Abstract
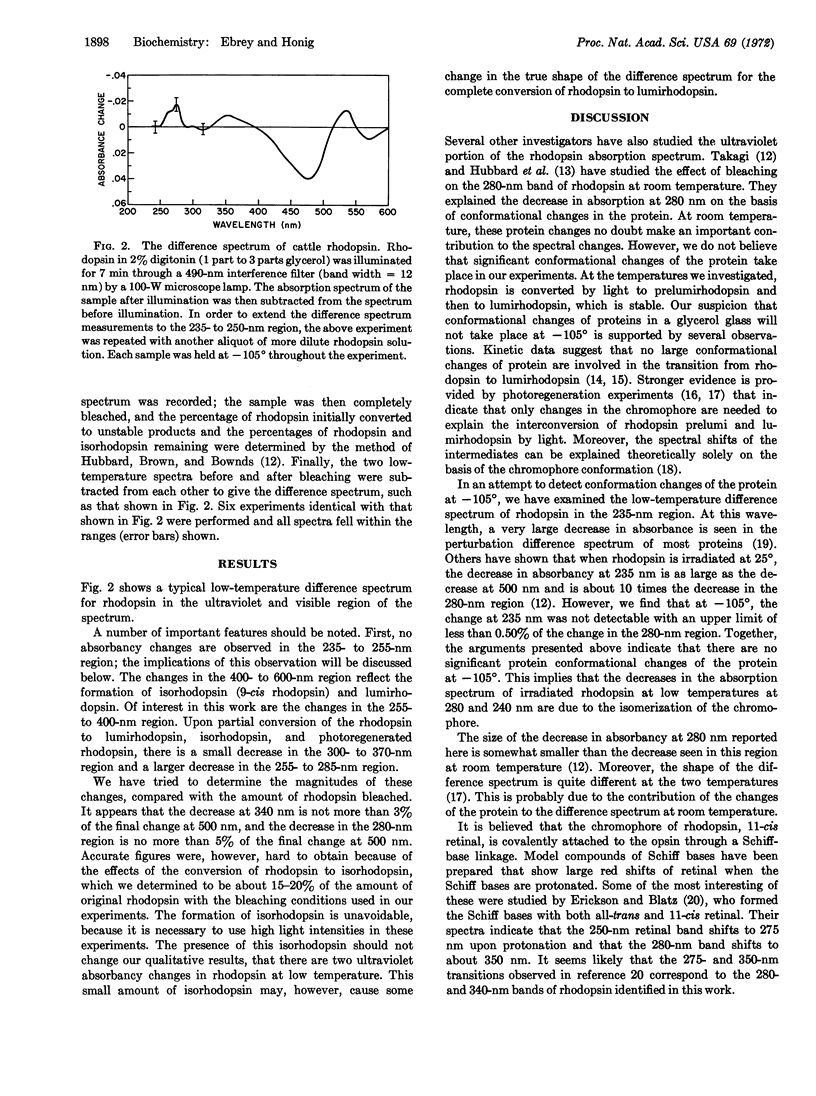
Difference spectra measured at -105° show two decreases in the ultraviolet absorption spectrum of rhodopsin upon bleaching that cannot be attributed to changes in protein conformation. These absorbancy decreases in rhodopsin are consistent with a cis-trans isomerization of the chromophore.
