Abstract
Flaviviruses affect hundreds of millions of people each year causing tremendous morbidity and mortality worldwide. This genus includes significant human pathogens such as dengue, West Nile, yellow fever, tick-borne encephalitis and Japanese encephalitis virus among many others. The disease caused by these viruses can range from febrile illness to hemorrhagic fever and encephalitis. A deeper understanding of the virus life cycle is required to foster development of antivirals and vaccines, which are an urgent need for many flaviviruses, especially dengue. The focus of this review is to summarize our current knowledge of flaviviral replication and assembly, the proteins and lipids involved therein, and how these processes are coordinated for efficient virus production.
Introduction
Flaviviruses (Family- Flaviviridae) are one of the major causes of arthropod-borne illness in humans. Flavivirus pathogenesis ranges from mild illness such as fever, rash and joint pain, to more severe symptoms such as hemorrhagic fever and fatal encephalitis. The Flavivirus genus consists of more than 70 enveloped, positive-strand RNA viruses including yellow fever virus (YFV), dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV) and tick-borne encephalitis virus (TBEV). Altogether flaviviruses affect hundreds of millions of individuals every year. Despite the availability of vaccines against YFV, JEV and TBEV, diseases resulting from these viruses are still prevalent worldwide. Protective vaccines or therapies are not yet available against the more pathogenic flaviviruses and prevention from insect bites remains the major defense against some of these viruses. Therefore, a better understanding of the flavivirus life cycle is essential in order to develop effective strategies for antiviral intervention and for development of novel vaccines.
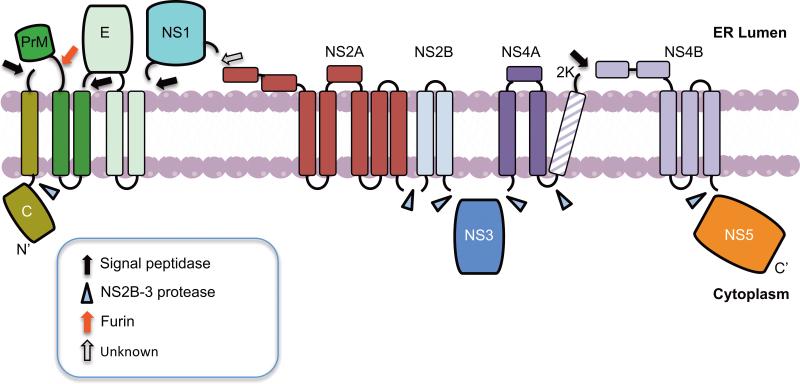
The virus enters host cells by receptor-mediated endocytosis and the ~11 Kb positive-sense RNA genome gains entry into the cytoplasm by viral glycoprotein-mediated membrane fusion. Flavivirus replication begins when the genome is recognized as messenger RNA and translated by host cell machinery to yield a single polyprotein. The polyprotein is co- and post-translationally cleaved by viral and cellular proteases into 10 gene products (Figure 1). The structural proteins capsid (C), precursor membrane (prM/M) and envelope (E) are incorporated into the virion, whereas the non-structural proteins NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 serve to coordinate the intracellular aspects of virus replication, assembly and modulation of host defense mechanisms. NS1 is essential for virus replication and inhibition of complement-mediated immune response [1]. NS3 contains serine protease, Nucleoside 5’ triphosphatase (NTPase), RNA helicase, and 5’ RNA triphosphatase (RTPase) activities, while NS2B serves as a cofactor for the protease activity of NS3. NS5 contains methyltransferase and RNA-dependent RNA polymerase (RdRp) domains required for genome replication and capping of nascent RNA [2]. Three non-enzymatic, integral membrane proteins NS2A, NS4A and NS4B are poorly understood. NS2A is required for virus replication and assembly [3-5]. NS4A induces membrane rearrangement [6,7] and autophagy to enhance viral replication [8], whereas NS4B modulates host immune response by suppressing the α/β interferon signaling and the helicase activity of NS3 [9,10].
Figure 1. Flavivirus polyprotein processing.
Translation of the viral genome results in a polyprotein, which is cleaved by cellular and viral proteases. The proposed topologies of the viral proteins with respect to the ER lumen and cytoplasm and the proteases responsible for their cleavages are indicated. The membrane bilayer is shown in pink, transmembrane domains for individual proteins are shown as cylinders spanning the membrane and connecting loops are shown as lines. Black, blue and red arrow / arrowheads represent signal peptidase, NS2B-3 protease and furin protease cleavage sites respectively. Open arrow represents a protease cleavage site that required additional characterization.
Polyprotein processing into component proteins takes place on ER membranes (Figure 1). The replication complex (RC) is formed on modified ER membranes where negative-sense RNA is copied from the genomic RNA template. The process of RNA synthesis is asymmetric favoring positive-sense RNA [2]. The RNA genome then interacts with C protein, which buds through the glycoprotein-containing ER membranes as immature virus particles into the lumen. The immature virus particles then go through maturation steps in the ER and Golgi complex and are released as mature particles from the cell. The biogenesis of the RC and its assembly involve modifications of cellular metabolic and structural components. This review presents an overview of the current knowledge of interactions between the viral and cellular components to promote cellular changes required for replication and assembly and how these two processes are coupled. Replication and assembly are important aspects of the virus life cycle that are targeted for future antiviral development.
Flavivirus Translation and Replication
Virus-induced membrane rearrangements
It is now established that flavivirus replication and viral RNA synthesis occurs on an extended network of modified ER membranes. At least three distinct membranous structures are found in flavivirus-infected cells: membranous sacs or vesicle packets (Vp), membrane vesicles (Ve) and convoluted membranes (CM) (Figure 2) [11-16]. Vps are small clusters of Ve formed by modification of ER membranes and used as sites of replication by the virus [13,14]. Ve are open to the cytoplasm via a small neck. The CMs are suggested to form the sites of translation and polyprotein processing and/or storage sites for viral proteins [14,15]. These structures are formed by membrane remodeling that can be induced by changes in lipid composition, influence of integral membrane proteins, activities of cytoskeletal protein and microtubule motors, scaffolding by peripheral and integral membrane proteins [17]. Certain lipids such as lysophosphatidic acid and phosphatidic acid favor negative membrane curvature [18]. Similarly, enzymes such as flippase can also introduce membrane asymmetry [19].
