories). The final reaction used diaminobenzidine (DAB; Vector Laboratories) for two minutes as the chromogen (brown stains). Counterstaining with cresyl violet was used to distinguish cells nuclei. Negative controls were performed by omitting the primary antibody in the procedure. Positive controls were performed using formalin-fixed brain sections of APP/PS1dE9 mice (Garcia-Alloza et al., 2006). All sections were mounted on slides and viewed using a standard microscope (Zeiss Axioplan, Germany).
As 4G8 cross-reacts with APP (Aho et al., 2010), we performed control experiments to assess the specificity of 4G8 immunostaining. We carried out double immunofluorescent staining (n= 5 animals) with a cocktail of primary antibodies: 4G8 (1/5000) and A8717, a polyclonal antibody directed against the amino acids 676–695 of the C-terminus part of the APP (1/500, SigmaAldrich, St Louis, MO, USA). Primary antibodies were incubated overnight at room temperature. Detection-visualization of primary antibodies was performed using a cocktail of secondary antibodies: Goat anti-Mouse Fluor Hylite 555 (1/200, Jackson Immunoresearch, West Grove, PA, USA) to detect 4G8 and donkey anti-Rabbit antibody conjugated to an Alexa Fluor 488 (1/200; Invitrogen) to detect A8717. Sections were counterstained with 4',6-diamidino-2-phenylindole (DAPI) and treated for 30 sec in a saturated Sudan Black solution to decrease a-specific autofluorescence (Delatour et al., 2001).
2.4 Morphological analysis
Intracellular 4G8 positive deposits, revealed by immunoperoxidase stainings, were detected mainly in the parietal cortex, hippocampus, and in the caudate nucleus (see results). The optical fractionator method was used to quantify the deposit-positive cells in these regions (West et al., 1991). Aggregate-positive cells were counted using a Zeiss Axioplan microscope equipped with a digital color camera, x–y motorized stage controller and Mercator (ExploraNova) stereology software. The regions of interest were delineated using a 1.5× objective, in accordance with a mouse lemur brain atlas (Bons et al., 1998). Sampling was performed bilaterally within the delineated areas with a 20× objective. The analysis was done on slices spaced out of 800 µm. Analysis was performed from squares sampling the structure (14.8% of the surface). The density of cells presenting intracellular aggregates (number of cells per mm2 of surface sampled) was calculated.