Keywords: cis peaks, perturbation difference spectra, retinal, Schiff bases
Full text
PDF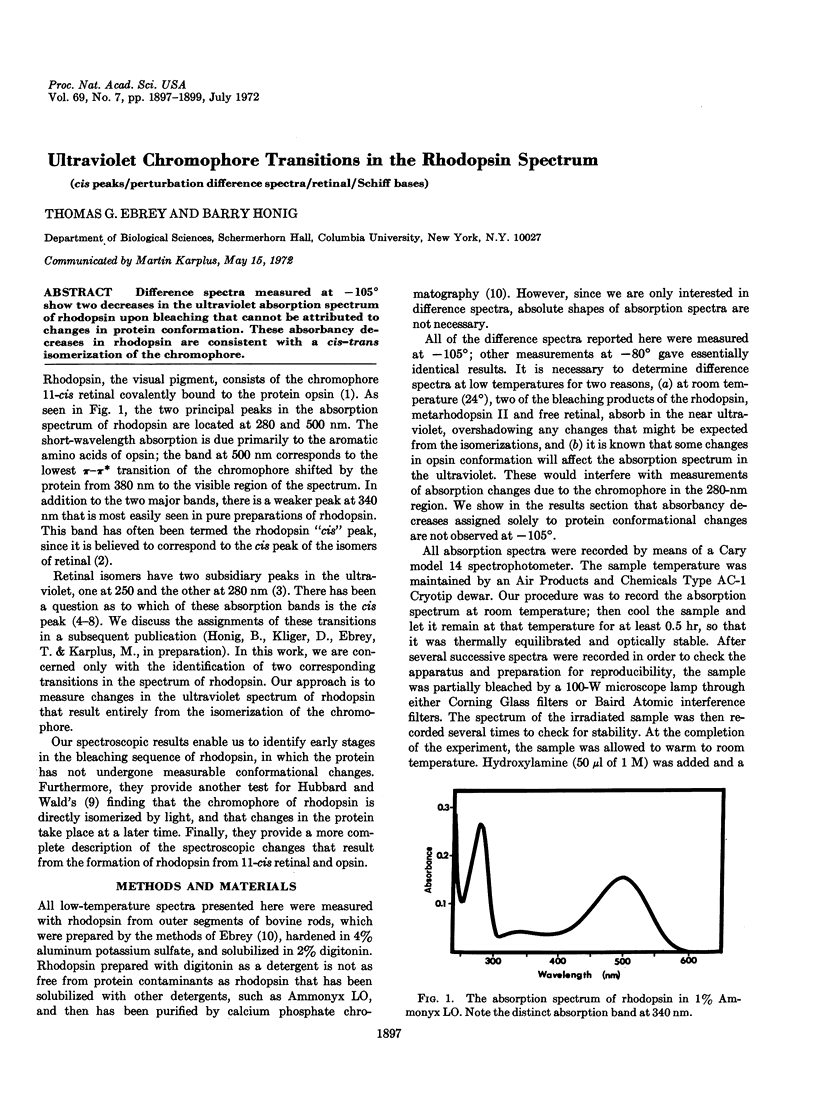

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN P. K., WALD G. The neo-b isomer of vitamin A and retinene. J Biol Chem. 1956 Oct;222(2):865–877. [PubMed] [Google Scholar]
- DIETERLE J. M., ROBESON C. D. Crystalline neoretinene b. Science. 1954 Aug 6;120(3110):219–220. doi: 10.1126/science.120.3110.219-a. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G. The use of Ammonyx LO in the purification of rhodopsin and rod outer segments. Vision Res. 1971 Sep;11(9):1007–1009. doi: 10.1016/0042-6989(71)90220-3. [DOI] [PubMed] [Google Scholar]
- Erickson J. O., Blatz P. E. N-retinylidene-1-amino-2-propanol: a Schiff base analog for rhodopsin. Vision Res. 1968 Oct;8(10):1367–1375. doi: 10.1016/0042-6989(68)90056-4. [DOI] [PubMed] [Google Scholar]
- GRELLMANN K. H., LIVINGSTON R., PRATT D. A flashphotolytic investigation of rhodopsin at low temperatures. Nature. 1962 Mar 31;193:1258–1260. doi: 10.1038/1931258a0. [DOI] [PubMed] [Google Scholar]
- HUBBARD R., BROWN P. K., KROPF A. Vertebrate lumi- and meta-rhodopsins. Nature. 1959 Feb 14;183(4659):442–446. [PubMed] [Google Scholar]
- HUBBARD R., WALD G. Cis-trans isomers of vitamin A and retinene in the rhodopsin system. J Gen Physiol. 1952 Nov;36(2):269–315. doi: 10.1085/jgp.36.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Karplus M. Implications of torsional potential of retinal isomers for visual excitation. Nature. 1971 Feb 19;229(5286):558–560. doi: 10.1038/229558a0. [DOI] [PubMed] [Google Scholar]
- Hubbard R., Bownds D., Yoshizawa T. The chemistry of visual photoreception. Cold Spring Harb Symp Quant Biol. 1965;30:301–315. doi: 10.1101/sqb.1965.030.01.032. [DOI] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W., Rafferty C. N. Relationship between absorption spectrum and molecular conformations of 11-cis-retinal. Nature. 1969 Nov 8;224(5219):590–594. doi: 10.1038/224591a0. [DOI] [PubMed] [Google Scholar]
- TAKAGI M. Studies on the ultraviolet-spectral displacements of cattle rhodopsin. Biochim Biophys Acta. 1963 May 21;66:328–340. doi: 10.1016/0006-3002(63)91202-2. [DOI] [PubMed] [Google Scholar]
- Wald G. Molecular basis of visual excitation. Science. 1968 Oct 11;162(3850):230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]