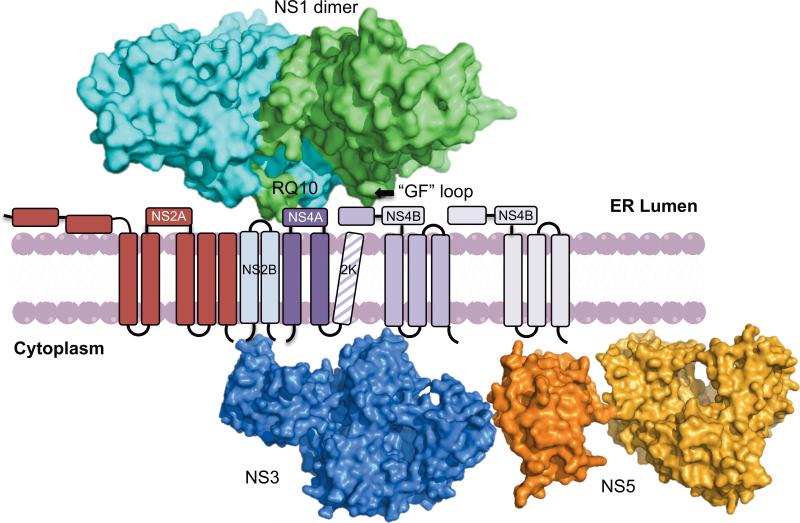
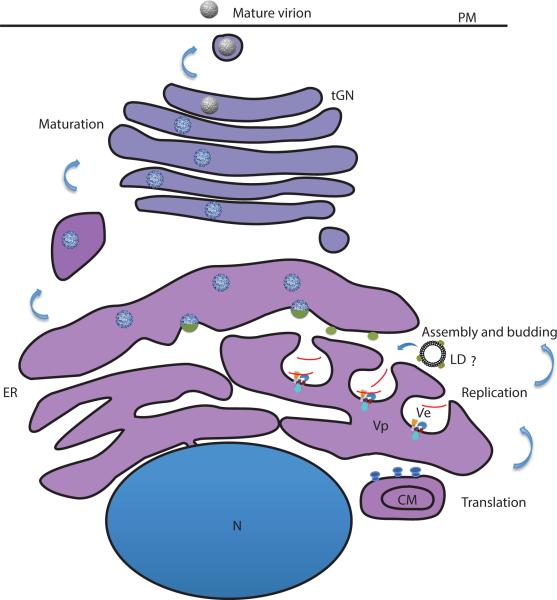
Figure 2. Flavivirus genome replication and assembly.
A) Spatial model for RC on Ve membranes. Surface representation of DENV NS1 dimer (PDB: 4O6B) [23] located in the ER bound to the Ve membrane through its “GF” loops. Dipeptide RQ10 residues of NS1 potentially interact with the NS4B dimer. NS1 also interacts with NS4A. NS2A is represented as a 5 membrane-pass protein interacting with NS3. NS4A and NS4B are also transmembrane proteins interacting with NS3 on the cytoplasmic side. Surface representations of DENV NS3 (PDB: 2VBC) [68], NS5 Methyltransferase domain (PDB: 1L9K) [69] and RdRp domain (PDB: 2J7W) [70] are shown towards the cytoplasmic side of the membrane. The transmembrane domains of membrane proteins are shown as cylinders and the connecting loops are shown as black lines. This model does not depict the exact topology or oligomeric state of the RC proteins and their interactions with host factors.
B) Coupling of Flavivirus replication and assembly. Genome replication takes place on virus-induced Ve membranes that have opening towards the cytoplasm. The replication complex is composed of NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 along with genomic RNA. The newly synthesized RNA (shown in red) exits the pore and is captured by C (shown as green ovals) attached to LDs or localized on the cytoplasmic side of the ER in close vicinity of the Ve pore to form nucleocapsid. The nucleocapsid complex acquires the viral glycoproteins expressed on the ER membrane by budding into the ER lumen. The spiky immature particles (shown in blue) undergo maturation as they traffic through the TGN and become smooth mature particles (shown in gray) by conformational changes in E protein and furin cleavage of prM protein. C: Capsid, CM: Convoluted Membranes, GF: Greasy Finger, LD: Lipid Droplets, ER: Endoplasmic Reticulum, PM: Plasma membrane, RC: Replication complex, tGN: Trans-Golgi Network, Vp: Vesicle packets, Ve: membrane vesicles.
It is still unclear what triggers the membrane modifications in flavivirus infection, viral proteins along with modulation of host lipid metabolism and cytoskeletal components are involved. Expression of KUNV and DENV NS4A containing the uncleaved carboxyl-terminal transmembrane domain, 2K, induced ER membrane rearrangements, while a role of NS4B in membrane rearrangement has also been suggested [6,7,20]. The amino terminal amphipathic helix of NS4A induces homo-oligomerization and is also predicted to form a scaffold in the membrane to induce membrane bending [21]. Additionally, NS4A of DENV was shown to rearrange vimentin intermediate filaments at perinuclear sites. This rearrangement of vimentin along with vimentin phosphorylation was implicated in enrichment of RCs [22]. KUNV NS2A has been implicated in membrane rearrangements, as a mutation at residue 59 (I59A) blocked membrane rearrangements and virus assembly [5]. Recently, NS1 was also shown to have membrane remodeling ability [23]. When purified NS1 was mixed with liposomes rich in cholesterol and phosphatidyl serine, they were fragmented into small nanoparticles suggesting that NS1 possesses an ability to remodel lipid membranes [23].