For the purpose of control experiments colocalization of 4G8-positive intracellular objects and A8717-positive objects were assessed in the caudate nucleus. The analysis was performed on 5 animals with ImageJ and the JACOP plugin (Bolte and Cordelieres, 2006) We studied the cytofluorograms and calculated a mean Pearson’s and overlap coefficient for all neurons exhibiting both 4G8 and/or A8717 signals.
2.5 Statistical analysis
Spearman's test was used to evaluate the correlation between plasmatic Aβ and age or cerebral 4G8 positive objects. Chi-square test was used to compare the proportion of animals with low and high plasma Aβ in young and aged animals. Student's t test was used to perform group comparisons. Statistical analysis was made using PRISM 5 software (Graphpad, La Jolla, CA, USA). P value < 0.05 was set as statistically significant level for each test.
3. RESULTS
3.1 Plasmatic Aβ
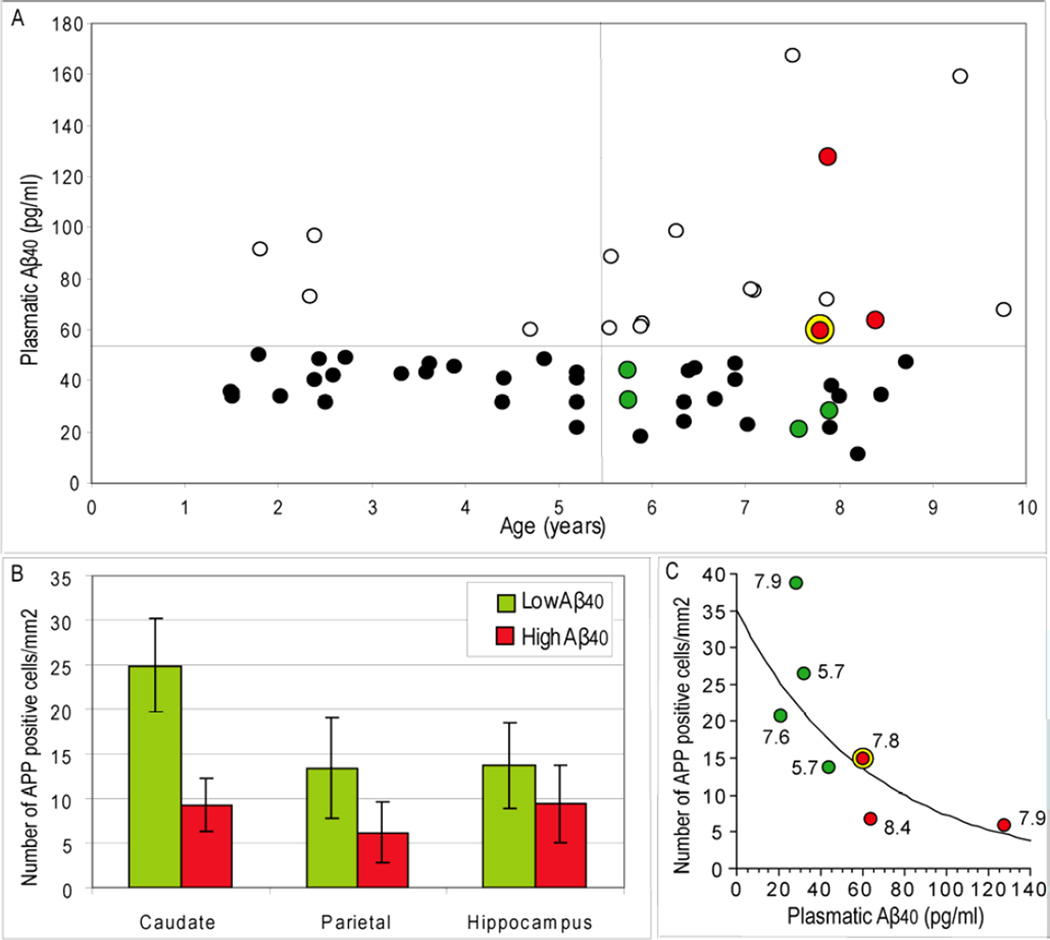
Plasmatic Aβ was evaluated in 1 to 10 years-old mouse lemurs. No significant correlation was noted between plasmatic Aβ40 and age (Fig. 1A). However, a large heterogeneity was observed in aged animals, as compared to young ones. Indeed, when we separated plasma Aβ in two categories: low level (≤55 pg/ml) and high level (>55 pg/ml), we found a significantly larger number of aged animals with a high plasma Aβ as compared to young animals (43 versus 16 % of the old and young animals respectively; X2=4.64; p=0.031). The concentrations of plasma Aβ in the animals classified as low or high plasma Aβ levels were significantly different (Mean±Standard error of the mean (SEM)=36.2±1.5 pg/ml and 86.7±7.9 pg/ml, Student's t test, p<0.0001, respectively).
Figure 1.
A. Plasma Aβ40 in mouse lemurs primates (n=25 young animals (1–5.5 years) and n=33 aged animals (5.5–10 years)). Aβ levels were classified either as low (≤55 pg/ml; dark or green spots) or high levels (>55 pg/ml; white or red spots). A larger number of animals older than 5.5 years had a high Aβ level as compared to young animals. The green and red spots correspond to animals that were studies by histology. The yellow ring corresponds to an animal that had extracellular amyloid deposits. B. Number of neurons presenting intracellular Aβ positive profiles in the caudate, parietal cortex and hippocampus of animals with low and high plasmatic Aβ loads (data represent mean±SEM). C. Plot fitting plasmatic Aβ and the density of intracellular Aβ aggregates in the caudate of aged animals. The values on the side of the dots are ages of the animals at blood sampling. The curve represents a logarithmic fit between intracellular Aβ and plasmatic Aβ. A significant negative correlation was found between intracellular Aβ and plasmatic Aβ (r=−0.86, p<0.05).