Comparable membranous structures were reported in both insect and mammalian cells infected with flaviviruses. However, CMs were not observed in DENV-infected insect cells [15]. A higher number of closed ended tubular structures were seen in the TBEV-infected tick cells suggesting that there exist differences between acute and persistent infections with tubular structures being predominant in the latter case [16]. Further studies are required to understand the molecular mechanisms these proteins employ to bring about the structural changes in the ER membrane, the host components involved in the process and the nature of interactions between the viral and host components.
RC arrangement on the membrane
Evidence suggests that viral RNA, NS3 and NS5 are localized within Ve and open to the cytoplasm [13,14,24-27]. The luminal side of the membrane contains NS1, while NS2A, NS2B, NS4A and NS4B are integral membrane proteins. It is still unclear how these proteins are arranged with respect to each other, but specific interactions among nonstructural proteins have been proposed based on genetic, localization and biophysical studies [5,10,28]. Recent genetic studies suggest that alteration of the hydrophobic “greasy finger” loops of NS1 inhibited DENV replication, highlighting the importance of the hydrophobic loop and its potential role in membrane interactions [23]. Additionally, reports demonstrating interactions between WNV NS1 and NS4B as well as between YFV NS1 and NS4A suggest that NS1 interacts with the RC via NS4A and NS4B [29,30]. Based on these observations, the model depicts the NS1 dimer docking on the membrane and interacting with NS4B via the NS1 β-roll (dipeptide RQ10) sequence. It is possible that NS1 stabilizes the replication complex on the membrane by holding the NS4A and NS4B proteins together and creating a scaffold for the replication machinery. NS2A was also predicted to be a transmembrane protein that colocalized with dsRNA and other proteins of the RC [4,25]. In addition, NS3 interactions with NS2A, NS4A, and NS4B [5,10,31] on membrane were predicted based on genetic studies on YFV, DENV and WNV. Figure 2A represents how RC is assembled on Ve membranes based on the above observations. This model depicts the viral components and their orientation with respect to the membrane. It does not represent their exact topology, oligomeric state or their interactions with host factors. An overview of the replication sites, Vp and Ve membranes, is shown in figure 2B, where RCs are shown on the Ve membrane along with nascent RNA being transcribed.
Flavivirus Assembly
We understand exceedingly little about the assembly process of flaviviruses. A packaging sequence on the genome of flaviviruses has not been identified with C-RNA interactions believed to be non-specific and electrostatic [32,33], yet the genomic RNA is specifically packaged into virions. It has been suggested that this specificity is achieved by coupling genomic replication and encapsidation. There is evidence to indicate that only actively synthesized RNAs are packaged, hinting that replication and virion assembly are closely intertwined processes [34]. Spatial coordination of the two processes has been observed by electron tomography in both DENV-infected mammalian and insect cells. As pointed out earlier, replication is believed to occur within the lumen of the Ve, which have openings/pores that possibly allow entry of building blocks from the cytoplasm and the subsequent release of genomic RNA for packaging (Figure 2B). Budding of virions from ER sites directly opposite to the Ve pores has been visualized and a connection between the Ve pore and the neck of the budding virions has also been seen [14,15]. It should be pointed out that not all virus-induced Ve structures identified in positive-strand RNA viruses have open pores [35-37]. Therefore, it would be interesting to probe the mechanism of formation and molecular architecture of these pores. To this end, it has been suggested that the small hydrophobic non-structural proteins (2A, 2B and 4B) of JEV have viroporin-like activity or capability for membrane permeabilization [38] but this needs to be explored further. It is also unclear how viral RNA is transported from the lumen of Ve to the sites of assembly. Genetic evidence suggests the involvement of several viral non-structural proteins and host proteins in the assembly pathway (Table 1), but their exact role needs to be elucidated. Based on the current evidence, it appears that NS2A, alone or in complex with NS3, may play a role in genome transport.
Table 1.
Proteins implicated in flavivirus assembly.
| Protein | Flavivirus | Suggested Role in assembly | Ref. |
|---|---|---|---|
|
Viral
| |||
| NS1 | WNV/DENV (RepliVAX) | Elimination of NS1' decreases packaging efficiency* | [71] |
| NS2A | YFV, KUNV | Nucleocapsid formation/incorporation | [3,5,72] |
| DENV | Formation of intracellular virions | [4] | |
| NS3 | YFV | Interaction with NS2 for virion morphogenesis | [5] |
| KUNV | Required in cis for assembly and/or release | [73-75] | |
| YFV | Not required for assembly in cis | [76] | |
| YFV | Nucleocapsid formation/incorporation | [77] | |
| NS2B-3 | YFV, MVEV** | Sequential capsid cleavage for efficient nucleocapsid incorporation into budding viral membranes | [46] |
| NS5 | KUNV | Coupling between genome replication and assembly – Active replication is required for packaging | [34] |
|
Host | |||
| Alix | YFV | Interaction with NS3 for virion morphogenesis | [78] |
| DDX56 | WNV | Interaction with C for virion morphogenesis | [79,80] |
| Nucleolin | DENV | Interaction with C for virion morphogenesis | [81] |
NS1' is an alternative gene product in WNV resulting from a ribosomal frameshift
MVEV: Murray Valley encephalitis virus
The cellular location of nucleocapsid formation (C-RNA complex) has also been debated in the field. Lipid droplets (LDs) have been implicated as sites for nucleocapsid assembly since C was found to localize to lipid droplets and mutations in the α2 helix (L50S-L54S) that disrupt C association with LDs abrogated virion production [39]. This suggestion conflicts with tomographic data as well as the observation that DENV assembles in cell lines that are not rich in LDs. The significance, mechanism and dynamics of C-LD interaction are not clear. The role of this association in sequestration of C to prevent premature binding to RNA has been speculated since mistargeted mutant C (L50S-L54S) displayed a dominant negative effect on DENV replication when expressed in trans [39]. C-RNA interaction studies have been challenging since nucleocapsids have not been visualized in flavivirus-infected cells though nucleocapsid-like particles of irregular geometry have been assembled in vitro from purified C [33,40,41]. Cryo-electron microscopy based single particle reconstruction of flaviviruses has also demonstrated a lack of an organized C-RNA nucleocapsid core [42-44]. Failure to visualize assembly intermediates suggests that the assembly processes are rapid and/or simultaneous with replication and budding.
Lack of tangible interaction between C and the prM/E glycoproteins is another confounding aspect of the flavivirus assembly. Budding of viral particles appears to be nucleocapsid independent, since subviral particles (SVPs) can be produced by over-expressing only the prM/E glycoproteins [45]. Mechanisms that ensure efficient nucleocapsid incorporation into budding viral membranes are not well understood. Requirement of a sequential cleavage of C by NS2B-3 on the cytosolic side (releases mature capsid) before cleavage by host signalase on the ER side (C-prM junction) had been postulated to be a mechanism for temporal regulation of virus assembly [46-48]. One possibility is that the first cleavage allows C-RNA interaction through NS2B-3 (and its interacting partners), and the delayed second cleavage allows nucleocapsid incorporation into budding membranes containing viral glycoproteins.
Following budding, through a complex interplay of events requiring conformational changes in E and furin cleavage of prM, non-infectious, spiky, immature particles get converted to smooth infectious virions as they pass through the secretory pathway. The maturation process of flaviviruses is outside the scope of this review but readers are referred to excellent reviews on this subject [49,50].
Roles of Cellular Lipids in Replication and Assembly
Based on studies so far, it appears that the high demand for lipids due to membrane remodeling and budding virions in flavivirus-infected cells is met in several ways. Reabsorption of LDs, the natural reservoirs and processing centers of lipids in the cell by the ER membrane was observed in DENV-infected cells [51]. Redistribution of fatty acid synthase (FASN), in a Rab18-dependent manner, by DENV NS3 at the sites of replication appears to be a mechanism utilized by the virus to maintain the supply of fatty acids [52,53]. FASN is a multienzyme protein that catalyzes the synthesis of palmitate from acetyl-CoA and malonyl-CoA in the presence of NADH into long chain saturated fatty acids. WNV replication also requires fatty acid synthesis [54]. Additionally, DENV has been shown to induce autophagy that leads to break down of LDs and triglycerides into fatty acids leading to higher β-oxidation and ATP release [55]. An increase in lysophosphatidylcholine that induces membrane curvature, was also noticed in DENV-infected mosquito cells along with increased levels of palmitic and stearic acids, indicating lipogenesis in the infected cells [56]. In YFV, DMVJC14 acts as a chaperone and targets the proteins of the RC to detergent-resistant membranes or lipid rafts [31].
Cholesterol and membrane rafts play an important role in flavivirus attachment and entry [57-62], but their role in virus replication has only come to light recently. Modulation of cholesterol biosynthesis is important for DENV replication, as inhibition of mevalonate diphospho decarboxylase (MVD) by siRNAs reduced Den-Rluc replication in A549 cells. Interestingly, other genes in the cholesterol pathways did not affect viral replication [63]. Surprisingly, supplementing cholesterol at the virus adsorption stage externally blocked both entry and replication of JEV and DENV2 in N18 cells [64]. When the cholesterol biosynthesis inhibitor, lovastatin, was used ER membrane biogenesis remained unaffected. However, production of infectious DENV particles was abrogated suggesting that cholesterol has additional functions in replication [51,65]. Furthermore, increased levels of cholesterol were induced in the early stages of DENV infection and an increase in LDL particle uptake and HMG-COA reductase activity was seen [58]. Several drugs that affect cellular lipid biosynthesis affect flavivirus replication (Table 2). Interestingly, no involvement of cellular pathways such as the unfolded protein response (UPR) and sterol regulatory element binding protein (SREBP-2) pathways that primarily mediate cholesterol and fatty acid biosynthesis was seen in DENV infection [51].
Table 2.
Inhibitors used to target cellular lipids and their effects on flavivirus lifecycle.
| Inhibitor | Target | Virus | Effect on Virus | Ref. |
|---|---|---|---|---|
| U18666A | Cholesterol transport | DENV | Inhibits entry and replication | [82] |
| C75 | FASN | DENV WNV | Reduces replication | [54,56,82] |
| Cerulenin | FASN | WNV | Reduces replication | [54] |
| GGTI | Geranylgeranylation | WNV | Reduced replication | [26] |
| AY9944 | Conversion from 7-dehydrocholesterol to cholesterol | WNV | Reduced replication | [26] |
| Methyl-beta cyclodextrin (MBCD), Nystatin | Cholesterol incorporation into the membrane (cholesterol depletion) | DENV | Reduced replication | [83] |
| SFV785 | Kinase Inhibitor, suppressor of flaviviridae 785 | DENV | Reduced virus assembly | [84] |
| GW4869, Spiroepoxide | nSMase | WNV | Subviral particle release | [67] |
| Lovastatin | HMG-CoA reductase, blocks conversion from HMG CoA to mevalonate | DENV | Reduces virus assembly | [65] |
| Spautin-1 | Autophagy | DENV | Reduced virus maturation | [85] |
Levels of a cellular sphingolipid precursor, ceramide, were found to be elevated in DENV-infected mosquito and mammalian cells but its role in the infection process is unclear [56]. Increased levels of ceramide in the cell were shown to inhibit JEV replication [66]. Thus, it is not clear whether increased levels of ceramides are induced by DENV for its advantage or if it's a cellular stress response and has a role in the induction of apoptosis [56]. Additionally, the importance of neutral sphingomylinases (nSMases) has been observed in WNV biogenesis. Inhibitors of nSMases reduced the formation of Vps and SVP [67]. Follow up studies are required to understand the role of sphingolipids and ceramides in flavivirus infection.
Conclusions
A common feature of flaviviruses is to remodel ER membranes to generate distinct organelle-like structures. These structures may be utilized by the virus for specific steps of its life cycle to ensure efficient replication, protection of viral RNA from cellular defense mechanisms and well-coordinated assembly and budding processes. Although there is evidence indicating the role of viral and host factors, the exact molecular mechanisms behind these membrane modifications are still poorly understood. Biochemical evidence is required to study the composition and the distinct roles of the individual membranous structures in replication and assembly. Several steps in the process such as how RNA is translocated from the site of replication to budding, and what host factors are involved in the process are not well known. Cellular lipids are highly involved in the replication and assembly process but how the virus modulates the levels and locations of various lipids is obscure. More studies to understand the key components of the lipid metabolic pathways and their roles in modulation of cell cycle and survival in the context of flavivirus infection are required.