3.2 Neuropathology
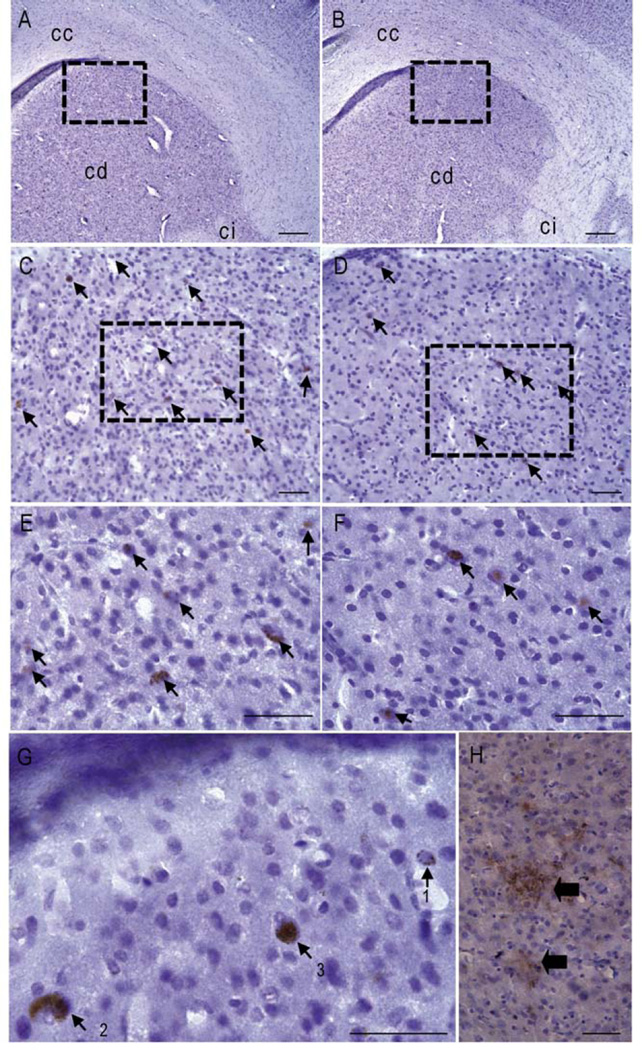
The brains of seven aged animals (6.1 to 9.3 years old) studied for plasmatic Aβ could be evaluated by neuropathology (four animals had low plasmatic Aβ (Fig. 1A, green dots) and three had high plasma Aβ (Fig. 1A, red dots). Extracellular Aβ deposits were observed in the cortex of only one out of the seven aged animals analyzed (Fig. 2H). It was an 8 year-old animal with a high concentration of plasmatic Aβ (59.9 pg/ml). The two other animals with high plasmatic Aβ (127.5 and 63.5 pg/ml) did not have plaques. In all the animals, 4G8-positive objects were mainly observed in the form of intracellular deposits (Fig. 2C-G). These deposits were present in several brain regions such as the parietal cortex, hippocampus, caudate nucleus and in a ventral brain areas corresponding to the nucleus basalis of Meynert (Bons et al., 1998). They appeared as an accumulation of spherical vesicles within the cells (Fig. 2A-G, Fig. 3A-B). The density of vesicles varied within the cells: the vesicles density could be low (Fig. 2G, label 1); it could be higher, providing an aspect of diffuse deposition (Fig. 2G, label 2); in some cases, the vesicles were densely packed within the cells (Fig. 2G, label 3). Overall, the densely packed deposits were less numerous than the less packed more diffuse deposits.
Figure 2.
Aβ deposits in brain tissues of aged mouse lemurs (4G8 staining). A-F. Comparison of Aβ deposits in animals with low (A, C, E) and high (B, D, F) plasmatic amyloid load. A-B. Low resolution image showing the caudate (cd), corpus callosum (cc) and internal capsula (ci). C-F. Higher magnification images showing intracellular amyloid deposits (arrows) in the caudate. G. High magnification image processed with the 3D mode algorithm of exploranova software showing various types of intracellular deposits: spherical vesicles with a low density (1); spherical vesicles with an increased density providing an aspect of diffuse deposition (2); densely packed vesicles (3). H. Extracellular amyloid plaques as seen in the cortex of only one animal. Scale bars: A-B, H: 200µm, C-G: 50 µm.
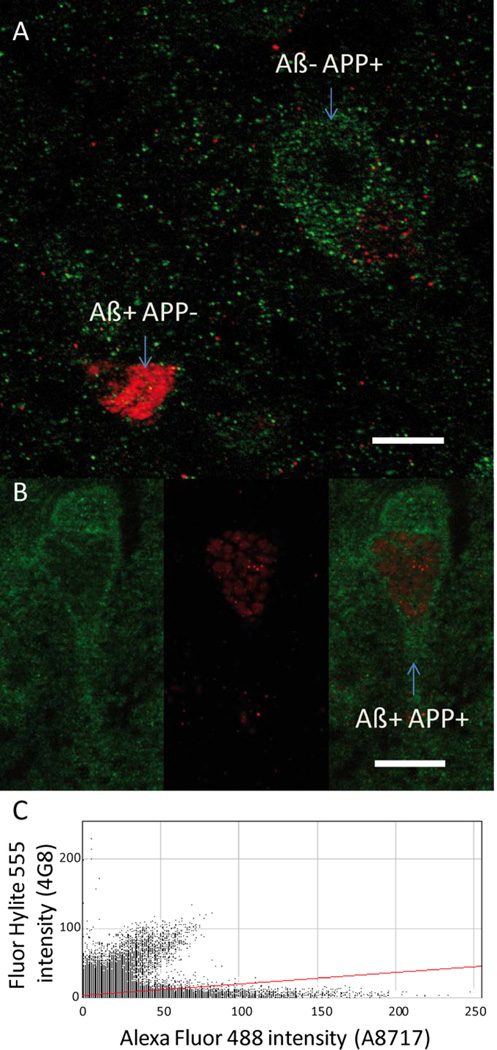
Figure 3.