Highlights.
■ Flavivirus replication occurs on modified ER membranes called vesicle packets (Vp) and membrane vesicles (Ve).
■ Flavivirus non-structural proteins are implicated in membrane rearrangements.
■ Viral replication and assembly appear to be tightly coupled.
■ Non-structural proteins are important for virus assembly and budding.
■ Cellular lipids play an essential role in membrane rearrangements, virus assembly and budding.
Acknowledgements
This work was supported by NIH grants R21 AI083984 and R01 AI76331 to R.J.K. from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avirutnan P, Fuchs A, Hauhart RE, Somnuke P, Youn S, Diamond MS, Atkinson JP. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J ExpMed. 2010;207:793–806. doi: 10.1084/jem.20092545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindenbach BD, Murray CL, Thiel H-J, Rice CM, Knipe DM, et al., editors. Flaviviridae: The Viruses and Their Replication. 6th Edition. Lippincott Williams & Wilkins; New York, NY: 2013. 2013. Fields Virology. Chapter 26 in. [In Fields Virology, edn 6th. Edited by al DMKe: Lippincott Williams & Wilkins.] [Google Scholar]
- 3.Leung JY, Pijlman GP, Kondratieva N, Hyde J, Mackenzie JM, Khromykh AA. Role of Nonstructural Protein NS2A in Flavivirus Assembly. J Virol. 2008;82:4731–4741. doi: 10.1128/JVI.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Gayen S, Kang C, Yuan Z, Shi P-Y. Membrane Topology and Function of Dengue Virus NS2A Protein. J Virol. 2013;87:4609–4622. doi: 10.1128/JVI.02424-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kümmerer BM, Rice CM. Mutations in the Yellow Fever Virus Nonstructural Protein NS2A Selectively Block Production of Infectious Particles. J Virol. 2002;76:4773–4784. doi: 10.1128/JVI.76.10.4773-4784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller S, Kastner S, Krijnse-Locker J, Bühler S, Bartenschlager R. The Non-structural Protein 4A of Dengue Virus Is an Integral Membrane Protein Inducing Membrane Alterations in a 2K-regulated Manner. J Biol Chem. 2007;282:8873–8882. doi: 10.1074/jbc.M609919200. [DOI] [PubMed] [Google Scholar]
- 7.Roosendaal J, Westaway EG, Khromykh A, Mackenzie JM. Regulated Cleavages at the West Nile Virus NS4A-2K-NS4B Junctions Play a Major Role in Rearranging Cytoplasmic Membranes and Golgi Trafficking of the NS4A Protein. J Virol. 2006;80:4623–4632. doi: 10.1128/JVI.80.9.4623-4632.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLean JE, Wudzinska A, Datan E, Quaglino D, Zakeri Z. Flavivirus NS4A-induced Autophagy Protects Cells against Death and Enhances Virus Replication. J Biol Chem. 2011;286:22147–22159. doi: 10.1074/jbc.M110.192500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muñoz-Jordán JL, Laurent-Rolle M, Ashour J, Martínez-Sobrido L, Ashok M, Lipkin WI, García-Sastre A. Inhibition of Alpha/Beta Interferon Signaling by the NS4B Protein of Flaviviruses. J Virol. 2005;79:8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umareddy I, Chao A, Sampath A, Gu F, Vasudevan SG. Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J Gen Virol. 2006;87:2605–2614. doi: 10.1099/vir.0.81844-0. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie LK, Hoenen A, Morgan G, Mackenzie JM. The Endoplasmic Reticulum Provides the Membrane Platform for Biogenesis of the Flavivirus Replication Complex. J Virol. 2010;84:10438–10447. doi: 10.1128/JVI.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackenzie J. Wrapping Things up about Virus RNA Replication. Traffic. 2005;6:967–977.. doi: 10.1111/j.1600-0854.2005.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westaway EG, Mackenzie JM, Kenney MT, Jones MK, Khromykh AA. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CKE, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. Composition and Three-Dimensional Architecture of the Dengue Virus Replication and Assembly Sites. Cell Host & Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [Electron tomography of DENV infected mammalian cells shows ER membrane rearrangements induced to promote viral replication and the spatial proximilty of replication and assembly sites.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Junjhon J, Pennington JG, Edwards TJ, Perera R, Lanman J, Kuhn RJ. Ultrastructural Characterization and Three-Dimensional Architecture of Replication Sites in Dengue Virus-Infected Mosquito Cells. J Virol. 2014;88:4687–4697. doi: 10.1128/JVI.00118-14. [Electron tomography of DENV infected mosquito cells links the formation of membrane vesicles (Ve) to the rate of viral RNA synthesis. It also highlights key differences between viral induced membrane rearrangements in mammalian (host) and insect (vector) cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Offerdahl DK, Dorward DW, Hansen BT, Bloom ME. A Three-Dimensional Comparison of Tick-Borne Flavivirus Infection in Mammalian and Tick Cell Lines. PLoS ONE. 2012;7:e47912. doi: 10.1371/journal.pone.0047912. [Comparison between acute and persistent TBEV-infected cells using electron tomography.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 18.Kooijman EE, Chupin V, de Kruijff B, Burger KNJ. Modulation of Membrane Curvature by Phosphatidic Acid and Lysophosphatidic Acid. Traffic. 2003;4:162–174. doi: 10.1034/j.1600-0854.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 19.Devaux PF, Herrmann A, Ohlwein N, Kozlov MM, 1591 16. How lipid flippases can modulate membrane structure. Bioch Bioph Acta (BBA) - Biomembranes. 2008;1778 doi: 10.1016/j.bbamem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Kaufusi PH, Kelley JF, Yanagihara R, Nerurkar VR. Induction of Endoplasmic Reticulum-Derived Replication-Competent Membrane Structures by West Nile Virus Non-Structural Protein 4B. PLoS ONE. 2014;9:e84040. doi: 10.1371/journal.pone.0084040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stern O, Hung Y-F, Valdau O, Yaffe Y, Harris E, Hoffmann S, Willbold D, Sklan EH. An N-Terminal Amphipathic Helix in Dengue Virus Nonstructural Protein 4A Mediates Oligomerization and Is Essential for Replication. J Virol. 