Identification of the nature of the 4G8-positive intracellular deposits. A-B. Immunofluorescent stained sections showing the location of 4G8 (red) and A8717-positive (green) objects. A. Example showing cells labeled only with 4G8 or with A8717. B. Example showing different locations of 4G8 and A8717-positive objects within the same neuron. C. The overlap coefficients were low between objects detected by the 4G8 or the A8717 antibodies (C). Scale Bars = 10 µm.
The 4G8 positive objects were found in the same brain locations regardless of the immunostaining method (immunoperoxidase or immunofluorescence). No fluorescent staining could be detected in sections incubated without primary antibodies, indicating the absence of confounding autofluorescent background that may interfere with the analysis of double staining. Immunodetection of APP with the A8717 antibody revealed intracellular staining of numerous neurons in almost all brain regions. APP immunostaining was hence not exclusively observed in the neurons displaying 4G8 immunoreactivity (Fig. 3A). In the caudate nucleus 445 cells were randomly selected for APP/4G8 co-labelling analysis. 396 of these neurons (i.e. 89%) were A8717-positive and 4G8-negative (Fig 3A); while only 9 neurons (2%) of the sampled population were A8717-negative and 4G8-positive (Fig 3A). Nine percent (40/445) of the neurons were both A8717-positive and 4G8-positive; however in this subpopulation of double-stained neurons the intracellular topographies of A8717 and 4G8 immunostainings were different and poorly colocalized: low overlap coefficients were indeed observed between 4G8 and A8717 signals (r= 0.569 CI95% 0.528–0.621) in the five studied animals (Fig. 3C). These results showed that, in our experimental conditions, 4G8 immunostaining does not cross-react with APP and therefore points to local accumulations of Aβ.
In the three quantified brain regions, the 4G8-positive cells were more numerous in the animals with low plasma amyloid load as compared to animals with high plasma amyloid load (Fig. 1B). Interestingly, the total number of 4G8-positive cells in the caudate nucleus was negatively correlated to plasmatic Aβ40 (Fig. 1C; r=−0.86, p<0.05).
4. DISCUSSION
Plasma Aβ is used as a biomarker for drug efficiency studies in animal models of AD (Chang et al., 2004; Kounnas et al., 2010; Lanz et al., 2010). However, age-associated plasmatic Aβ modulation in animal models is practically never addressed in the literature. Also data on evolution of plasma Aβ during aging and AD in humans are still controversial (Buerger et al., 2009; Roher et al., 2009; Sobow et al., 2005; Song et al., 2011). Assessing the effect of age and pathology progression on plasmatic Aβ in primate models is thus a crucial issue. Here, we evaluated plasmatic Aβ in mouse lemur primates, a model of cerebral aging that can spontaneously develop intracellular (Joseph-Mathurin et al., 2013; Mestre-Frances et al., 2000) and extracellular (Bons et al., 1991) Aβ deposits while aging. In mouse lemurs, plasma Aβ is already used as a biomarker in drug studies (Trouche et al., 2009; Joseph-Mathurin et al., 2013) although the age effect on plasmatic Aβ has never been studied. In mouse lemurs, plasma Aβ concentrations are much lower than in transgenic mice, as mouse lemurs are non transgenic animals who are not overexpressing Aβ. Because of the low plasma Aβ concentration in lemurs, Aβ42 levels are usually below the limit of detection with classical ELISA tests (Trouche et al., 2009). We thus focused on Aβ40 that is the main Aβ component in the brain. In humans Aβ40 in the CSF is assumed to reflect the total amount of Aβ proteins in the brain (Wiltfang et al., 2007) and a positive relationship has been reported between plasma Aβ40 levels and cerebral amyloid load measured by imaging methods (Devanand et al., 2011). Aβ40 in the plasma is also used to follow-up the action of drugs modulating Aβ synthesis or promoting amyloid clearance in animals (Chang et al., 2004; Lanz et al., 2010, Trouche et al., 2009) and humans (Fleisher et al., 2008). Interestingly, as opposed to transgenic mice (data not shown), we found two categories of aged mouse lemurs: low (57%) and high (43%) plasmatic Aβ (Fig. 1A). This suggests that in lemurs, Aβ is modulated differently amongst animals with aging.
Only one animal out of the seven lemurs evaluated by histology, displayed extracellular Aβ plaques. This animal had high plasma Aβ. However, the other animals with higher plasmatic Aβ did not show any extracellular Aβ deposits. Extracellular deposits were not found in mouse lemurs with low plasma Aβ. This possibly suggests a lack of correlation between these two parameters. However, as the number of extracellular deposits is very rare in mouse lemurs, the number of animals included in our histological evaluation is likely too low to establish such a correlation.
Strikingly intracellular accumulation of 4G8 positive material was detected in all studied animals (with density of intraneuronal staining varying from one case to the other). We selected the 4G8 antibody to immunodetect intraneuronal deposits as it is a standard antibody used in animal and human tissues to reveal Aβ deposition. 4G8 immunohistochemistry is even considered as a reference method for multicentric studies (Alafuzoff et al., 2008) and for the standardization of neuropathological assessment of brain amyloidosis (Alafuzoff et al., 2009). However due to epitope specificity 4G8 can cross-react with APP. The presence of Aβ in neurons is currently highly discussed. It can be clearly evidenced in neuronal cell cultures (Lee et al., 2003) and also in a few AD transgenic mouse lines with very aggressive neuropathology, such as the APPxPS1-Ki and 5xFAD models (Faure et al., 2011; Oakley et al., 2006). However, the presence of Aβ in neurons of AD mice remains a matter of debate (Winton et al., 2011; Wirths et al., 2012; Cuello et al., 2012).
Results from our double labelling control experiments revealed that, in mouse lemurs, 4G8-positive intracellular objects do not strictly correspond to APP accumulation. It can be concluded that 4G8 objects in mouse lemurs are mainly composed of Aβ and not of APP. Intracellular Aβ deposition is often considered as an early stage of the evolution towards AD either in experimental models (Casas et al., 2004; Walsh et al., 2000) or in humans (Gouras et al., 2000). Interestingly, intracellular Aβ vanishes as extracellular Aβ deposition increases (Wirths et al., 2001). This suggests that it is released in the extracellular space before to aggregate in extracellular Aβ deposits. Also, there is a balance between the soluble extracellular Aβ in the brain and in the plasma (Craft et al., 2002). Our data may support a three-phases model of amyloidosis development: (1) First, a low plasmatic amyloid load can be associated either to a low amyloid synthesis which may be the case in young animals and in some aged animals, or to the sequestration of Aβ in the intra-neuronal space, as seen in the old animals included in our study. (2) Then, Aβ is released in the extracellular space from where it is exported to the periphery increasing plasma concentration. This would explain the high plasma Aβ levels found in animals with a low intracellular amyloid load. (3) Data from the literature suggest that later the extracellular Aβ may also aggregate into parenchymal Aβ plaques leading to a reduced plasma Aβ (Song et al., 2011). The phases 1 and 2 of this process match with our observations in lemurs and could explain some findings reported in humans (Song et al., 2011) and dogs (Gonzalez-Martinez et al., 2011) underlining an increased plasmatic Aβ in early phases of AD or of AD-like age-related pathologies. These events could correspond to a time frame during which amyloid can be released from cells but is not yet aggregated into plaques, hence favouring its passage to the plasmatic compartment.
Other factors could however explain plasmatic Aβ modulation in our animals. In humans, it was proposed that plasmatic Aβ pool does not only result from intracerebral Aβ clearance, but also from peripheral sources such as the skeletal muscle, platelets, and vascular walls, which produce appreciable amounts of Aβ (Kuo et al., 2000). Also, some plasma proteins such as immunoglobulins, apolipoproteins and proteins of the complement can bind and mask Aβ peptides or can modulate their metabolism (Roher et al., 2009). Pathological states such as atherosclerotic vascular diseases can also modulate the bioavailability of peripheral Aβ which appears to bind fatty streaks in atherosclerotic vessels (Roher et al., 2009). We cannot rule out a role of these factors in the regulation of plasma Aβ in lemurs. Finally, plasmatic Aβ in lemurs could also be regulated by internal factors such as corticoid levels. Indeed, a study in macaques has shown that plasmatic Aβ is reduced by chronic exposure to corticoids (Kulstad et al., 2005). Environmental factors leading to stress and increased glucocorticoid levels might thus also modulate plasmatic Aβ in lemurs. Longitudinal studies will thus have to be performed to further elucidate the origin of plasmatic Aβ pool and to study the reason of heterogeneous plasmatic Aβ in old lemurs. However, our results suggest that in aged animals plasmatic Aβ can be used as a stratification biomarker to include homogeneous animals in experimental groups for preclinical drug trials that aim at modifying amyloid load.
Mouse lemurs are small primates used as spontaneous models of cerebral aging.
Plasmatic Aβ levels are increased in a subpopulation of aged lemurs.
Intracellular Aβ deposits are detected in several brain regions of aged lemurs.
Plasmatic Aβ is negatively correlated with the density of neurons accumulating Aβ.
ACKNOWLEDGEMENTS
This study was financially supported by the France-Alzheimer Association, the National Foundation for Alzheimer’s Disease and Related Disorders, and the National Institute on Aging (R01-AG020197). We thank Marion Chaigneau, Diane Houitte and Martine Guillermier for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors declare no conflicts of interest.