2013;87:4080–4085. doi: 10.1128/JVI.01900-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teo CSH, Chu JJH. Cellular Vimentin Regulates Construction of Dengue Virus Replication Complexes through Interaction with NS4A Protein. J Virol. 2014;88:1897–1913. doi: 10.1128/JVI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akey DL, Brown WC, Dutta S, Konwerski J, Jose J, Jurkiw TJ, DelProposto J, Ogata CM, Skiniotis G, Kuhn RJ, et al. Flavivirus NS1 Structures Reveal Surfaces for Associations with Membranes and the Immune System. Science. 2014;343:881–885. doi: 10.1126/science.1247749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackenzie JM, Jones MK, Young PR. Immunolocalization of the Dengue Virus Nonstructural Glycoprotein NS1 Suggests a Role in Viral RNA Replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 25.Mackenzie JM, Khromykh AA, Jones MK, Westaway EG. Subcellular Localization and Some Biochemical Properties of the Flavivirus Kunjin Nonstructural Proteins NS2A and NS4A. Virology. 1998;245:203–215. doi: 10.1006/viro.1998.9156. [DOI] [PubMed] [Google Scholar]
- 26.Mackenzie JM, Khromykh AA, Parton RG. Cholesterol Manipulation by West Nile Virus Perturbs the Cellular Immune Response. Cell Host & Microbe. 2007;2:229–239. doi: 10.1016/j.chom.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Matusan AE, Pryor MJ, Davidson AD, Wright PJ. Mutagenesis of the Dengue Virus Type 2 NS3 Protein within and outside Helicase Motifs: Effects on Enzyme Activity and Virus Replication. J Virol. 2001;75:9633–9643. doi: 10.1128/JVI.75.20.9633-9643.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L, Takeda K, Markoff L. Protein–protein interactions among West Nile non-structural proteins and transmembrane complex formation in mammalian cells. Virology. 2013;446:365–377. doi: 10.1016/j.virol.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Youn S, Li T, McCune BT, Edeling MA, Fremont DH, Cristea IM, Diamond MS. Evidence for a Genetic and Physical Interaction between Nonstructural Proteins NS1 and NS4B That Modulates Replication of West Nile Virus. J Virol. 2012;86:7360–7371. doi: 10.1128/JVI.00157-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindenbach BD, Rice CM. Genetic Interaction of Flavivirus Nonstructural Proteins NS1 and NS4A as a Determinant of Replicase Function. J Virol. 1999;73:4611–4621. doi: 10.1128/jvi.73.6.4611-4621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi Z, Yuan Z, Rice CM, MacDonald MR. Flavivirus replication complex assembly revealed by DNAJC14 functional mapping. J Virol. 2012;86:11815–11832. doi: 10.1128/JVI.01022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pong W-L, Huang Z-S, Teoh P-G, Wang C-C, Wu H-N. RNA binding property and RNA chaperone activity of dengue virus core protein and other viral RNA-interacting proteins. FEBS Letters. 2011;585:2575–2581. doi: 10.1016/j.febslet.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teoh P-G, Huang Z-S, Pong W-L, Chen P-C, Wu H-N. Maintenance of Dimer Conformation by the Dengue Virus Core Protein α4-α4′ Helix Pair Is Critical for Nucleocapsid Formation and Virus Production. J Virol. 2014;88:7998–8015. doi: 10.1128/JVI.00940-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Khromykh AA, Varnavski AN, Sedlak PL, Westaway EG. Coupling between Replication and Packaging of Flavivirus RNA: Evidence Derived from the Use of DNA-Based Full-Length cDNA Clones of Kunjin Virus. J Virol. 2001;75:4633–4640. doi: 10.1128/JVI.75.10.4633-4640.2001. [Active replication is required for viral RNA packagining. Transfection of KUNV plasmid DNA with mutation in the active site of RNA polymerase region of NS5 allowed continuous production of genomic RNA with tranlation capability but no infectious virions were recovered (infectious virions were obtained upon transfection with plasmid encoding wild type Kunjin DNA).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belov GA, Nair V, Hansen BT, Hoyt FH, Fischer ER, Ehrenfeld E. Complex dynamic development of poliovirus membranous replication complexes. J Virol. 2012;86:302–312. doi: 10.1128/JVI.05937-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limpens RW, van der Schaar HM, Kumar D, Koster AJ, Snijder EJ, van Kuppeveld FJ, Barcena M. The transformation of enterovirus replication structures: a three-dimensional study of single- and double-membrane compartments. MBio. 2011;2 doi: 10.1128/mBio.00166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero-Brey I, Merz A, Chiramel A, Lee JY, Chlanda P, Haselman U, Santarella-Mellwig R, Habermann A, Hoppe S, Kallis S, et al. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 2012;8:e1003056. doi: 10.1371/journal.ppat.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YS, Liao CL, Tsao CH, Chen MC, Liu CI, Chen LK, Lin YL. Membrane permeabilization by small hydrophobic nonstructural proteins of Japanese encephalitis virus. J Virol. 1999;73:6257–6264. doi: 10.1128/jvi.73.8.6257-6264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samsa MM, Mondotte JA, Iglesias NG, Assunção-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. Dengue Virus Capsid Protein Usurps Lipid Droplets for Viral Particle Formation. PLoS Pathog. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López C, Gil L, Lazo L, Menéndez I, Marcos E, Sánchez J, Valdés I, Falcón V, de la Rosa M, Márquez G, et al. In vitro assembly of nucleocapsid-like particles from purified recombinant capsid protein of dengue-2 virus. Arch Virology. 2009;154:695–698. doi: 10.1007/s00705-009-0350-8. [DOI] [PubMed] [Google Scholar]