REFERENCES
- Aho L, Pikkarainen M, Hiltunen M, Leinonen V, Alafuzoff I. Immunohistochemical visualization of amyloid-beta protein precursor and amyloid-beta in extra- and intracellular compartments in the human brain. J. Alzh. Dis. 2010;20:1015–1028. doi: 10.3233/JAD-2010-091681. [DOI] [PubMed] [Google Scholar]
- Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kavantzas N, King A, Korkolopoulou P, Kovacs GG, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Stadelmann C, Streichenberger N, Tagliavini F, Kretzschmar H. Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol. 2008;115:533–546. doi: 10.1007/s00401-008-0358-2. [DOI] [PubMed] [Google Scholar]
- Alafuzoff I, Thal DR, Arzberger T, Bogdanovic N, Al-Sarraj S, Bodi I, Boluda S, Bugiani O, Duyckaerts C, Gelpi E, Gentleman S, Giaccone G, Graeber M, Hortobagyi T, Hoftberger R, Ince P, Ironside JW, Kavantzas N, King A, Korkolopoulou P, Kovacs GG, Meyronet D, Monoranu C, Nilsson T, Parchi P, Patsouris E, Pikkarainen M, Revesz T, Rozemuller A, Seilhean D, Schulz- Schaeffer W, Streichenberger N, Wharton SB, Kretzschmar H. Assessment of beta-amyloid deposits in human brain: a study of the BrainNet Europe Consortium. Acta Neuropathol. 2009;117:309–320. doi: 10.1007/s00401-009-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid beta-amyloid(1–42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch. Neurol. 1999;56:673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J. Microscopy. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Bons N, Mestre N, Petter A. Senile plaques and neurofibrillary changes in the brain of an aged lemurian primate Microcebus murinus. Neurobiol. Aging. 1991;13:99–105. doi: 10.1016/0197-4580(92)90016-q. [DOI] [PubMed] [Google Scholar]
- Bons N, Sihol S, Barbier V, Mestre-Frances N, Albe-Fessard D. A stereotaxic atlas of the grey lesser mouse lemur brain (Microcebus murinus) Brain Res. Bull. 1998;46:1–173. doi: 10.1016/s0361-9230(97)00458-9. [DOI] [PubMed] [Google Scholar]
- Buerger K, Frisoni G, Uspenskaya O, Ewers M, Zetterberg H, Geroldi C, Binetti G, Johannsen P, Rossini PM, Wahlund LO, Vellas B, Blennow K, Hampel H. Validation of Alzheimer's disease CSF and plasma biological markers: the multicentre reliability study of the pilot European Alzheimer's Disease Neuroimaging Initiative (EADNI) Exp. Gerontol. 2009;44:579–585. doi: 10.1016/j.exger.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am. J. Pathol. 2004;165:1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Koelsch G, Wong S, Downs D, Da H, Weerasena V, Gordon B, Devasamudram T, Bilcer G, Ghosh AK, Tang J. In vivo inhibition of Abeta production by memapsin 2 (beta-secretase) inhibitors. J. Neurochem. 2004;89:1409–1416. doi: 10.1111/j.1471-4159.2004.02452.x. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Allard S, Ferretti MT. Evidence for the accumulation of Abeta immunoreactive material in the human brain and in transgenic animal models. Life Sci. 2012;91(23–24):1141–1147. doi: 10.1016/j.lfs.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Craft DL, Wein LM, Selkoe DJ. A mathematical model of the impact of novel treatments on the A beta burden in the Alzheimer's brain, CSF and plasma. Bull. Math. Biol. 2002;64:1011–1031. doi: 10.1006/bulm.2002.0304. [DOI] [PubMed] [Google Scholar]
- Das R, Nachbar RB, Edelstein-Keshet L, Saltzman JS, Wiener MC, Bagchi A, Bailey J, Coombs D, Simon AJ, Hargreaves RJ, Cook JJ. Modeling effect of a gamma-secretase inhibitor on amyloid-beta dynamics reveals significant role of an amyloid clearance mechanism. Bull. Math. Biol. 2011;73:230–247. doi: 10.1007/s11538-010-9540-5. [DOI] [PubMed] [Google Scholar]
- Delatour B, Mercken L, El Hachimi KH, Colle MA, Pradier L, Duyckaerts C. FE65 in Alzheimer's disease: neuronal distribution and association with neurofibrillary tangles. Am. J. Pathol. 2001;158:1585–1591. doi: 10.1016/S0002-9440(10)64113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Schupf N, Stern Y, Parsey R, Pelton GH, Mehta P, Mayeux R. Plasma Abeta and PET PiB binding are inversely related in mild cognitive impairment. Neurology. 2011;77:125–131. doi: 10.1212/WNL.0b013e318224afb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Verret L, Bozon B, El Tannir El Tayara N, Ly M, Kober F, Dhenain M, Rampon C, Delatour B. Impaired neurogenesis, neuronal loss, and brain functional deficits in the APPxPS1-Ki mouse model of Alzheimer's disease. Neurobiol. Aging. 2011;32:407–418. doi: 10.1016/j.neurobiolaging.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, Farlow MR, Galvin JE, Peskind ER, Quinn JF, Sherzai A, Sowell BB, Aisen PS, Thal LJ. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch. Neurol. 2008;65(8):1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez A, Rosado B, Pesini P, Suarez ML, Santamarina G, Garcia-Belenguer S, Villegas A, Monleon I, Sarasa M. Plasma beta-amyloid peptides in canine aging and cognitive dysfunction as a model of Alzheimer's disease. Exp. Gerontol. 2011;46:590–596. doi: 10.1016/j.exger.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am. J. Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Arch. Pathol. Lab. Med. 2001;125:489–492. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph-Mathurin N, Dorieux O, Trouche S, Boutajangout A, Kraska A, Fontès P, Verdier J-M, Sigurdsson EM, Mestre-Francés N, Dhenain M. Aβ immunization in old mouse lemur primates induces cerebral microhemorrhages and accelerates age-associated iron deposits in the choroid plexus. Neurobiol. Aging. 2013;34:2613–2622. doi: 10.1016/j.neurobiolaging.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain CSF plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J. Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, Yu C, Pleynet D, Digregorio PJ, Velicelebi G, Stauderman KA, Comer WT, Mobley WC, Li YM, Sisodia SS, Tanzi RE, Wagner SL. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraska A, Dorieux O, Picq J-L, Petit F, Bourrin E, Chenu E, Volk A, Perret M, Hantraye P, Mestre-Frances N, Aujard F, Dhenain M. Age associated cerebral atrophy in mouse lemur Primates. Neurobiol. Aging. 2011;32:894–906. doi: 10.1016/j.neurobiolaging.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulstad JJ, McMillan PJ, Leverenz JB, Cook DG, Green PS, Peskind ER, Wilkinson CW, Farris W, Mehta PD, Craft S. Effects of chronic glucocorticoid administration on insulin-degrading enzyme and amyloid-beta peptide in the aged macaque. J. Neuropathol. Exp. Neurol. 2005;64:139–146. doi: 10.1093/jnen/64.2.139. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Kokjohn TA, Watson MD, Woods AS, Cotter RJ, Sue LI, Kalback WM, Emmerling MR, Beach TG, Roher AE. Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am. J. Pathol. 2000;156:797–805. doi: 10.1016/s0002-9440(10)64947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J. Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz TA, Wood KM, Richter KE, Nolan CE, Becker SL, Pozdnyakov N, Martin BA, Du P, Oborski CE, Wood DE, Brown TM, Finley JE, Sokolowski SA, Hicks CD, Coffman KJ, Geoghegan KF, Brodney MA, Liston D, Tate B. Pharmacodynamics and pharmacokinetics of the gamma-secretase inhibitor PF-3084014. J. Pharmacol. Exp. Ther. 2010;334:269–277. doi: 10.1124/jpet.110.167379. [DOI] [PubMed] [Google Scholar]
- Lee EB, Skovronsky DM, Abtahian F, Doms RW, Lee VM. Secretion and intracellular generation of truncated Abeta in beta-site amyloid-beta precursor protein-cleaving enzyme expressing human neurons. J. Biol. Chem. 2003;278:4458–4466. doi: 10.1074/jbc.M210105200. [DOI] [PubMed] [Google Scholar]
- Lemere CA. Developing novel immunogens for a safe and effective Alzheimer's disease vaccine. Prog. Brain. Res. 2009;175:83–93. doi: 10.1016/S0079-6123(09)17506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. Alzheimer's disease abeta vaccine reduces central nervous system abeta levels in a nonhuman primate, the Caribbean vervet. Am. J. Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Duff K. A Technique for Serial Collection of Cerebrospinal Fluid from the Cisterna Magna in Mouse. Journal of Visualized Experiments. 2008 doi: 10.3791/960. http://www.jove.com/index/Details.stp?ID=960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, Chang YF, Tracy R, DeKosky ST. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70:1664–1671. doi: 10.1212/01.wnl.0000306696.82017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre-Frances N, Keller E, Calenda A, Barelli H, Checler F, Bons N. Immunohistochemical analysis of cerebral cortical and vascular lesions in the primate Microcebus murinus reveal distinct amyloid beta 1–42 and beta 1–40 immunoreactivity profiles. Neurob. Dis. 2000;7:1–8. doi: 10.1006/nbdi.1999.0270. [DOI] [PubMed] [Google Scholar]
- Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, Chang L, Miller B, Clark C, Green R, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann. Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picq JL, Aujard F, Volk A, Dhenain M. Age-related cerebral atrophy in nonhuman primates predicts cognitive impairments. Neurobiol. Aging. 2012;33:1096–1109. doi: 10.1016/j.neurobiolaging.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, Younkin S, Younkin L, Schiff R, Weksler ME. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol. Aging. 2009;30:1728–1736. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, Sabbagh MN. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvee M, DidierLaurent G, Latarche C, Escanye MC, Olivier JL, Malaplate-Armand C. Additional use of abeta42/abeta40 ratio with cerebrospinal fluid biomarkers p-tau and abeta42 increases the level of evidence of Alzheimer's disease pathophysiological process in routine practice. J. Alzheimers Dis. 2014;41(2):377–386. doi: 10.3233/JAD-131838. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman MJ, Morici M, Hone E, Berger T, Taddei K, Martins IJ, Lim WL, Singh S, Wenk MR, Ghiso J, Buxbaum JD, Gandy S, Martins RN. APOE genotype results in differential effects on the peripheral clearance of amyloid-beta42 in APOE knock-in and knock-out mice. J. Alzheimers Dis. 2010;21:403–409. doi: 10.3233/JAD-2010-100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaets S, Le Bastard N, Martin JJ, Sleegers K, Van Broeckhoven C, De Deyn PP, Engelborghs S. Cerebrospinal fluid Abeta1–40 improves differential dementia diagnosis in patients with intermediate P-tau181P levels. J. Alzheimers Dis. 2013;36(4):759–767. doi: 10.3233/JAD-130107. [DOI] [PubMed] [Google Scholar]
- Sobow T, Flirski M, Kloszewska I, Liberski PP. Plasma levels of alpha beta peptides are altered in amnestic mild cognitive impairment but not in sporadic Alzheimer's disease. Acta Neurobiol. Exp. 2005;65:117–124. doi: 10.55782/ane-2005-1544. [DOI] [PubMed] [Google Scholar]
- Song F, Poljak A, Valenzuela M, Mayeux R, Smythe GA, Sachdev PS. Meta- Analysis of Plasma Amyloid-beta levels in Alzheimer's Disease. J. Alzh. Dis. 2011;25:1–11. doi: 10.3233/JAD-2011-101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimer's and Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- Trouche SG, Asuni A, Boutajangout A, Frangione B, Wisniewski T, Rouland S, Verdier JM, Sigurdsson EM, Mestre-Frances N. Neuropathological evaluation of the nonhuman primate Microcebus murinus immunized with K6Abeta1–30, an Abeta derivative. Alzheimers Dement. 2008;4(4-Suppl2):T211. [Google Scholar]
- Trouche SG, Asuni A, Rouland S, Wisniewski T, Frangione B, Verdier JM, Sigurdsson EM, Mestre-Frances N. Antibody response and plasma Abeta1–40 levels in young Microcebus murinus primates immunized with Abeta1–42 and its derivatives. Vaccine. 2009;27:957–964. doi: 10.1016/j.vaccine.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochem. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Wang A, Das P, Switzer RC, 3rd, Golde TE, Jankowsky JL. Robust amyloid clearance in a mouse model of Alzheimer's disease provides novel insights into the mechanism of amyloid-beta immunotherapy. J. Neurosci. 2011;31:4124–4136. doi: 10.1523/JNEUROSCI.5077-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anatomic. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wiltfang J, Esselmann H, Bibl M, Hull M, Hampel H, Kessler H, Frolich L, Schroder J, Peters O, Jessen F, Luckhaus C, Perneczky R, Jahn H, Fiszer M, Maler JM, Zimmermann R, Bruckmoser R, Kornhuber J, Lewczuk P. Amyloid beta peptide ratio 42/40 but not A beta 42 correlates with phospho-Tau in patients with lowand high-CSF A beta 40 load. J. Neurochem. 2007;101(4):1053–1059. doi: 10.1111/j.1471-4159.2006.04404.x. [DOI] [PubMed] [Google Scholar]
- Winton MJ, Lee EB, Sun E, Wong MM, Leight S, Zhang B, Trojanowski JQ, Lee VM. Intraneuronal APP not free Abeta peptides in 3xTg-AD mice: implications for tau versus Abeta-mediated Alzheimer neurodegeneration. J. Neurosci. 2011;31:7691–7699. doi: 10.1523/JNEUROSCI.6637-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wirths O, Dins A, Bayer TA. AbetaPP accumulation and/or intraneuronal amyloidbeta accumulation? The 3xTg-AD mouse model revisited. J. Alzheimers Dis. 2012;28(4):897–904. doi: 10.3233/JAD-2011-111529. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Bayer TA. A modified beta-amyloid hypothesis: intraneuronal accumulation of the beta-amyloid peptide--the first step of a fatal cascade. J. Neurochem. 2004;91:513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Czech C, Blanchard V, Moussaoui S, Tremp G, Pradier L, Beyreuther K, Bayer TA. Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci. Lett. 2001;306:116–120. doi: 10.1016/s0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yabuki C, Seubert P, Schenk D, Hori Y, Ohtsuki S, Terasaki T, Hashimoto T, Iwatsubo T. Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J. Neurosci. 2009;29:11393–11398. doi: 10.1523/JNEUROSCI.2021-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]