- 41.Kiermayr S, Kofler RM, Mandl CW, Messner P, Heinz FX. Isolation of Capsid Protein Dimers from the Tick-Borne Encephalitis Flavivirus and In Vitro Assembly of Capsid-Like Particles. J Virol. 2004;78:8078–8084. doi: 10.1128/JVI.78.15.8078-8084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Ge P, Yu X, Brannan JM, Bi G, Zhang Q, Schein S, Zhou ZH. Cryo-EM structure of the mature dengue virus at 3.5-A resolution. Nat Struct Mol Biol. 2013;20:105–110. doi: 10.1038/nsmb.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schalich J, Allison SL, Stiasny K, Mandl CW, Kunz C, Heinz FX. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J Virol. 1996;70:4549–4557. doi: 10.1128/jvi.70.7.4549-4557.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee E, Stocks CE, Amberg SM, Rice CM, Lobigs M. Mutagenesis of the signal sequence of yellow fever virus prM protein: enhancement of signalase cleavage In vitro is lethal for virus production. J Virol. 2000;74:24–32. doi: 10.1128/jvi.74.1.24-32.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lobigs M, Lee E. Inefficient signalase cleavage promotes efficient nucleocapsid incorporation into budding flavivirus membranes. J Virol. 2004;78:178–186. doi: 10.1128/JVI.78.1.178-186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lobigs M, Lee E, Ng ML, Pavy M, Lobigs P. A flavivirus signal peptide balances the catalytic activity of two proteases and thereby facilitates virus morphogenesis. Virology. 2010;401:80–89. doi: 10.1016/j.virol.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Pierson TC, Diamond MS. Degrees of maturity: the complex structure and biology of flaviviruses. Curr Opin Virol. 2012;2:168–175. doi: 10.1016/j.coviro.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodenhuis-Zybert IA, Wilschut J, Smit JM. Partial maturation: an immune-evasion strategy of dengue virus? Trends Microbiol. 2011;19:248–254. doi: 10.1016/j.tim.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Peña J, Harris E. Early Dengue Virus Protein Synthesis Induces Extensive Rearrangement of the Endoplasmic Reticulum Independent of the UPR and SREBP-2 Pathway. PLoS ONE. 2012;7:e38202. doi: 10.1371/journal.pone.0038202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Heaton NS, Perera R, Berger KL, Khadka S, LaCount DJ, Kuhn RJ, Randall G. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Nat Acad Sci. 2010;107:17345–17350. doi: 10.1073/pnas.1010811107. [Role of fatty acid synthase in DENV infection and its interaction with NS3 at sites of replication.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang W-C, Lin R-J, Liao C-L, Lin Y-L. Rab18 Facilitates Dengue Virus Infection by Targeting Fatty Acid Synthase to Sites of Viral Replication. J Virol. 2014;88:6793–6804. doi: 10.1128/JVI.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martín-Acebes MA, Blázquez A-B, Jiménez de Oya N, Escribano-Romero E, Saiz J-C. West Nile Virus Replication Requires Fatty Acid Synthesis but Is Independent on Phosphatidylinositol-4-Phosphate Lipids. PLoS ONE. 2011;6:e24970. doi: 10.1371/journal.pone.0024970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heaton NS, Randall G. Dengue Virus-Induced Autophagy Regulates Lipid Metabolism. Cell Host & Microbe. 2010;8:422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, Pasa-Tolic L, Metz TO, Adamec J, Kuhn RJ. Dengue Virus Infection Perturbs Lipid Homeostasis in Infected Mosquito Cells. PLoS Pathog. 2012;8:e1002584. doi: 10.1371/journal.ppat.1002584. [Mass-spectrometric analysis and comparision of lipid species in mosquito cells infected with dengue virus compared to non-infected cells and UV treated cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das S, Chakraborty S, Basu A. Critical role of lipid rafts in virus entry and activation of phosphoinositide 3′ kinase/Akt signaling during early stages of Japanese encephalitis virus infection in neural stem/progenitor cells. J Neurochem. 2010;115:537–549. doi: 10.1111/j.1471-4159.2010.06951.x. [DOI] [PubMed] [Google Scholar]
- 58.Soto-Acosta R, Mosso C, Cervantes-Salazar M, Puerta-Guardo H, Medina F, Favari L, Ludert JE, del Angel RM. The increase in cholesterol levels at early stages after dengue virus infection correlates with an augment in LDL particle uptake and HMG-CoA reductase activity. Virology. 2013;442:132–147. doi: 10.1016/j.virol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Kalia M, Khasa R, Sharma M, Nain M, Vrati S. Japanese Encephalitis Virus Infects Neuronal Cells through a Clathrin-Independent Endocytic Mechanism. J Virol. 2013;87:148–162. doi: 10.1128/JVI.01399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Medigeshi GR, Hirsch AJ, Streblow DN, Nikolich-Zugich J, Nelson JA. West Nile Virus Entry Requires Cholesterol-Rich Membrane Microdomains and Is Independent of αvβ3 Integrin. J Virol. 2008;82:5212–5219. doi: 10.1128/JVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Y-Z, Cao M-M, Wang W-B, Wang W, Ren H, Zhao P, Qi Z-T. Association of heat-shock protein 70 with lipid rafts is required for Japanese encephalitis virus infection in Huh7 cells. J Gen Virol. 2012;93:61–71. doi: 10.1099/vir.0.034637-0. [DOI] [PubMed] [Google Scholar]
- 62.Zaitseva E, Yang S-T, Melikov K, Pourmal S, Chernomordik LV. Dengue Virus Ensures Its Fusion in Late Endosomes Using Compartment-Specific Lipids. PLoS Pathog. 2010;6:e1001131. doi: 10.1371/journal.ppat.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rothwell C, LeBreton A, Young Ng C, Lim JYH, Liu W, Vasudevan S, Labow M, Gu F, Gaither LA. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 3898-19 doi: 10.1016/j.virol.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Lee C-J, Lin H-R, Liao C-L, Lin Y-L. Cholesterol Effectively Blocks Entry of Flavivirus. J Virol. 2008;82:6470–6480. doi: 10.1128/JVI.00117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez-Gutierrez M, Correa-Londoño LA, Castellanos JE, Gallego-Gómez JC, Osorio JE. Lovastatin Delays Infection and Increases Survival Rates in AG129 Mice Infected with Dengue Virus Serotype 2. PLoS ONE. 2014;9:e87412. doi: 10.1371/journal.pone.0087412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tani H, Shiokawa M, Kaname Y, Kambara H, Mori Y, Abe T, Moriishi K, Matsuura Y. Involvement of Ceramide in the Propagation of Japanese Encephalitis Virus. J Virol. 2010;84:2798–2807. doi: 10.1128/JVI.02499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martín-Acebes MA, Merino-Ramos T, Blázquez A-B, Casas J, Escribano-Romero E, Sobrino F, Saiz J-C. The Composition of West Nile virus Lipid Envelope Unveils a Role of Sphingolipid Metabolism on Flavivirus Biogenesis. J Virol. 2014 doi: 10.1128/JVI.02061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo D, Xu T, Hunke C, Grüber G, Vasudevan SG, Lescar J. Crystal Structure of the NS3 Protease-Helicase from Dengue Virus. J Virol. 2008;82:173–183. doi: 10.1128/JVI.01788-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO. 2002;21:2757–2768. doi: 10.1093/emboj/21.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yap TL, Xu T, Chen Y-L, Malet H, Egloff M-P, Canard B, Vasudevan SG, Lescar J. Crystal Structure of the Dengue Virus RNA-Dependent RNA Polymerase Catalytic Domain at 1.85-Angstrom Resolution. J Virol. 2007;81:4753–4765. doi: 10.1128/JVI.02283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winkelmann ER, Widman DG, Suzuki R, Mason PW. Analyses of mutations selected by passaging a chimeric flavivirus identify mutations that alter infectivity and reveal an interaction between the structural proteins and the nonstructural glycoprotein NS1. Virology. 2011;421:96–104. doi: 10.1016/j.virol.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Liu WJ, Chen HB, Khromykh AA. Molecular and Functional Analyses of Kunjin Virus Infectious cDNA Clones Demonstrate the Essential Roles for NS2A in Virus Assembly and for a Nonconservative Residue in NS3 in RNA Replication. J Virol. 2003;77:7804–7813. doi: 10.1128/JVI.77.14.7804-7813.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khromykh AA, Sedlak PL, Westaway EG. cis- and trans-Acting Elements in Flavivirus RNA Replication. J Virol. 2000;74:3253–3263. doi: 10.1128/jvi.74.7.3253-3263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu WJ, Sedlak PL, Kondratieva N, Khromykh AA. Complementation Analysis of the Flavivirus Kunjin NS3 and NS5 Proteins Defines the Minimal Regions Essential for Formation of a Replication Complex and Shows a Requirement of NS3 in cis for Virus Assembly. J Virol. 2002;76:10766–10775. doi: 10.1128/JVI.76.21.10766-10775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pijlman GP, Kondratieva N, Khromykh AA. Translation of the Flavivirus Kunjin NS3 Gene in cis but Not Its RNA Sequence or Secondary Structure Is Essential for Efficient RNA Packaging. J Virol. 2006;80:11255–11264. doi: 10.1128/JVI.01559-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones CT, Patkar CG, Kuhn RJ. Construction and applications of yellow fever virus replicons. Virology. 2005;331:247–259. doi: 10.1016/j.virol.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 77.Patkar CG, Kuhn RJ. Yellow Fever Virus NS3 Plays an Essential Role in Virus Assembly Independent of Its Known Enzymatic Functions. J Virol. 2008;82:3342–3352. doi: 10.1128/JVI.02447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carpp LN, Galler R, Bonaldo MC. Interaction between the yellow fever virus nonstructural protein NS3 and the host protein Alix contributes to the release of infectious particles. Microbes and Infection. 2011;13:85–95. doi: 10.1016/j.micinf.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 79.Xu Z, Anderson R, Hobman TC. The Capsid-Binding Nucleolar Helicase DDX56 Is Important for Infectivity of West Nile Virus. J Virol. 2011;85:5571–5580. doi: 10.1128/JVI.01933-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Z, Hobman TC. The helicase activity of DDX56 is required for its role in assembly of infectious West Nile virus particles. Virology. 2012;433:226–235. doi: 10.1016/j.virol.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balinsky CA, Schmeisser H, Ganesan S, Singh K, Pierson TC, Zoon KC. Nucleolin interacts with the dengue virus capsid protein and plays a role in formation of infectious virus particles. J Virol. 2013;87:13094–13106. doi: 10.1128/JVI.00704-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poh MK, Shui G, Xie X, Shi P-Y, Wenk MR, Gu F. U18666A, an intra-cellular cholesterol transport inhibitor, inhibits dengue virus entry and replication. Antiviral Research. 2012;93:191–198. doi: 10.1016/j.antiviral.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 83.Carro AC, Damonte EB. Requirement of cholesterol in the viral envelope for dengue virus infection. Virus Research. 2013;174:78–87. doi: 10.1016/j.virusres.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 84.Anwar A, Hosoya T, Leong KM, Onogi H, Okuno Y, Hiramatsu T, Koyama H, Suzuki M, Hagiwara M, Garcia-Blanco MA. The Kinase Inhibitor SFV785 Dislocates Dengue Virus Envelope Protein from the Replication Complex and Blocks Virus Assembly. PLoS ONE. 2011;6:e23246. doi: 10.1371/journal.pone.0023246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mateo R, Nagamine CM, Spagnolo J, Méndez E, Rahe M, Gale M, Yuan J, Kirkegaard K. Inhibition of Cellular Autophagy Deranges Dengue Virion Maturation. J Virol. 2013;87:1312–1321. doi: 10.1128/JVI.02177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]